IgG4-related sclerosing cholangitis and chronic sclerosing sialadenitis mimicking cholangiocarcinoma and neck malignancy
2017-08-16LiSun,Hong-YanLi,DavidRBrigstock等
IgG4-related sclerosing cholangitis and chronic sclerosing sialadenitis mimicking cholangiocarcinoma and neck malignancy
To the Editor:
IgG4-related sclerosing cholangitis (IgG4-SC) has recently been recognized as a biliary manifestation of IgG4-related disease (IgG4-RD). Type 3 IgG4-SC presented biliary strictures in both the porta hepatis and the distal common bile duct (CBD).[1,2]Its manifestation, especially in the absence of autoimmune pancreatitis, is extremely rare and very similar to that of cholangiocarcinoma (CC).
Chronic sclerosing sialadenitis (Küttner tumor, KT) is an uncommon benign tumor-like lesion that most often affects the unilateral or bilateral submandibular glands.[3,4]Recently, KT has been recognized within the spectrum of IgG4-RD.[3]Clinically this disease is easily confused with neck malignancy.
Here, we would like to describe a rare case of type 3 IgG4-SC that lacked pancreatic lesion and was accompanied by KT and lymphadenitis manifesting itself as a mass in the neck, which was originally suspected as CC and neck malignancy.
A 59-year-old female from Northeast China was admitted for a six-month history of jaundice and pruritus. She presented a mass in the right submandibular region with slight dysfunction of salivary secretion for three months. One year prior to this admission, she had undergone surgical excision of a mass in the left submandibular region. The patient has never suffered from ulcerous colonitis.
On physical examination, the patient had a painless, hard, fi xed mass in the right submandibular region (suspected cervical malignancy). Abdominal examination revealed right upper quadrant tenderness to deep palpation. Laboratory values included serum total bilirubin 100.7 μmol/L, alkaline phosphatase 400.1 U/L, alanine aminotransferase 107.6 U/L, amylase 85 U/L, CA19-9 147.3 U/mL (normal range <37 U/mL), negative reactions for ANA, p-ANCA, anti-SSA/SSB and anti-HIV antibodies. A CT scan showed wall thickening and marked lumen stenosis in the upper part of the CBD and a normal size in the head and body of the pancreas (Fig. 1), suggesting the possibility of CC. MRCP showed severe stenosis in both the upper portion and the distal portion of the CBD with an intra- and extra-hepatic bile duct dilation (Fig. 2).
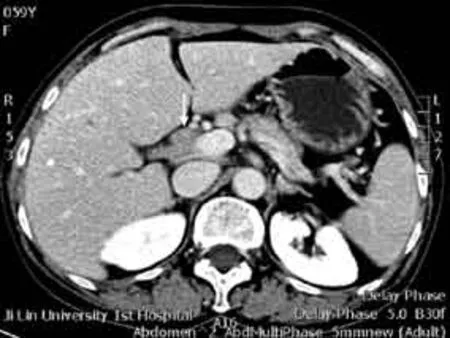
Fig. 1. A CT image of rare hepatic portal sclerosing cholangitis:the obstruction in the upper part of the common bile duct with nodular projection (white arrow) and a normal size in the head and body of the pancreas.
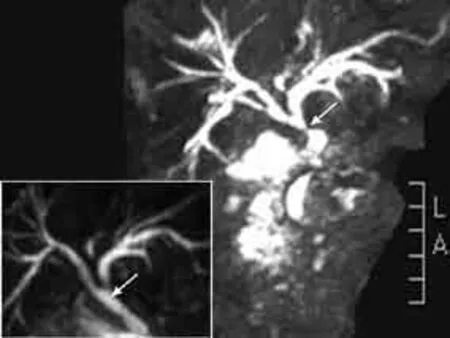
Fig. 2. An MRCP image showing an intra- and extra-hepatic bile duct dilation and a marked stenosis in the upper portion of the common bile duct (white arrow) and a severe stenosis in the distal portion of the common bile duct. The inset showing their dramatic recovery after 4 week of steroid therapy.
On the 5th day after admission, the patient underwent an ultrasound examination of the neck, which showed a right swollen submandibular gland (2.0×2.0 ×1.5 cm3). She then underwent an ultrasound-guided needle biopsy of the submandibular gland. HE staining of the left submandibular gland that had been previously resected one year ago revealed acinar atrophy, dense lymphoplasmacytic inf i ltration and fi brosis, but no pathological changes in normal submandibular gland from a healthy individual (Fig. 3A, B). Immunohistological staining of this specimen showed numerous IgG4-postive plasma cell inf i ltrations, with a ratio of IgG4/ IgG-positive plasma cells of more than 40% (Fig. 3C, D). Similar histological and immunohistochemical fi ndings were obtained for the needle biopsy samples of the right submandibular gland obtained after admission (data not shown).
The resected lymph node specimens collected from the left submandibular region one year prior to hospital admission were also determined by immunohistochemistry. As shown in Fig. 4, there were numerous IgG4-positive plasma cell inf i ltrations in the patient’s lymph node, with a ratio of IgG4/IgG-positive plasma cells of more than 40%.
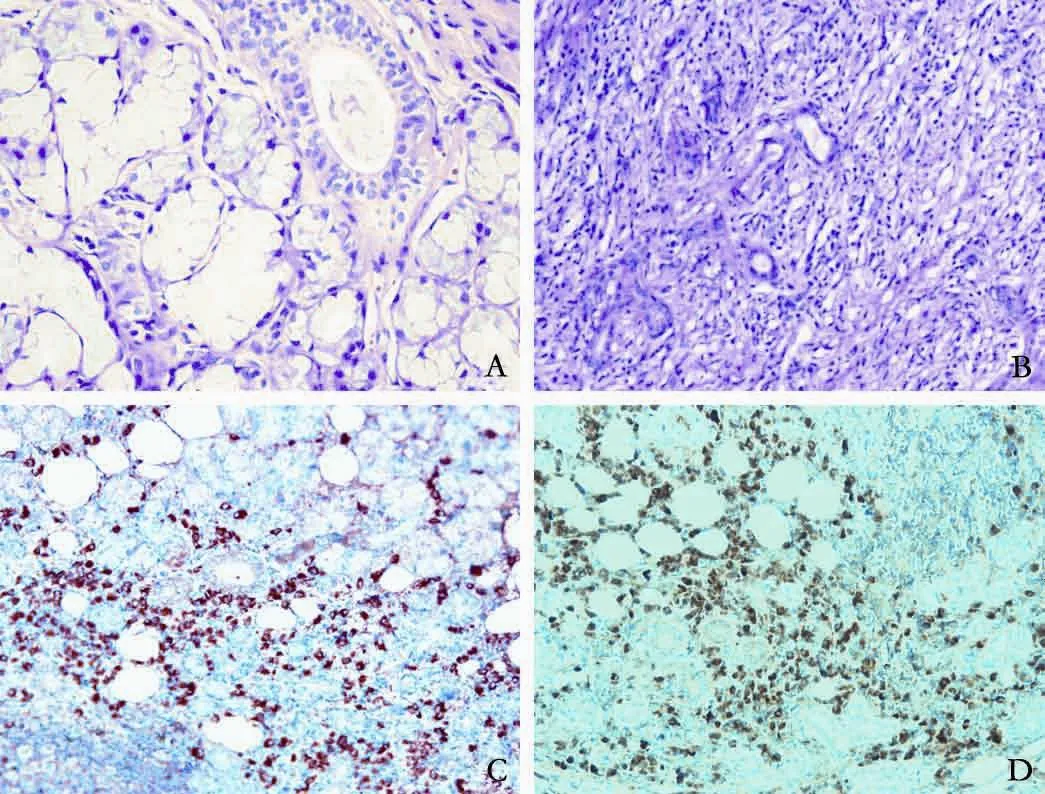
Fig. 3. A, B: HE staining showing normal submandibular gland (A × 400); acinar atrophy and lymphoplasmacytic inf i ltration and fi brosis in the resected submandibular gland from the patient (B ×200); C, D: immunohistological staining showing IgG4-positive plasma cells (C ×200) and IgG-positive plasma cells in the resected submandibular gland from the patient (D ×200).
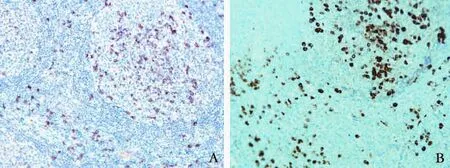
Fig. 4. Immunohistochemical staining showing IgG4-positive plasma cells (A ×200) and IgG-positive plasma cells in the lymph node sections of the patient (B ×200).
On the 8th day after admission, seroimmunological tests revealed a high level of IgG4 (38.4 g/L). The patient was then diagnosed with type 3 IgG4-SC accompanied with KT and lymphadenitis. She received 30 mg/d of prednisone for four weeks. The swollen submandibular gland was no longer palpable, liver function and CA19-9 had returned to normal levels, and the elevated IgG4 concentration (38.4 g/L) was dramatically decreased (14.9 g/L). Additionally, the intra- and extra-hepatic bile duct dilations and stenotic CBD had returned to nearnormal size (inset Fig. 2). The patient received a longterm maintenance dose of 10 mg/d prednisone after steroid tapering. At 12-month follow-up, her illness had not recurred.
KT is an uncommon benign tumor-like lesion usually affecting the unilateral or bilateral submandibular glands. Recently it has been recognized within the spectrum of IgG4-RD.[3]The differential diagnosis of KT mainly includes infectious/reactive lymphadenopathy, lymphoma, and metastatic involvement of the cervical lymph nodes.[5]Recently, ultrasonography has been proposed to evaluate the nature of focal salivary masses, with sensitivity and accuracy of nearly 100%.[6]Fine-needle aspiration biopsy (FNAB) is also widely accepted for the diagnosis of salivary gland masses.[6]The patient in this study fully met the diagnostic criteria of KT based on an elevated level of serum IgG4 and an abundant IgG4-positive plasma cell inf i ltration in the right submandibular gland tissue samples that was obtained by FNAB after admission. However, she underwent an unnecessary excision of the left submandibular gland and lymph nodes one year ago. Therefore, serum IgG4 test and ultrasonic examination should always be performed, while FNAB offers an additional optional approach to distinguish KT from malignancies or other inf l ammatory lesions in case of a mass in the submandibular region.
IgG4-SC is a distinctive type of cholangitis of unknown pathogenesis. Recently, a new clinical diagnostic criteria of IgG4-SC was established based on the diffuse or segmental narrowing of the intra-hepatic and/or extra-hepatic bile duct associated with the thickening of bile duct wall plus the coexistence of other IgG4-RD.[7]Thus, the patient in this study fully met the diagnostic criteria of IgG4-SC. However, the patient initially presented with a normal sized pancreas, bile duct strictures in both the porta hepatis and the distal CBD, an elevated level of serum CA19-9 and a mass in the right submandibular region, which was very rare and extremely similar to CC and neck malignancy.
Recently, Nakazawa et al[8]proposed the following intraductal ultrasonographic features that may be helpful for diagnosing IgG4-SC: circular-symmetric wall thickness, a smooth inner margin, a smooth outer margin, and a homogenous internal echo in the stricture. A cutoff level of serum IgG4 (207 mg/dL) is also suggested as a useful parameter in type 3 IgG4-SC.[9]Additionally, other organ involvement (diffuse swollen pancreas, dacryoadenitis/sialadenitis and lymphadenitis) were highly specif i c in the diagnosis of IgG4-SC. SpyGlass™ cholangioscopy has recently been shown to allow adequate biopsy sampling and def i nite diagnosis with high accuracy in patients with indeterminate biliary lesions.[10]Therefore, a combination of characteristic clinical, serological, morphological and histological features, other organ involvement and response to steroid should be analyzed to differentiate IgG4-SC from CC.
Li Sun, Hong-Yan Li, David R Brigstock and Run-Ping Gao
Department of Hepatic-biliary-pancreatic Medicine, First Hospital of Jilin University, Changchun 130021, China (Sun L, Li HY and Gao RP); The Research Institute at Nationwide Children’s Hospital and Division of Pediatric Surgery, Departmentof Surgery, The Ohio State University, Columbus, OH 43205, USA (Brigstock DR)
Contributors: SL and LHY contributed to the study design and data collection. GRP designed the study and wrote the manuscript. BDR coordinated the study and edited the manuscript. GRP is the guarantor.
Funding: This study was supported by a grant from the National Natural Science Foundation of China (81270544).
Ethical approval: Written consent was obtained from the patient.
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Okazaki K, Uchida K, Koyabu M, Miyoshi H, Ikeura T, Takaoka M. IgG4 cholangiopathy: current concept, diagnosis, and pathogenesis. J Hepatol 2014;61:690-695.
2 Miki A, Sakuma Y, Ohzawa H, Sanada Y, Sasanuma H, Lefor AT, et al. Immunoglobulin G4-related sclerosing cholangitis mimicking hilar cholangiocarcinoma diagnosed with following bile duct resection: report of a case. Int Surg 2015;100:480-485.
3 Wei TW, Lien CF, Hsu TY, He HL. Chronic sclerosing sialadenitis of the submandibular gland: an entity of IgG4-related sclerosing disease. Int J Clin Exp Pathol 2015;8:8628-8631.
4 Furukawa S, Moriyama M, Kawano S, Tanaka A, Maehara T, Hayashida JN, et al. Clinical relevance of Küttner tumour and IgG4-related dacryoadenitis and sialoadenitis. Oral Dis 2015;21:257-262.
5 McKinnon T, Randazzo WT, Kim BD, Biddinger P, Forseen S. IgG4-related disease presenting as a solitary neck mass. J Radiol Case Rep 2015;9:1-8.
6 Uhliarova B, Svec M. Kuttner tumor. Bratisl Lek Listy 2013;114:36-38.
7 Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci 2012;19:536-542.
8 Nakazawa T, Naitoh I, Hayashi K. Usefulness of intraductal ultrasonography in the diagnosis of cholangiocarcinoma and IgG4-related sclerosing cholangitis. Clin Endosc 2012;45:331-336.
9 Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol 2013;19:7661-7670.
10 Manta R, Frazzoni M, Conigliaro R, Maccio L, Melotti G, Dabizzi E, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc 2013;27:1569-1572.
Published online July 13, 2017.
Run-Ping Gao
(Email: gao_runping@126.com)
10.1016/S1499-3872(17)60042-0)
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- The International Study Group of Pancreatic Surgery def i nition of delayed gastric emptying and the effects of various surgical modif i cations on the occurrence of delayed gastric emptying after pancreatoduodenectomy
- Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Effects of multimodal fast-track surgery on liver transplantation outcomes
