Succinic anhydride-grafted nanocrystalline cellulose for the binding of luteolin and luteoloside
2017-09-12LIHuanCHENKuiWANGYuanWANGYong
LI Huan, CHEN Kui, WANG Yuan, WANG Yong
(College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
Succinic anhydride-grafted nanocrystalline cellulose for the binding of luteolin and luteoloside
LI Huan, CHEN Kui, WANG Yuan, WANG Yong*
(CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
Succinic anhydride-grafted nanocrystalline cellulose labeled NCSA was developed and further modified with cetyltrimethylammonium bromide (CTAB) labeled CTAB@NCSA as a novel drug delivery excipient which was used to modulate the loading of hydrophobic drugs. The morphology and structure of the NCSA before and after modification were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD) and zeta potential. Furthermore, the adsorption kinetics and isotherm analysis of NCSA for CTAB were employed to explain in-depth information associated with the adsorption process. The adsorption kinetics fitted well to the pseudo-second-order model and isothermal study demonstrated a multilayered adsorption following Temkin model. Hydrophobic luteolin (LUT) and luteoloside (LUS) were used as model drugs for the investigation of drug loading and release performance. The CTAB@NCSA exhibited high drug loading capability and long sustained release time. These findings prefigure the promising potentials of CTAB@NCSA as a carrier for hydrophobic LUT and LUS.
luteolin and luteoloside; succinic anhydride; nanocrystalline cellulose; surfactant; in vitro release
Luteolin (LUT) and luteoloside (LUS, Fig.1) belong to flavonoid compound, which mostly exist in various vegetables and medical plants[1]. They possess pharmacological properties, including anticancer[2-4], anti-inflammatory[5-6], anti-HBV[7-8]and antioxidant[9-10]etc. Nevertheless, potential properties of LUT and LUS are hampered due to their poor solubility and oral absorption that results fast metabolism from blood[11]. To overcome the problems, there are significant research efforts focusing on the drug delivery using various nano-carriers such as polymeric micelles[12-14], lipid carriers and microemulsions[15-17]. It is regrettable that most of reported the nano-carriers show poor stability and low drug loadings.

Fig.1 Chemical structural formula of LUT (a) and LUS (b)
Recently, cellulose especially nanocrystalline cellulose (NCC) has been actively investigated as drug delivery excipients due to its abundant renewable, biocompatible, biodegradable, safe and easily modified properties[18-19]. In our previous work, we established a novel drug delivery system based on cetyl trimethylammonium bromide (CTAB) modified NCC to control the release of LUT and LUS[20]. However, the drug loading capability is low, the chemical modification of the cellulose is important to improve its practical use for drug carrier. It is worth noting that grafted functional groups onto cellulose can afford more efficient sites to load small molecules for drug delivery[21-22]. In this work, we have developed functionalized NCC with succinic anhydride labeled NCSA. Here succinic anhydride is utilized for introducing carboxylic groups which can provide more negatively charged onto NCC via ring-opening esterification reaction. Beyond that, NCC is generally prepared by the sulfuric acid hydrolysis of native cellulose, resulting in the formation of negatively charged surface which mainly refers to the negative sulfate esters. On the basis of large negative charges, CTAB is used to modify NCSA labeled CTAB@NCSA. Here CTAB is a quaternary ammonium and hydrophobic cationic type surfactant which can increase the hydrophobicity of NCC[23]. The aim of our investigation is to study the potential of CTAB@NCSA as a nano-carrier of hydrophobic LUT and LUS. The CTAB@NCSA exhibited higher drug loading capability and longer sustained release time.
1 Experimental
1.1 Materials
Microcrystalline cellulose, succinic anhydride, CTAB and LUT were purchased from Sigma-Aldrich. LUS was isolated and prepared from crude extract ofLonicerajaponicaleaves by high-speed counter current chromatography (HSCCC). The purity was 98.0% determined by HPLC and calculated with peak area normalization method. Other chemicals were of analytical grade, and water is double distilled.
1.2 Preparation of NCSA
Suspension of NCC was prepared by acid hydrolysis and ultrasonic technology of microcrystalline cellulose[20], as described below. First, MCC (5 g) was treated with sulfuric acid solution (64%) at 45 ℃ for 30 min and then continued to hydrolyze under ultrasonic treatment at 50 Hz for 10 min. Second, reaction was terminated by adding much water and the suspension was centrifuged and washed. Finally, the suspension was dialyzed and dried by freezing, reserved.
NCSA was synthesized by grafting succinic anhydride on the hydroxyl groups of NCC, as described below. Dried powder of NCC (1.0 g) was dispersed in 50 mL of dimethylacetamide (DMAC) and 1 mL of pyridine with constant magnetic stirring. Then excess succinic anhydride (6.2 g) was added to the mixtures at 100 ℃ in an oil bath for 10 h. The result mixtures were filtered and washed with chloroform repeatedly. Finally, the power of NCSA was obtained by vacuum drying.
1.3 Preparation of CTAB@NCSA
The adsorption experiments of CTAB including effect of pH, temperature and adsorption time were studied, and the optimized condition was determined. NCSA (0.05 g) was fully reacted with fixed concentration of CTAB at room temperature for 70 min. The unbound CTAB was removed by centrifugation at 10 000 rpm. After the suspension was repeatedly washed and centrifuged, a novel drug carrier labeled CTAB@NCSA was collected by freeze drying.
1.4 Characterization
The morphologies of samples were studied on JSM-7001F and JEM-2100 (JEOL Co., Japan), respectively. X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance diffractometer with Cu Kαradiation and 2θrange was 10°-60°. The zeta potential of samples was recorded on a Nano ZS 90 at 25 ℃. The FT-IR spectra of samples were recorded on a Thermo Nicolet Avatar 360 spectrometer in transmittance mode by use of KBr pellets. Measurements were taken in the wavelength ranging from 500 cm-1to 4 000 cm-1.
1.5 Adsorption experiments of CTAB
The adsorption behaviors of NCSA for CTAB were systematically investigated by changing the factors of solution pH, temperature and adsorption time. The solution pH value was changed from 3.0 to 9.0, and 0.10 mol·L-1NaOH or HCl solution was used to adjust the pH value. The solution temperature and adsorption time were all changed from 16 to 70 ℃ and 10 to 240 min, respectively. Adsorption kinetic studies were conducted with CTAB concentration of 1.0 g·L-1at room temperature, and the adsorption isotherms experiments were performed with various CTAB concentrations (c0= 0.073-2.47 mmol·L-1) for 70 min. Mixtures were separated and the residual concentration of CTAB was determined by the color-reaction of CTAB with methyl orange in phosphate buffer, and the absorbance was recorded on UV-Vis spectrophotometer at 470 nm. All experiments were repeated three times and the average values are reported. The adsorption efficiency (E, %) and capacity (q) of CTAB were calculated according to the equation evaluated of Eqs. (1) and (2).
(1)
(2)
Wherec0is initial concentration andceis equilibrium of CTAB,mis the weight of NCSA, andVis the volume of solution.
1.6 Loading of LUT and LUS
CTAB@NCSA (5.0 mg) was fully dispersed in double distilled water, and LUT (or LUS) solution was added under constant magnetic stirring. Then suspension was incubated at room temperature under stirring for 60 min, and high-speed centrifuged for 10 min. The amount of unbound drug in the supernatant was determined by UV-vis spectrophotometer. Flocculent precipitates were dried by freeze drying, which were labeled with LUT@CTAB@NCSA (or LUS@CTAB@NCSA), and reserved. The drug loading capacity was expressed using Eqs.(2), and the corresponding parameters are altered, wherec0is initial concentration andceis equilibrium of LUT (or LUS),mis the weight of CTAB@NCSA, andVis still the volume of solution.
1.7 In vitro drug release
The in vitro release profile of LUT and LUS loaded CTAB@NCSA was studied in phosphate buffer (pH 6.4 and 7.4) at 37 ℃. Suspensions were prepared in buffer solution and maintained in a shaker water bath at the constant temperature of 37 ℃. At predetermined time, the suspension was high-speed centrifuged for less than 1 min, meanwhile, the supernatant was removed for drug quantification by UV-Vis for LUT (or LUS), as previously described. At each sampling time point, fresh phosphate buffer of equivalent volume was added to the test tube, and the drug delivery system was re-suspended. The accumulative percentage release (Q/%) was calculated from the Eqs.(3).
(3)
Wherecn(g·L-1) is the concentration of drug in the sample,V0(mL) is the volume of the release medium,Vi(mL) is the volume of the replaced medium,m(mg) is the amount of drug in the sample. This study was repeated three times, and the results were expressed as mean values ± standard deviation.
2 Results and discussion
2.1 Carboxyl content
Carboxyl content of NCSA was measured with the inverse titration method, and the value was calculated to be 3.67 mmol·g-1. When the raw cellulose was transformed into nanometer size, large amount of released hydroxyl groups on the surface of NCC react with succinic anhydride more easily[24].
2.2 Characterization of NCSA and CTAB@NCSA

Fig.2 SEM of MCC(A), NCC(B), NCSA(C) and TEM of NCC(D), NCSA(E), CTAB@NCSA(F)
The SEM images (Fig.2A, B and C) show that great changes have taken place in appearance of NCSA, which was obvious different from MCC and NCC. The TEM images (Fig.2D, E and F) show that NCC and NCSA particles are between 20 to 60 nm in diame ter with spherical morphology, while CTAB@NCSA particle exhibits obviously thicker diameters of nearby 130 nm with relatively good dispersions compared with precursor NCSA.
As shown in Fig.3A, the X-ray powder diffraction peaks of NCC, NCSA and CTAB@NCSA correspond to the (ITO), (110), (200) and (004) crystallographic planes of cellulose I[25],respectively. The relative degree of crystallinity of samples is determined according to the method of peak intensity[26], and the crystallinity value is calculated to be 72.4%, 68.1% and 60.0%, respectively. In contrast to NCC, the crystalline regions of NCSA and CTAB@NCSA are breaking down when subjected to the proper combination of chemical treatments.
The FT-IR spectra of samples are presented in Fig.3B. The difference observed between NCC and NCSA is the appearance of band at 1 730 cm-1which is assigned to the C=O stretching vibration of carbonyl group suggesting that succinic anhydride was grafted on NCC successfully. A broad peak between 3 340 and 3 430 cm-1is related to -O-H stretching, and the band between 2 850 and 2 920 cm-1corresponds to asymmetric and symmetric C-H stretching vibration. The band at 1 636 cm-1corresponds to deformation vibration of C=C and the C-O asymmetric bridge stretching appears at 1 165 cm-1.
2.3 Adsorption experiments of CTAB
2.3.1 Effect of pH, temperature and adsorption time
As shown in Fig.4A, the adsorption efficiency of CTAB by NCSA increase in the pH range of 3.6-8.0 and the maximum adsorption efficiency achieve at pH 6.2 indicating that electrostatic attraction of between positively charged CTAB and negatively charged NCSA is the driving force for adsorption process. As a result, the optimum pH was selected to be 6.2 in the following experiments. The effect of temperature on the sorption of CTAB is shown in Fig.4B, showing the neglected influences within the range of 16-70 ℃ of the adsorption processes. So the room temperature was selected in the following experiments. As shown in Fig.4C, the adsorption efficiency of NCSA increases rapidly within the first 70 min and then is a gradual decline process. Based on the results, 70 min was selected as the shaking time to ensure the maximal adsorption efficiency in the following experiments.
2.3.2 Adsorption kinetics studies
The changes of adsorption amount with time were simulated with pseudo-second-order kinetic model which is expressed as Eqs.(4). The plot oft/qtagainsttis shown in Fig.5, which shows a better fit to pseudo-second-order model with high regression coefficients (R2=0.999 1). Moreover, the equilibrium adsorption amounts (qe) calculated is about 787.4 which approach the experimental data, andk2value is 0.018 13.

Fig.4 Effect of pH (A), temperature (B) and shaking time (C) on adsorption
(4)
Whereqe(mg·g-1) is the equilibrium adsorption capacity,qt(mg·g-1) is the adsorption capacity at any timet,k2(dm3·mg-1·min-1) is the rate constant of pseudo-second-order adsorption.
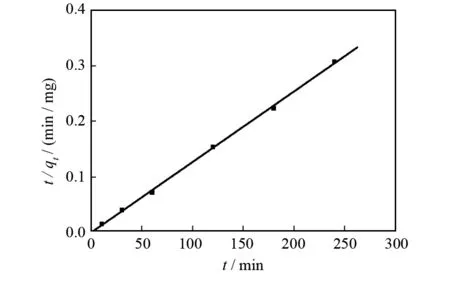
Fig.5 Linear fitting of pseudo-second-order
2.3.3 Adsorption isotherms
The results for the adsorption of CTAB onto NCSA at various initial concentrations are shown in Fig.6. As the initial CTAB concentration increasing from 0.073 to 2.47 mmol·L-1, the adsorption capacity of CTAB increases from 34.58 to 710.5 mg·g-1for NCSA. When the equilibrium concentration of CTAB was about 1.2×10-3mol·L-1adsorption isotherm reached the first platform. With the increase of CTAB concentration, the adsorption capacity continues to increase. Furthermore, the adsorption capacity is no longer increased until the concentration of CTAB reach about 2.0×10-3mol·L-1which appear the second platform.

Fig.6 Adsorption isotherm of CTAB
Langmuir, Freundlich and Temkin commonly used isotherm models are employed to fit the CTAB adsorption process by NCSA, which are described as Eqs.(5-7), respectively. According to the correlation coefficient values (Fig.7), Temkin model fits better for the CTAB adsorption isotherm, indicating a multi molecular layer adsorption of CTAB onto NCSA surface, and the parameters calculated from the three models were presented in table 1.
(5)
(6)
(7)
2.4 Loading of LUT and LUS
As can be seen from the zeta potential distribution (Fig.8), the electrostatic force between positively charged CTAB and negatively charged NCSA, as well as between positively charged CTAB@NCSA and negatively charged LUT or LUS, all played an important role in the binding reaction. Both LUT@CTAB@NCSA and LUS@CTAB@NCSA have negative charges in water as evidenced by zeta potentials of approximately -28.0 and -20.4 mV, respectively. By calculations, the loading content of LUT and LUS are much higher which are (42.8±4.2) and (129.2±1.2) mg·g-1, respectively, compared to previous report ((12.9±1.5) and (56.9±0.9) mg·g-1)[20]. It is possible more hydrophobic domain of CTAB@NCSA for more hydrophobic LUT and LUS molecules are accessible.

Fig.7 Linear fitting of Langmuir (A), Freundlich (B) and Temkin (C) model

Table 1 Isotherm models for adsorption
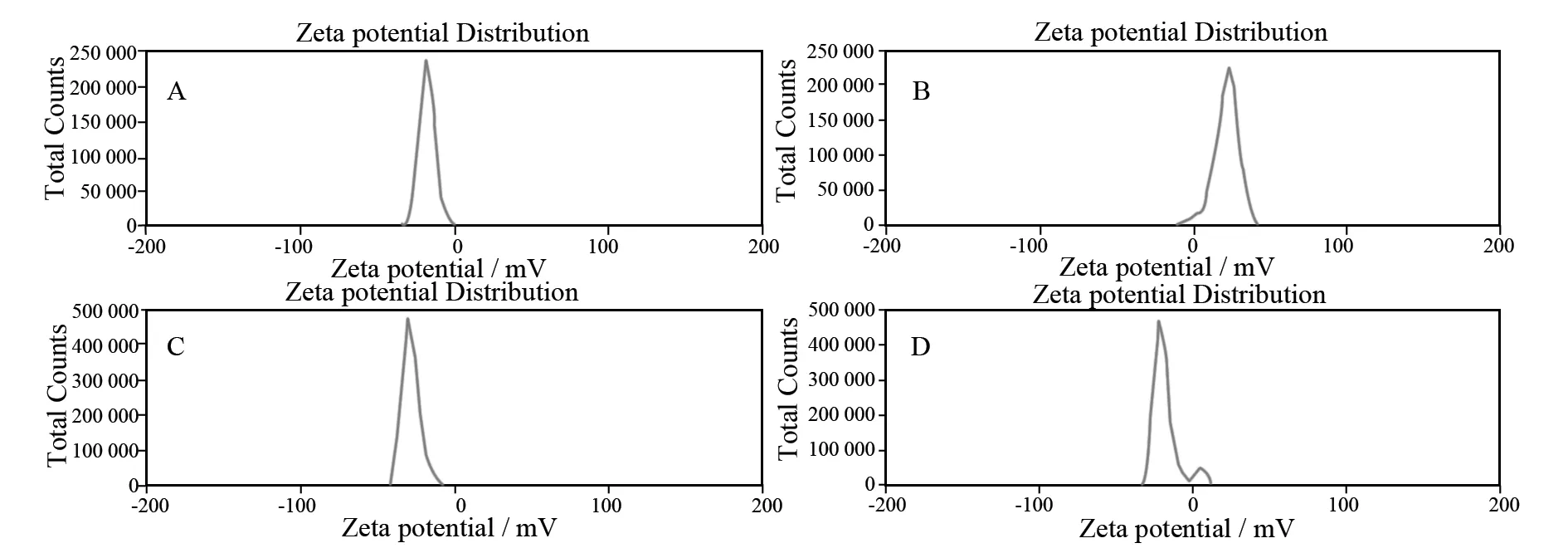
Fig.8 Zeta potential of NCSA (A), CTAB@NCSA (B), LUT@CTAB@NCSA (C) and LUS@CTAB@NCSA (D)
2.5 Release of the drug delivery system
The content of LUT and LUS was analyzed by UV-vis at 351 nm and the assay method was validated, respectively. The calibration curves of LUT and LUS areA= 0.001 7 + 74.65cLUT(R2= 0.999 8) andA= 0.007 1 + 47.78cLUS(R2= 0.999 3), respectively. As seen in Fig.9, the release profiles of LUT@CTAB@NCSA and LUS@CTAB@NCSA are studied. In general, the amount of LUT and LUS complexes released at pH 7.4 is higher than delivered at pH 6.4. The cumulative release percentage is found sustained over a period of 72 h, showing a considerable increase in controlled release time.
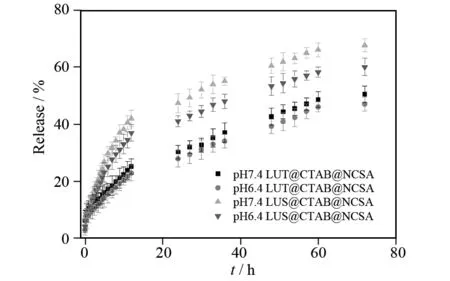
Fig.9 Release of LUT@CTAB@NCSA and LUS@ CTAB@NCSA at 37 ℃ using PBS solution
3 Conclusion
On the basis of precedent researches, this paper aimed to modify NCC with succinic anhydride to develop cellulose composites for control release of drug. A novel cellulose composites CTAB@NCSA was prepared and characterized with SEM, TEM, FT-IR, XRD and zeta potentials. Functionalized CTAB@NCSA particle shows spherical shape with diameters of nearby 130 nm and possess more active sites. The adsorption efficiency for CTAB was encouraging, and adsorption kinetic studies indicated that the adsorption behavior following the pseudo-second-order kinetic model and the equilibrium data was well fitted to the Temkin isotherm model. Furthermore CTAB@NCSA can be efficiently used for binding water-insoluble LUT and LUS, and loading capacity and controlled release time are much better compared with the previous report. These above findings suggest that CTAB@NCSA could be used as an efficient carrier for hydrophobic LUT and LUS delivery.
[1] LIN Y, SHI R, WANG X, et al. Luteolin, a flavonoid with potentials for cancer prevention and therapy [J]. Current Cancer Drug Targets, 2008, 8(7): 634-646.
[2] MENG G, CHAI K, LI X, et al. Luteolin exerts proapoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway [J]. Chemico-Biological Interactions, 2016, 257: 26-34.
[3] CHAKRABARTI M, RAY S. Anti-tumor activities of luteolin and silibinin in glioblastoma cells:overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo [J]. Apoptosis, 2015, 21(3): 312-328.
[4] BASKAR A, IGNACIMUTHU S, MICHAE G, et al. Can-cer chemo-preventive potential of luteolin-7-O-glucoside isolated from ophiorrhiza mungos linn [J]. Nutrition & Can-cer, 2011, 63(1): 130-138.
[5] KURE A, NAKAGAWA K, KONDO M, et al. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect [J]. Journal of Agricultural and Food Chemistry, 2016, 64(21): 4246-4254.
[6] ODONTUYA G, HOULT J, HOUGHTON P. Structure-activity relationship for anti-inflammatory effect of luteolin and its derived glycosides [J]. Phytotherapy Research, 2005, 19(9): 782-786.
[7] TEWTRAKUL S, MIYASHIRO H, NAKAMURA N, et al. HIV-1 integrase inhibitory substances from coleus parvifolius [J]. Hytotherapy Research, 2003, 17(3): 232-239.
[8] TIAN Y, SUN L, LIU X, et al. Anti-HBV active flavone glucosides from euphorbia humifusa willd [J]. Fitoterapia, 2010, 81(7): 799-802.
[9] ROOBAN B, SASIKALA V, GAYATHRI DEVI V, et al. Prevention of selenite induced oxidative stress and cataractogenesis by luteolin isolated from vitex negundo [J]. Chemico-Biological Interactions, 2012, 196(1/2): 30-38.
[10] HU C, KITTS D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells [J]. Molecular and Cellular Biochemistry, 2004, 265: 107-113.
[11] CHEN T, LI L, LU X. Absorption and excretion of luteolin and apigenin in rats after oral administration of chrysanthemum morifolium extract [J]. Journal of Agricultural and Food Chemistry, 2007, 55(2): 273-277.
[12] MAJUMDAR D, JUNG K, ZHANG H, et al. Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity [J]. Cancer Prevention Research, 2014, 7(1): 65-73.
[13] QIU J, GAO X, WANG B, et al. Preparation and characterization of monomethoxy poly (ethylene glycol)-poly(ε-caprolactone) micelles for the solubilization and in vivo delivery of luteolin [J]. International Journal of Nanomedicine, 2013, 8: 3061-3069.
[14] QING W, WANG Y, LI H, et al. Preparation and characterization of copolymer micelles for the solubilization and in vitro release of luteolin and luteoloside [J]. AAPS PharmSciTech, 2017, 18(6): 2095-2101.
[15] KHAN J, ALEXANDER A, AJAZUDDIN, et al. Luteolin-phospholipid complex: preparation, characterization and biological evaluation [J]. Journal of Pharmacy and Pharmacology, 2014, 66(10): 1451-1462.
[16] DANG H, MENG M, ZHAO H, et al. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies [J]. Journal of Nanoparticle Research, 2014(4): 16: 2347.
[17] LIU Y, WANG L, ZHAO Y, et al. Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin [J]. International Journal of Pharmaceutics, 2014, 476(1/2): 169-177.
[18] LIN N, DUFRESNE A. Nanocellulose in biomedicine: Current status and future prospect [J]. European Polymer Journal, 2014, 59: 302-325.
[19] EDGAR J. Cellulose esters in drug delivery [J]. Cellulose, 2007, 14(1): 49-64.
[20] QING W, WANG Y, LI H, et al. The modified nanocrystalline cellulose for hydrophobic drug delivery [J]. Applied Surface Science, 2016, 366: 404-409.
[21] MOGHADDAM N, AVVAL E, FAREGHI R. Modification of cellulose by graft polymerization for use in drug delivery systems [J]. Colloid and Polymer Science, 2014, 292(1): 77-84.
[22] MOVAGHARNEZHAD N, MOGHADAM N. Folate-decorated carboxymethyl cellulose for controlled doxorubicin delivery [J]. Colloid and Polymer Science, 2016, 294(1): 199-206.
[23] HU Z, BALLINGER S, PELTON R. Surfactant-enhanced cellulose nanocrystal Pickering emulsions [J]. Journal of Colloid and Interface Science, 2015, 439: 139-148.
[24] ANG-ATIKARNKUL P, WATTANAPHANIT A, RUJIRAVANIT R. Fabrication of cellulose nanofiber/chitin whisker/silk sericin bionanocomposite sponges and characterizations of their physical and biological properties [J]. Composites Science Technology, 2014, 96(1/2): 88-96.
[25] FRENCH A. Idealized powder diffraction patterns for cellulose polymorphs [J]. Cellulose, 2014, 21(2): 885-896.
[26] DONG S, CHO H, LEE Y, et al. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting [J]. Biomacromolecules, 2014, 15(5): 1560-1567.
[责任编辑:吴文鹏]
丁二酸酐接枝纳米纤维素负载木犀草素及其苷
李 缓,陈 奎,王 园,王 勇*
(河南大学 化学化工学院,河南 开封 475004)
将纳米纤维素(NCC)表面接枝丁二酸酐得到丁二酸酐化纳米纤维素(NCSA),再将阳离子表面活性剂十六烷基三甲基溴化铵(CTAB)负载到NCSA,得到一种新的纳米药物载体(CTAB@NCSA). 考察了NCSA的物理化学性能,包括扫描、透射电镜,红外光谱,X射线粉末衍射及电位测定;同时研究了NCSA对CTAB的吸附行为. 最后以CTAB@NCSA为药物载体,以LUT和LUS为模型药物,通过分子间作用力及疏水作用力得到负载LUT和LUS的纳米复合物微球CTAB@NCSA@LUT和CTAB@NCSA@LUS,并对其体外释药进行了研究.
木犀草素及其苷;丁二酸酐;纳米纤维素;表面活性剂;体外释放
date: 2017-04-22.
The Foundation of Education Department of Henan Province (14A150011).
, E-mail:wangyong@henu.edu.cn.
O629 Document code: A
1008-1011(2017)04-0493-08
Biography: LI Huan(1992-), female, postgraduate, majoring in chemistry of natural product.*