脑胶质瘤诊断的分子遗传学研究进展
2017-09-03占昌友
官 娟,占昌友
(复旦大学基础医学院药理系,上海200033)
脑胶质瘤诊断的分子遗传学研究进展
官 娟,占昌友
(复旦大学基础医学院药理系,上海200033)
胶质瘤是中枢神经系统中最常见的恶性肿瘤,全基因组分子图谱已揭示不同类型胶质瘤的遗传学和表观分子生物学特征.2016年世界卫生组织在经典的组织病理学诊断基础上,将分子标志物纳入胶质瘤的分类中,为胶质瘤的诊断提供了一套新的金标准,有望对临床上胶质瘤的干预和预后产生重要意义.
胶质瘤;分子遗传学;生物标志物;诊断
0 前言
胶质瘤占所有原发性恶性脑肿瘤的80%,在美国胶质瘤年发病率为6/100000[1-2].2007年世界卫生组织(WHO)根据胶质瘤的组织学特征将其分为Ⅰ~Ⅳ四个等级,其中Ⅰ级肿瘤生长非常缓慢,手术切除有治愈的可能,Ⅱ级和Ⅲ级恶性程度较高,预后较差,而Ⅳ级恶性程度最高,中位生存期只有14~16个月[1,3].组织形态学分类一度成为临床上胶质瘤诊断的金标准,但胶质瘤组织形态类型繁多,各类型的临床表现、分子标志物、治疗策略、预后不尽相同,不同观察者对肿瘤分级也存在偏差[4].2016年,世界卫生组织在2007版形态学分类方法的基础上将生物标志物纳入胶质瘤分类系统中,规定:结合组织形态学、WHO分级和生物标志三个层次对胶质瘤做出综合诊断,有利于临床医生对胶质瘤的临床表现和预后评估作出精准判断[5].本文将对2016版WHO分类系统中主要类型胶质瘤的相关生物标志物进行综述.
1 WHOⅠ级
1.1 纤维性星形胶质细胞瘤(pilocytic astrocytoma)
纤维性星形胶质细胞瘤的主要特征是遗传学改变导致MAPK信号通路激活[6],位于染色体7q上的BRAF和KIAA1549基因融合,是预后良好的生物标志物[7].该类型胶质瘤常伴有 MAPK信号通路中RAF1,PTPN11,NTRK2等基因的融合或 BRAF,KRAS,FGFR1,NF1基因突变[6](表1).而非MAPK信号通路中基因突变并不多见,因此,该型细胞瘤是一种“单信号通路疾病”.毛细胞粘液样细胞瘤(pilo⁃myxoid astrocytoma)是纤维性星形细胞瘤的罕见变型体,术后易局部复发、可沿脊髓传播[4].
1.2 室管膜下的巨细胞型星形胶质细胞瘤(sub⁃ependymal giant⁃cell astrocytoma) 室管膜下的巨细胞型星形细胞瘤与结节性硬化复合体(tuberous sclerosis complex,TSC)的形成密切相关,基因突变和等位基因的缺失导致错构瘤蛋白(hamartin,TSC1)或薯球蛋白(tuberin,TSC2)表达缺失,最终导致mTOR信号通路激活[8](表1).
1.3 星形增生性室管膜细胞瘤(subependymoma)室管膜细胞瘤具有9种不同的亚型,主要分布于幕上、颅后窝和脊髓,各有3种不同的亚型[9].其中星形细胞增生性室管膜瘤在这3个部位均有发生,愈后较好.
2 WHOⅡ、Ⅲ级
WHOⅢ级胶质瘤多由Ⅱ级胶质瘤发展而来,在此不再分开赘述.
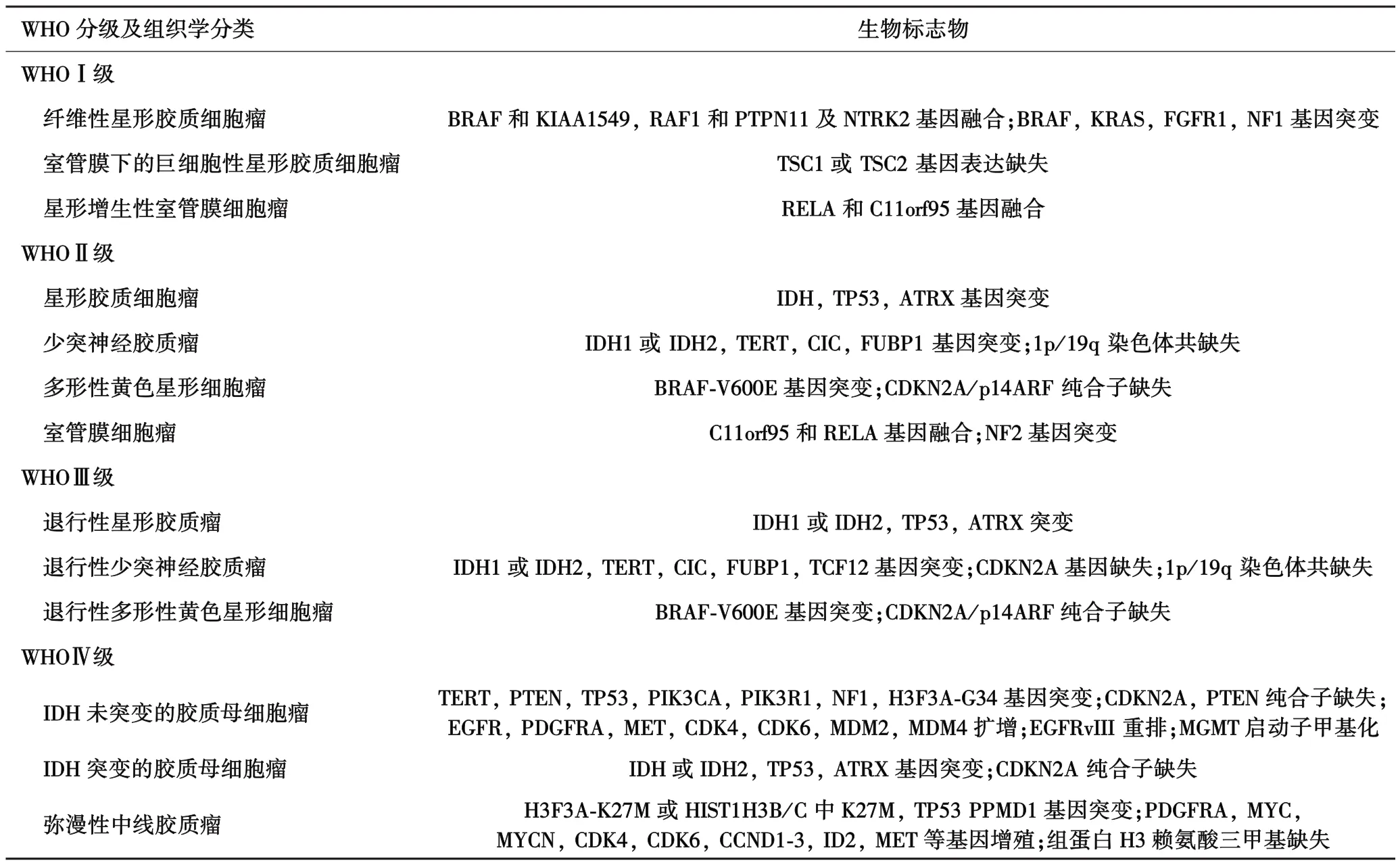
表1 各类脑胶质瘤的生物标志物
2.1 星形胶质细胞瘤(astrocytic gliomas) IDH是胶质瘤发病过程中突变最早的基因[10],但只有 IDH基因突变并不能导致胶质瘤的发生[11].IDH突变的星形胶质细胞瘤常伴有TP53和ATRX基因的突变.ATRX位于性染色体Xq21.1[12-14],编码染色体重组调节因子,它的突变导致细胞核表达的ATRX的缺失,致使端粒功能障碍和广泛的基因组失稳[15],容易导致弥漫性星形胶质瘤(WHOⅡ)转变为退行性星形胶质瘤(anaplastic astrocytoma)(WHOⅢ),IDH基因二次突变,并伴有染色体9p21缺失[16](表1).
2.2 少突神经胶质瘤(oligodendrogliomas) 少突神经胶质瘤伴有IDH基因突变和染色1p和19q共缺失.绝大多数的少突神经胶质瘤 TERT启动子区TERT基因编码的端粒酶反转录酶与染色体端粒酶mRNA共同维持端粒的长度和结构,位于染色体5p15.33.正常细胞中,细胞每分裂一次,端粒会缩短50~100 bp,逐渐失去DNA保护功能,细胞老化,凋亡.在胶质瘤细胞中,TERT基因突变,端粒酶活性不减弱,端粒不缩短,细胞失去老化和凋亡的能力,不断进行分裂[17],最终导致肿瘤的发生.约2/3的患者伴有CIC突变,介导转录抑制酶的同系物 Drosophila capicua失活.约1/3的患者伴有FUBR1突变,FUBR1可编码上游的结合蛋白,调节MYC的表达[18].这些基因的突变可促使该类型的胶质瘤进一步恶化,发展为WHOⅢ级,并伴有染色体9p21缺失[19],转录因子TF12突变,最终导致MYC信号通路的激活[20](表1).
2.3 多形性黄色星形细胞瘤(pleomorphic xanthoas⁃trocytoma,PXA) PXA与BRAF⁃600突变有关,常伴有纯合子CDKN2A缺失及p16INK4A表达减少.基因的突变常导致PXA(WHOⅡ)发展为退行性型PXA(WHOⅢ)[21-23](表1).
2.4 室管膜细胞瘤(ependymomas) 室管膜细胞瘤中,儿童有 2/3的幕上室管膜细胞瘤(supratentorial ependymomas)有基因重排现象:NF⁃κB亚基RELA和C11orf95融合,致使 NF⁃κB转录异常,愈后较差,YAP1融合的患者较少见,但愈后较好.发生与颅后窝上的肿瘤有两种类型:PF⁃A型和PF⁃B型,前者染色体较稳定,愈后较差;后者染色体不稳定,愈后相对较好.发生于脊髓的胶质瘤多伴有NF2基因的突变,而粘液乳头状室管膜细胞瘤常伴有多个染色体的畸变,愈后较差(表1).
3 WHOⅣ级
3.1 胶质母细胞瘤(glioblastoma) 根据IDH基因是否突变,可将胶质母细胞瘤分为两大类型:IDH基因未突变型胶质母细胞瘤和IDH突变型胶质母细胞瘤.IDH基因未突变的胶质母细胞瘤在中老年较常见,恶性程度最高,病程一般小于3个月[4].在年龄大于55岁的患者中,若胶质瘤的发病位置在非中线位置(nonmidline locations)且 R132H阴性,可确诊为IDH未突变型胶质母细胞瘤.而年龄小于55岁的胶质瘤患者,需排除IDH基因突变,方可确诊为IDH基因未突变的胶质母细胞瘤[4].患者常具有以下几个特征:7号染色体增益;10号染色体变为单体;PTEN突变或纯合子缺失;CDKN2A和CKN2B纯合子缺失;TERT启动子突变[24](表1).
IDH基因突变的胶质母细胞瘤约占10%左右,常见于青壮年[4],可由各种WHOⅡ和Ⅲ级中的原发性胶质瘤发展而来.其基因图谱与IDH突变的星形胶质瘤极为相似,伴有 TP53和 ATRX基因突变及G⁃CIMP,但其DNA甲基化水平较低,愈后较IDH基因未突变的好[25](表1).
3.2 弥漫性中线胶质瘤(H3⁃K27M⁃mutant diffuse midline glioma) H3⁃K27M突变的弥漫性中线细胞瘤,发病位置主要集中于丘脑、脑干和脊髓.该类型的肿瘤中常见于编码组蛋白⁃H3的基因 H3F3A或HIST1H3B/C中K27M突变,通过破坏PRC2的募集和抑制组蛋白⁃赖氨酸⁃N甲基转移酶EZH2的活性使细胞内三甲基化的H3K27减少.该类型胶质瘤常伴有TP53和PPM1D基因突变和PDGFRA,MYC,MYCN,CDK4,CDK6等原癌基因的增殖[13,26-27].
4 对术后治疗有指导价值的生物标志物
4.1 启动子MGMT甲基化增加MGMT是一种修复6O甲基鸟嘌呤的酶,位于染色体10q26,可作为甲基转移酶和甲基受体蛋白,将烷化剂作用下形成的6O位甲基化鸟嘌呤上的甲基移除到自身的活性半胱氨基酸残基上,修复损伤的DNA,同时自身不可逆失活为烷基化MGMT,在修复DNA烷基化损伤的过程中至关重要.但MGMT修复DNA损伤的同时,阻止化疗药物形成的二级损伤导致了烷化剂耐药性.测定MGMT水平或MGMT启动子甲基化状态可预测肿瘤对烷化剂化疗药物的药敏性,为实现个体化化疗提供理论和实践依据,也可用于胶质瘤患者愈后评估[28].
4.2 1p/19q共缺失染色体1p/19q联合缺失是由染色体不平衡异位构成[29-30],少突胶质细胞瘤常伴有IDH突变和染色体1p/1q共缺失.95%以上的少突胶质瘤细胞存在启动子TERT区突变.临床对照试验结果显示:染色体1p/19生物标志物是临床上实施PVC方案的指标,且患者生存期较长,预后良好[31-32].
4.3 新出现的辅助术后治疗的分子标志物BRAF⁃V600E突变极有希望会成为适用于BRAF抑制剂治疗的分子标记物[33],检测IDH基因是否突变有助于判断该类型的胶质瘤是否适用于IDH突变抑制剂治疗,或判断临床中是否适用于靶向IDH1⁃R132H的疫苗治疗[13].靶向EGFR或EGFRvIII的治疗方法也需要检测是否有EGFR增殖或EGFRvIII阳性[34-36].其他分子生物标记物如:FGFR⁃TACC融合,可以判断是否适用于FGFR抑制剂的治疗[37-38].
5 结语
将分子标志物纳入胶质瘤的分型中,拓宽了胶质瘤的诊断和治疗前景,临床上结合组织形态和遗传学改变对胶质瘤做出“综合性”诊断,使得病理诊断更接近胶质瘤的生物学特征,指导胶质瘤的精准治疗.
[1]Gilbert MR,Wang M,Aldape KD,et al.Dose⁃dense temozolomide for newly diagnosed glioblastoma:a randomized phaseⅢclinical trial[J].J Clin Oncol,2013,31(32):4085-4091.
[2]Ostrom QT,Gittleman H,Fulop J,et al.CBTRUS statistical report:primary brain and central nervous system tumors diagnosed in the United States in 2008-2012[J].J Neurooncol,2015,17 Suppl 4:iv1-iv62.
[3]Chinot OL,Wick W,Mason W,et al.Bevacizumab plus radiothera⁃py⁃temozolomide for newly diagnosed glioblastoma[J].N Engl J Med,2014,370(8):709-722.
[4] Reifenberger G,Wirsching HG,Knobbe⁃Thomsen CB,et al.Advances in the molecular genetics of gliomas⁃implications for classi⁃fication and therapy[J].Nat Rev Clin oncol,2016.
[5]Malzkorn B,Reifenberger G.Practical implications of integrated glioma classification according to the World Health Organization classification of tumors of the central nervous system 2016[J].Curr Opin Oncol,2016,28(6):494-501.
[6]Jones DT,Hutter B,Jäger N,et al.Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma[J].Nat Genet,2013,45(8):927-932.
[7]Hawkins C,Walker E,Mohamed N,et al.BRAF⁃KIAA1549 fusion predicts better clinical outcome in pediatric low⁃grade astrocytoma[J].Clin Cancer Res,2011,17(14):4790-4798.
[8]Chan JA,Zhang H,Roberts PS,et al.Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas:biallelic inactivation of TSC1 or TSC2 leads to mTOR activation[J].J Neuropathol Exp Neurol,2004,63(12):1236-1242.
[9]Pajtler KW,Witt H,Sill M,et al.Molecular classification of ependymal tumors across all CNS compartments,histopathological grades,and age groups[J].Cancer Cell,2015,27(5):728-743.
[10]Suzuki H,Aoki K,Chiba K,et al.Mutational landscape and clonal architecture in grade II and III gliomas[J].Nat Genet,2015,47(5):458-468.
[11]Sasaki M,Knobbe CB,Munger JC,et al.IDH1(R132H)mutation increases murine haematopoietic progenitors and alters epigenetics[J].Nature,2012,488(7413):656-659.
[12]Wiestler B,Capper D,Sill M,et al.Integrated DNA methylation and copy⁃number profiling identify three clinically and biologically relevant groups of anaplastic glioma[J].Acta Neuropathol,2014,128(4):561-571.
[13]Schwartzentruber J,Korshunov A,Liu XY,et al.Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblas⁃toma[J].Nature,2012,482(7384):226-231.
[14]Wiestler B,Capper D,Holland⁃Letz T,et al.ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis[J].Acta Neuropathol,2013,126(3):443-451.
[15]Abedalthagafi M,Phillips JJ,Kim GE,et al.The alternative lengthe⁃ning of telomere phenotype is significantly associated with loss of ATRX expression in high⁃grade pediatric and adult astrocytomas:a multi⁃institutional study of 214 astrocytomas[J].Mod Pathol,2013,26(11):1425-1432.
[16]Watson LA,Goldberg H,Bérubé NG.Emerging roles of ATRX in cancer[J].Epigenomics,2015,7(8):1365-1378.
[17]Willeit P,Willeit J,Mayr A,et al.Telomere length and risk of inci⁃dent cancer and cancer mortality[J].JAMA,2010,304(1):69-75.
[18]Bettegowda C,Agrawal N,Jiao Y,et al.Mutations in CIC and FUBP1 contribute to human oligodendroglioma[J].Science,2011,333(6048):1453-1455.
[19]Alentorn A,Dehais C,Ducray F,et al.Allelic loss of 9p21.3 is a prognostic factor in 1p/19q codeleted anaplastic gliomas[J].Neurology,2015,85(15):1325-1331.
[20]Labreche K,Simeonova I,Kamoun A,et al.TCF12 is mutated in anaplastic oligodendroglioma[J].Nat Commun,2015,6:7207.
[21]Weber RG,Hoischen A,Ehrler M,et al.Frequent loss of chromo⁃some 9,homozygous CDKN2A/p14(ARF)/CDKN2B deletion and low TSC1 mRNA expression in pleomorphic xanthoastrocytomas[J].Oncogene,2007,26(7):1088-1097.
[22]Schindler G,Capper D,Meyer J,et al.Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra⁃cerebellar pilocytic astrocytoma[J].Acta Neuropathol,2011,121(3):397-405.
[23]Koelsche C,Sahm F,Wöhrer A,et al.BRAF⁃mutated pleomorphic xanthoastrocytoma is associated with temporal location,reticulin fiber deposition and CD34 expression[J].Brain Pathol,2014,24(3):221-229.
[24]Aldape K,Zadeh G,Mansouri S,et al.Glioblastoma:pathology,molecular mechanisms and markers[J].Acta Neuropathol,2015,129(6):829-848.
[25] Ceccarelli M,Barthel FP,Malta TM,et al.Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma[J].Cell,2016,164(3):550-563.
[26]Wu G,Broniscer A,McEachron TA,et al.Somatic histone H3 alter⁃ations in pediatric diffuse intrinsic pontine gliomas and non⁃brainstem glioblastomas[J].Nat Genet,2012,44(3):251-253.
[27]Buczkowicz P,Hoeman C,Rakopoulos P,et al.Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations[J].Nat Genet,2014,46(5):451-456.
[28]杨燕武,毛 庆.胶质瘤诊断和预后标志物的研究进展[J].中国神经肿瘤杂志,2012,10(1):45-50.
[29]Griffin CA,Burger P,Morsberger L,et al.Identification of der(1;19)(q10;p10)in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss[J].J Neuropathol Exp Neurol,2006,65(10):988-994.
[30]Jenkins RB,Blair H,Ballman KV,et al.A t(1;19)(q10;p10)mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma[J].Cancer Res,2006,66(20):9852-9861.
[31]van den Bent MJ,Brandes AA,Taphoorn MJ,et al.Adjuvant procar⁃bazine,lomustine,and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma:long⁃term follow⁃up of EORTC brain tumor group study 26951[J].J Clin Oncol,2013,31(3):344-350.
[32]Cairncross G,Wang M,Shaw E,et al.PhaseⅢtrial of chemoradio⁃therapy for anaplastic oligodendroglioma:long⁃term results of RTOG 9402[J].J Clin Oncol,2013,31(3):337-343.
[33]Preusser M,Bienkowski M,Birner P.BRAF inhibitors in BRAF⁃V600 mutated primary neuroepithelial brain tumors[J].Expert Opin Investig Drugs,2016,25(1):7-14.
[34]Sampson JH,Heimberger AB,Archer GE,et al.Immunologic escape after prolonged progression⁃free survival with epidermal growth factor receptor variantⅢ peptide vaccination in patients with newly diag⁃nosed glioblastoma[J].J Clin Oncol,2010,28(31):4722-4729.
[35]Phillips AC,Boghaert ER,Vaidya KS,et al.ABT⁃414,an antibody⁃drug conjugate targeting a tumor⁃selective EGFR epitope[J].Mol Cancer Ther,2016,15(4):661-669.
[36]Schuster J,Lai RK,Recht LD,et al.A phase II,multicenter trial of rindopepimut(CDX⁃110)in newly diagnosed glioblastoma:the ACT III study[J].Neuro Oncol,2015,17(6):854-861.
[37]Singh D,Chan JM,Zoppoli P,et al.Transforming fusions of FGFR and TACC genes in human glioblastoma[J].Science,2012,337(6099):1231-1235.
[38]Di Stefano AL,Fucci A,Frattini V,et al.Detection,characterization,and inhibition of FGFR⁃TACC fusions in IDH wild⁃type glioma[J].Clin Cancer Res,2015,21(14):3307-3317.
Research progress on molecular genetics in the diagnosis of glioma
GUAN Juan,ZHAN Chang⁃You
Department of Pharmacology,School of Basic Medical Sciences,Fudan University,Shanghai 200033,China
Gliomas are among the most frequent malignant tumors in central nervous system.Genome⁃wide molecular⁃profi⁃ling studies have revealed the characteristics of genetic alteration and epigenetic profile associated with different types of gliomas.In 2016,the WHO classification of central nervous system tumors has been revised,in which molecular biomarkers have been incor⁃porated based on classic histology for the categorization of gliomas.Those biomarkers would serve as a gold standard in the diagnosis of glioma and guide precise therapy and evaluation of prognosis in clinical practice.
glioma;molecular genetics;biomarker;diagnosis
R739.41
A
2095⁃6894(2017)07⁃07⁃04
2017-04-22;接受日期:2017-05-05
国家自然科学基金(81673361)
官 娟.博士生.研究方向:靶向药物递送.E⁃mail:16111010088@fudan.edu.cn
占昌友.研究员,青年千人.研究方向:靶向药物递送.E⁃mail:cyzhan@fudan.edu.cn
