核受体NR6A1通过上调RIPK3基因表达诱导血管平滑肌细胞凋亡*
2017-09-22张亚辉吴胜英彭吉霞武福云张秋芳吕艳霞
张亚辉, 汪 雄, 吴胜英, 彭吉霞, 武福云, 柯 镜, 张 鹏, 张秋芳, 吕艳霞
(湖北医药学院病理生理学教研室, 湖北 十堰 442000)
核受体NR6A1通过上调RIPK3基因表达诱导血管平滑肌细胞凋亡*
张亚辉, 汪 雄, 吴胜英, 彭吉霞, 武福云, 柯 镜, 张 鹏, 张秋芳, 吕艳霞△
(湖北医药学院病理生理学教研室, 湖北 十堰 442000)
目的: 探讨核受体亚家族6A1(NR6A1)对血管平滑肌细胞凋亡的影响及可能的分子机制。方法: 将腺病毒Ad-NR6A1感染大鼠血管平滑肌细胞,分别在感染后0 h、24 h和48 h时进行MTT实验,以时间为横坐标,A570为纵坐标绘制细胞生长曲线,观察NR6A1对细胞生长的影响;进行DAPI染色、TUNEL染色及caspase活性检测,观察细胞凋亡情况; 进一步通过基因芯片技术,寻找NR6A1的靶基因;采用siRNA介导的基因沉默技术,观察受体相互作用丝氨酸/苏氨酸蛋白激酶3(RIPK3)基因沉默对NR6A1诱导的血管平滑肌细胞凋亡的影响。结果: 腺病毒Ad-NR6A1感染细胞48 h时,NR6A1过表达组细胞数量较对照组(重组腺病毒载体Ad-LacZ)明显减少;DAPI染色显示NR6A1过表达诱导血管平滑肌细胞出现核浓缩和核碎裂的凋亡表型,TUNEL染色显示NR6A1过表达引起细胞凋亡,caspase活性检测结果显示NR6A1过表达细胞内caspase-3、caspase-8和caspase-9活性均较对照组高;基因芯片技术检测发现,NR6A1过表达上调血管平滑肌细胞中RIPK3基因表达;RIPK3基因沉默可以显著抑制NR6A1诱导的平滑肌细胞凋亡。结论: NR6A1通过上调RIPK3基因表达诱导血管平滑肌细胞凋亡。
核受体亚家族6A1; 受体相互作用丝氨酸/苏氨酸蛋白激酶3; 细胞凋亡
核受体亚家族6A1(nuclear receptor subfamily 6,group A,member 1,NR6A1)又名生殖细胞核因子(germ cell nuclear factor,GCNF),是目前发现的核受体超家族第6亚族唯一的成员。核受体家族在调节生长发育、生殖、代谢及维持内环境稳定等方面发挥了重要作用。已有研究表明NR6A1在生殖及神经发育中具有重要生物学作用。NR6A1通过调节鱼精蛋白、线粒体3-磷酸甘油脱氢酶、endozepine样肽等基因的表达,影响精子的成熟、活力[1-3];NR6A1还可以下调BMP-15和GDF-9基因的表达,从而调节卵泡发育[4]。NR6A1基因缺陷的小鼠胚胎出现神经发育障碍及心血管缺陷[5]。研究发现,NR6A1能够抑制Oct4基因表达,调节神经细胞分化[6];通过抑制miR-302a调节cyclin D1表达,影响胚胎干细胞分化[7]。Rajkovic等[2]报道,NR6A1能够与CREMτ竞争结合靶基因启动子,影响下游基因表达。我们前期研究发现,平滑肌细胞中的NR6A1能够与细胞核actin和RNA聚合酶Ⅱ形成复合物,抑制平滑肌细胞内源性骨桥蛋白(osteopontin)的转录水平,并调节平滑肌细胞迁移[8]。本研究观察了NR6A1对血管平滑肌细胞凋亡的影响及可能的分子机制。
材料和方法
1材料和试剂
Ad-NR6A1腺病毒由本实验室制备[9];DMEM及胎牛血清购自Gibco;小鼠抗FLAG标签抗体和TRITC偶联的羊抗小鼠IgG购自Santa Cruz;紫杉醇(paclitaxel)购自成都曼思特生物科技公司;TUNEL细胞凋亡检测试剂盒和caspase活性检测试剂盒购自Millipore;小鼠抗受体相互作用丝氨酸/苏氨酸蛋白激酶2(receptor-interacting serine/threonine-protein kinase 2,RIPK2)和RIPK3抗体购自Santa Cruz;RIPK3干扰片段购自上海生工生物科技有限公司;SYBR Green PCR试剂盒购自Clontech。
2方法
2.1免疫荧光化学染色技术 将体外培养的大鼠血管平滑肌细胞接种于6孔板中,用含10%胎牛血清的DMEM培养至90%融合。将Ad-NR6A1感染细胞,24 h后细胞经4%多聚甲醛固定,进行常规的免疫荧光化学染色,用抗FLAG标签抗体为 I 抗在4 ℃孵育过夜,随后用TRITC标记的 II 抗显示NR6A1的表达,用DAPI标记细胞核,采用相同种属的IgG作为阴性对照抗体,在共聚焦显微镜下观察染色结果并照相。
2.2RNA提取及RT-qPCR技术 将体外培养的大鼠血管平滑肌细胞接种于6孔板中,用含10%胎牛血清的DMEM培养至90%融合。然后向细胞加入10 nmol/L浓度的paclitaxel,分别在0 h、6 h、12 h和24 h后用Trizol试剂提取总RNA。采用逆转录试剂盒和SYBR Green PCR试剂盒做RT-qPCR反应,检测靶基因的表达。
2.3细胞凋亡的检测 如上述方法接种血管平滑肌细胞于6孔板中,将Ad-NR6A1-FLAG感染细胞,分别在感染后0 h、24 h和48 h时进行MTT实验,以时间为横坐标,A570为纵坐标绘制细胞生长曲线,观察NR6A1对细胞生长的影响;进行DAPI染色和TUNEL染色,观察细胞凋亡情况。Ad-NR6A1感染细胞48 h后用4%多聚甲醛固定,加入新鲜配制的TUNEL检测液,37 ℃避光孵育60 min,在共聚焦显微镜下观察染色结果并照相。Caspase活性检测按试剂盒操作要求,在Ad-NR6A1感染细胞48 h后裂解细胞,离心收集上清,使样品与底物37 ℃孵育约2 h,测定A570值,根据对硝基苯胺标准曲线和样品A值,计算出caspase酶活性。
2.4蛋白免疫印迹技术 收集腺病毒感染后的细胞,制备蛋白样品。Ad-NR6A1感染血管平滑肌细胞24~48 h后收集细胞,运用RIPA裂解液提取总蛋白,蛋白变性后采用SDS-PAGE及ECL显色检测相关蛋白水平。
2.5siRNA介导的基因沉默 将体外培养的大鼠血管平滑肌细胞接种于6孔板中,用含10%胎牛血清的DMEM培养至60%~80%融合,使用前换成无血清培养基培养。分别用Opti-MEM reduced serum medium 稀释Lipo2000与siRNA,将稀释好的siRNA与Lipo2000轻轻混匀,室温培养20~25 min,将siRNA-Lipo2000混合液加入细胞,37 ℃的CO2培养箱中培养4~6 h,换成完全培养基,继续培养24 h后裂解细胞提取总蛋白,运用Western blot检测基因沉默效果。
2.6基因表达谱芯片技术 Ad-LacZ或者Ad-NR6A1感染血管平滑肌细胞24h,采用Trizol法提取总RNA,由上海生工生物技术有限公司行Affymetrix表达谱分析。
3统计学分析
采用SPSS软件处理,数据以均数±标准差(mean±SD)表示,多组比较采用单因素方差分析,两两比较采用SNK法。以P<0.05为差异有统计学意义。
结 果
1NR6A1表达于血管平滑肌细胞核中
既往研究显示NR6A1表达于细胞核中。我们首先观察了NR6A1在平滑肌细胞中的亚细胞定位。免疫荧光化学技术发现,血管平滑肌细胞中所表达的融合蛋白NR6A1与细胞核有共定位,提示NR6A1定位在血管平滑肌细胞核内,见图1。
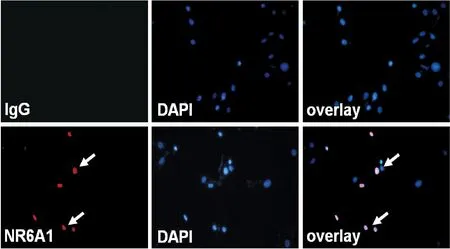
Figure 1. The sub-cellular localization of NR6A1 in the VSMCs (×400). VSMCs were infected with Ad-NR6A1 for 24 h. Indirect immunofluorescence staining of VSMCs with anti-NR6A1 and tetramethylrhodamine isothiocyanate-conjugated donkey anti-rabbit antibodies was performed. The nuclei stained with DAPI were shown. The negative controls were stained with normal IgG only. The arrows pointed at VSMCs were positive for NR6A1.
图1免疫荧光化学技术检测NR6A1在平滑肌细胞中的定位
2紫杉醇增加血管平滑肌细胞中NR6A1的表达
RT-qPCR结果显示,在紫杉醇处理平滑肌细胞12 h和24 h后,可以抑制细胞中actin的表达,同时NR6A1 mRNA的表达较对照组明显增加,提示NR6A1是紫杉醇的靶基因,见图2。
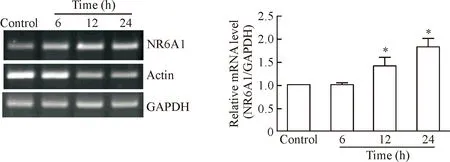
Figure 2. The effect of paclitaxel on mRNA expression of NR6A1 in the VSMCs. Serum-starved VSMCs were treated with paclitaxel for 6 h, 12 h and 24 h. The mRNA levels of NR6A1 were measured by RT-qPCR. Mean±SD.n=3.*P<0.05vscontrol group.
图2紫杉醇对平滑肌细胞中NR6A1mRNA表达的影响
3NR6A1过表达引起血管平滑肌细胞凋亡
在Ad-NR6A1感染细胞后0 h、24 h和48 h时进行MTT实验,Western blot显示腺病毒诱导NR6A1蛋白过表达(图3A)。以时间为横坐标,A570为纵坐标绘制细胞生长曲线,结果表明,48 h时NR6A1过表达组细胞数量较对照组明显减少(图3B)。DAPI染色显示NR6A1过表达诱导血管平滑肌细胞出现核浓缩和核碎裂的凋亡表型,TUNEL染色结果显示NR6A1过表达引起细胞凋亡(图3C)。进一步的caspase活性检测结果显示NR6A1过表达组细胞内caspase-3、caspase-8和caspase-9活性均较对照组高(图3D)。
4凋亡信号基因RIPK3是NR6A1靶基因
本研究通过基因芯片技术检测发现,NR6A1过表达可以上调血管平滑肌细胞中的169个基因,下调217个基因(图4A)。另外我们对挑选出来的差异表达基因做了gene ontology的统计分析,发现NR6A1参与调节免疫反应、氧化还原反应、蛋白水解、发育、转录调节等生物学过程(图4B)。其中,凋亡信号调节基因RIPK3是NR6A1的靶基因,芯片结果显示NR6A1过表达可以上调平滑肌细胞中RIPK3基因表达(图4C)。进一步采用蛋白免疫实验发现,NR6A1过表达可以增加平滑肌细胞RIPK2和RIPK3的蛋白表达,其中主要增加了RIPK3的表达(图4D)。
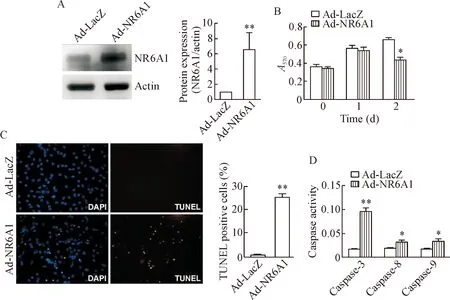
Figure 3. The effect of NR6A1 over-expression on VSMC apoptosis. VSMCs were infected by Ad-LacZ or Ad-NR6A1 at 100 MOI for 48 h. A: NR6A1 protein levels in the cells were determined by Western blot; B: the cell viability was assessed by MTT assay; C: the cell apoptosis was analyzed by TUNEL assay (×200); D: the activities of caspase-3, 8 and 9 in the VSMCs were measured by colorimetric enzymatic assay. Mean±SD.n=3.*P<0.05,**P<0.01vsAd-LacZ group.
图3NR6A1过表达对平滑肌细胞凋亡的影响
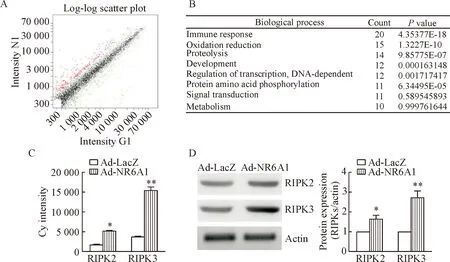
Figure 4. The effect of NR6A1 over-expression on VSMC signal pathways and RIPK expression. A: log-log scatter plot of NR6A1 over-expression group (X axis) and control group (Y axis) was shown (the red labeled valid points and green labeled valid points were the ratio values of the fluorescence signal intensity in the 2 groups ≥2 or ≤0.5, respectively, indicating the genes with differential expression; the black labeled valid points were the ratio values of the fluorescence signal intensity in the 2 groups between 0.5 and 2, indicating the genes without differential expression); B: statistic analysis of gene ontology (GO) for the differentially expressed genes (the minimalPvalues of the 10 categorizations of GO analysis were shown); C: the effect of NR6A1 over-expression on RIPK expression was measured by DNA microarray; D: the effect of NR6A1 over-expression on RIPK expression was analyzed by Western blot. Mean±SD.n=3.*P<0.05,**P<0.01vsAd-LacZ group.
图4NR6A1过表达对平滑肌细胞信号途径和RIPK表达的影响
5RIPK3基因沉默抑制NR6A1诱导的平滑肌细胞凋亡
本研究采用siRNA介导的基因沉默技术,敲低了RIPK3基因,观察RIPK3基因是否是NR6A1诱导平滑肌细胞凋亡的分子机制。结果显示,RIPK3基因沉默可以显著抑制NR6A1诱导的平滑肌细胞凋亡,见图5。
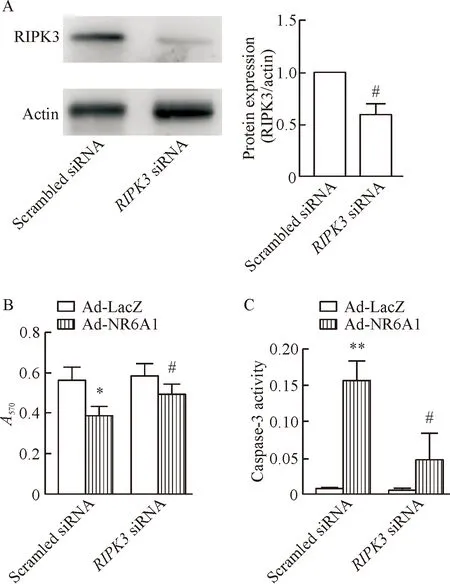
Figure 5.RIPK3 silencing inhibited NR6A1-induced apoptosis of the VSMCs. The VSMCs were transfected withRIPK3 siRNA fragments for 24 h. A: the protein expression of RIPK3 was determined by Western blot; B: the cell viability was analyzed by MTT assay; C: the caspase activities were measured. Mean±SD.n=3.*P< 0.05,**P<0.01vsAd-LacZ group;#P<0.05vsscrambled siRNA group.
图5RIPK3基因沉默对NR6A1诱导的平滑肌细胞凋亡的影响
讨 论
心脑血管疾病已成为目前死亡率最高的疾病,其具有“发病率高、死亡率高、致残率高”的特点,严重威胁中老年人健康。它们共同的病理基础是动脉粥样硬化、内膜增生。病变部位主要在大型及中型肌弹力型动脉,尤其以主动脉、冠状动脉及脑动脉多见。在动脉粥样硬化进展过程中,血管平滑肌细胞从动脉中膜增殖并迁移至内膜下是动脉粥样硬化及内膜增生的重要发病环节之一,造成血管腔狭窄,是引起血管腔闭塞的重要原因[10]。因此探寻抑制平滑肌细胞的增殖与迁移或促进平滑肌细胞凋亡的分子机制是心脑血管疾病研究领域的热点问题。
NR6A1是目前发现的核受体超家族第6亚族唯一的成员。核受体超家族在调节胚胎生长发育、生殖、代谢及维持内环境稳定等方面发挥了重要作用[11-13]。人与大鼠NR6A1基因的同源性、蛋白结构功能的一致性超过90%。NR6A1由A/B、C、D和E四个结构域组成:A/B区高度可变,包含至少一种本身有活性的配体非依赖性的转录激活域;保守的C域为DNA结合区,包含2个锌指结构可以和靶基因上的激素应答元件或其它特异性DNA序列结合,如AGGTCAAGGTCA及TCAAGGTCAAGGTCA;D域为一铰链区可与辅抑制子结合;最大的E域能够与配体结合,二聚体化并被激活,发挥转录因子的作用,调控下游靶基因[14]。但是与其它核受体不同的是,NR6A1对靶基因的作用主要表现为抑制效应。比如,NR6A1可以下调BMP-15和GDF-9基因的表达,从而调节卵泡发育[4];NR6A1能够抑制Oct4基因表达,调节神经细胞分化[6];还通过抑制miR-302a调节cyclin D1表达,影响胚胎干细胞分化[7]。
我们前期研究发现,NR6A1过表达能够抑制PDGF-BB和血清诱导的血管平滑肌细胞迁移,NR6A1可以与RNA聚合酶Ⅱ和细胞核中的actin形成大复合物,并进而结合cAMP反应元件结合蛋白(cAMP response element binding protein,CREB),抑制CREB对分泌型磷蛋白1(secreted phosphoprotein-1,SPP1)基因的转录激活,从而下调SPP1基因表达[8]。可见,NR6A1是调节血管平滑肌细胞的重要分子。
本研究进一步探讨了NR6A1过表达对平滑肌细胞凋亡的影响,结果发现NR6A1过表达48 h可引起平滑肌细胞明显凋亡,细胞内caspase-3、caspase-8和caspase-9活性均升高,以caspase-3活性升高最明显。进一步的实验表明NR6A1过表达能增加RIPK3的基因表达。RIPK3是丝氨酸/苏氨酸蛋白激酶受体相互作用蛋白家族的一员,其氨基端为特征性的丝/苏氨酸激酶结构域,羧基端为RIPK3所独有的结构域[14]。有研究显示,作为肿瘤坏死因子受体1信号复合物的一部分,RIPK3能诱导细胞凋亡[15-16]。本研究发现RIPK3基因沉默则抑制NR6A1过表达诱导的平滑肌细胞凋亡。因此我们认为NR6A1很可能通过上调RIPK3的基因表达,启动平滑肌细胞内的caspase凋亡信号通路导致细胞凋亡。NR6A1调节RIPK的分子机制还有待进一步研究。
[1] Hummelke GC, Cooney AJ. Reciprocal regulation of the mouse protamine genes by the orphan nuclear receptor germ cell nuclear factor and CREMτ[J]. Mol Reprod Dev, 2004, 68(4):394-407.
[2] Rajkovic M, Middendorff R, Wetzel MG, et al. Germ cell nuclear factor relieves cAMP-response element modulator τ-mediated activation of the testis-specific promoter of human mitochondrial glycerol-3-phosphate dehydrogenase[J]. J Biol Chem, 2004, 279(50):52493-52499.
[3] Valentin M, Balvers M, Pusch W, et al. Structure and expression of the mouse gene encoding the endozepine-like peptide from haploid male germ cells[J]. Eur J Biochem, 2000, 267(17):5438-5449.
[4] Lan ZJ, Gu P, Xu X, et al. GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility[J]. EMBO J, 2003, 22(16): 4070-4081.
[5] Cooney AJ, Lee CT, Lin SC, et al. Physiological function of the orphans GCNF and COUP-TF[J]. Trends Endocrinol Metab, 2001, 12(6):247-251.
[6] Fuhrmann G, Chung AC, Jackson KJ, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor[J]. Dev Cell, 2001, 1(3):377-387.
[7] Wang H, Wang X, Archer TK, et al. GCNF-dependent activation of cyclin D1 expression via repression of Mir302a during ESC differentiation[J]. Stem Cells, 2014, 32(6):1527-1537.
[8] Wang Y, Zhang Y, Dai X, et al. NR6A1 couples with cAMP response element binding protein and regulates vascular smooth muscle cell migration[J]. Int J Biochem Cell Biol, 2015, 69:225-232.
[9] 张亚辉, 钱 航, 张志峰, 等.NR6A1过表达腺病毒载体构建及在血管平滑肌细胞中的表达[J]. 湖北医药学院学报, 2012, 31(3):181-185.
[10] 王瑾瑜, 周 允, 冯 寒, 等.脂肪因子CTRP3促进血管钙化机制研究[J]. 中国病理生理杂志, 2012, 11(28):2033-2034.
[11] Zhao H, Li Z, Cooney AJ, et al. Orphan nuclear receptor function in the ovary[J]. Front Biosci, 2007, 12:3398-3405.
[12] Schote AB, Turner JD, Schiltz J, et al. Nuclear receptors-in human immune cells: expression and correlations[J]. Mol Immunol, 2007, 44(6):1436-1445.
[13] Mullen EM, Gu P, Cooney AJ. Nuclear receptors in regulation of mouse ES cell pluripotency and differentiation[J]. PPAR Res, 2007, 2007:61563.
[14] Hentschke M, Kurth I, Borgmeyer U, et al. Germ cell nuclear factor is a repressor of CRIPTO-1 and CRIPTO-3[J]. J Biol Chem, 2006, 281(44):33497-33504.
[15] Sun X, Lee J, Navas T, et al. RIP3, a novel apoptosis-inducing kinase[J]. J Biol Chem, 1999, 274(24):16871-16875.
[16] Vandenabeele P, Declercq W, Van Herreweghe F, et al. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis[J]. Sci Signal, 2010, 3(115):re4.
(责任编辑: 陈妙玲, 罗 森)
NR6A1 promotes vascular smooth muscle cell apoptosis by up-regulating RIPK3 gene expression
ZHANG Ya-hui, WANG Xiong, WU Sheng-ying, PENG Ji-xia, WU Fu-yun, KE Jing, ZHANG Peng, ZHANG Qiu-fang, LV Yan-xia
(Department of Pathophysiology, Hubei University of Medicine, Shiyan 442000, China. E-mail: 350182495@qq.com)
AIM: To determine the role of nuclear receptor subfamily 6, group A, member 1 (NR6A1) in vascular smooth muscle cell (VSMC) apoptosis.METHODS: NR6A1 protein was over-expressed in the VSMCs by infection of adenovirus. The effect of NR6A1 on the viability of VSMCs was measured by MTT assay. DAPI staining, TUNEL staining and caspase activity assay were conducted. DNA microarray was used to quickly screen the target genes of NR6A1. The effect of receptor-interacting serine/threonine-protein kinase 3 (RIPK3) silencing on NR6A1-induced apoptosis of the VSMCs was further analyzed.RESULTS: Adenovirus-mediated over-expression of NR6A1 induced the apoptosis of VSMCs. TheRIPK3 gene expression was up-regulated by NR6A1 over-expression in the VSMCs. NR6A1-induced VSMC apoptosis was inhibited byRIPK3 silencing.CONCLUSION: NR6A1 promotes VSMC apoptosis by up-regulating theRIPK3 gene expression.
Nuclear receptor subfamily 6, group A, member 1; Receptor-interacting serine/threonine-protein kinase 3; Apoptosis
1000- 4718(2017)09- 1581- 06
2017- 02- 16 [
] 2017- 07- 12
湖北省教育厅科学研究计划指导性项目(No. B2015492);湖北省自然科学基金资助项目(No. 2014CFB187);国家自然科学基金资助项目(No. 81641140)
R363.1; R543
A
10.3969/j.issn.1000- 4718.2017.09.007
△通讯作者 Tel: 0719-8875312; E-mail: 350182495@qq.com
