Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
2017-08-16MustafaKaplanIhsanAtesMuhammedYenerAkpinarMahmutYukselUfukBarisKuzuSabiteKacarOrhanCoskunandErtugrulKayacetin
Mustafa Kaplan, Ihsan Ates, Muhammed Yener Akpinar, Mahmut Yuksel, Ufuk Baris Kuzu, Sabite Kacar, Orhan Coskun and Ertugrul Kayacetin
Ankara, Turkey
Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
Mustafa Kaplan, Ihsan Ates, Muhammed Yener Akpinar, Mahmut Yuksel, Ufuk Baris Kuzu, Sabite Kacar, Orhan Coskun and Ertugrul Kayacetin
Ankara, Turkey
BACKGROUND:Serum C-reactive protein (CRP) increases and albumin decreases in patients with inf l ammation and infection. However, their role in patients with acute pancreatitis is not clear. The present study was to investigate the predictive signif i cance of the CRP/albumin ratio for the prognosis and mortality in acute pancreatitis patients.
METHODS:This study was performed retrospectively with 192 acute pancreatitis patients between January 2002 and June 2015. Ranson scores, Atlanta classif i cation and CRP/albumin ratios of the patients were calculated.
RESULTS:The CRP/albumin ratio was higher in deceased patients compared to survivors. The CRP/albumin ratio was positively correlated with Ranson score and Atlanta classif i cation in particular and with important prognostic markers such as hospitalization time, CRP and erythrocyte sedimentation rate. In addition to the CRP/albumin ratio, necrotizing pancreatitis type, moderately severe and severe Atlanta classif i cation, and total Ranson score were independent risk factors of mortality. It was found that an increase of 1 unit in the CRP/albumin ratio resulted in an increase of 1.52 times in mortality risk. A prediction value about CRP/albumin ratio >16.28 was found to be a signif i cant marker in predicting mortality with 92.1% sensitivity and 58.0% specif i city. It was seen that Ranson and Atlanta classif i cation were higher in patients with CRP/albumin ratio >16.28 compared with those with CRP/albumin ratio ≤16.28. Patients with CRP/albumin ratio >16.28 had a 19.3 times higher chance of death.
CONCLUSION:The CRP/albumin ratio is a novel but promising, easy-to-measure, repeatable, non-invasive inf l ammationbased prognostic score in acute pancreatitis.
(Hepatobiliary Pancreat Dis Int 2017;16:424-430)
Atlanta classif i cation; C-reactive protein; Glasgow prognostic score; Ranson score; acute pancreatitis
Introduction
Acute pancreatitis (AP) is a severe inf l ammation of the pancreas presented with sudden onset and severe abdominal pain and has high morbidity and mortality rate. Although its etiology is not known for certain, it is mostly associated with gallstones and alcohol.[1]It is associated with intra-acinar activation of proteolytic enzymes, leukocyte chemoattraction, release of cytokines, oxidative stress and microcirculatory injury.[2-4]The common factor in all these mechanisms is an excessive inf l ammatory response.
The prognosis of the disease varies depending on the severity of the disease. Approximately 75%-80% of cases progress mildly and can be cured solely with intravenous fl uid treatment and supportive care. The remaining cases progress with mortality and severe complications up to 30%-50%.[5]For this reason, early diagnosis of the disease and determination of a therapeutic strategy according to disease severity are of great importance. Several scoring systems are used in order to determine the disease severity. However, none of these scoring systems can determine the severity within the fi rst hour of admission. For this reason, there is a need for easy-to-use and inexpensive indices that can determine the disease severity and give an idea about the prognosis within minutes in clinical practice.
Serum C-reactive protein (CRP) is a positive acutephase reactant synthesized by the liver and its level in the blood increases within hours in response to inf l ammation and infection.[6,7]It is frequently used in infection and inf l ammation follow-up due to its short half-life, easy measurement and close relationship with prognosis of the disease.[8-10]It can be used for diagnosis, treatment follow-up and mortality prediction especially in inf l ammatory cases.[11,12]
Albumin is a negative acute phase reactant synthesized by the liver and its level in the blood decreases during inlf ammation. In previous studies, albumin was shown to be associated with inf l ammation severity, disease prognosis and mortality.[13-15]The reason for this is the close relationship between inf l ammation and malnutrition.
The CRP/albumin ratio is a new inf l ammation-based prognostic score and it is correlated to the inf l ammation severity[16]and mortality.[17]However, there is no study available in the literature which investigates the relationship of this marker with disease prognosis and mortality. The present study investigated the predictive signif i cance of the CRP/albumin ratio for prognosis and mortality in AP patients.
Methods
Patients
We reviewed the fi les of AP patients in the Gastroenterology Clinic of Turkey Yuksek Ihtisas Training and Research Hospital between January 2002 and June 2015. A total of 300 patients were retrieved initially, 108 were excluded from the study due to lack of follow-up and insuff i cient laboratory values. Patients with documented cardiovascular and cerebrovascular diseases, known renal and liver failure or malignancy diagnosis and those who had infectious disease within the last month were excluded from the study. Our patients were over the age of 18, hospitalized with AP diagnosis according to Atlanta criteria. Laboratory parameters had been recorded and computed tomographies were performed to evaluate pancreatitis severity at least at the date of admission. Finally 192 patients were included in the analysis.
A diagnosis of AP was made using the Atlanta criteria which requires the presence at least 2 of the followings: 1) abdominal pain highly suggestive of acute pancreatitis; 2) elevations in serum amylase and/or lipase to more than 3 times the upper limit of normal; 3) the presence of characteristic radiological fi ndings (ultrasonography or computerized tomography) of AP.[18,19]
Relevant information such as age, gender, laboratory fi ndings, complications, radiologic fi ndings, duration of hospital stay, Ranson score, Atlanta classif i cation, mortality status were retrieved from patient fi les. According to the Atlanta classif i cation, patients without local or systemic complications and organ failure were classif i ed as mild AP; those with transient organ failure - organ failure that resolves within 48 hours and/or local or systemic complications - were classif i ed as moderately severe AP; and those with persistent organ failure were classif i ed as severe AP. For patients with pancreatitis associated with gallstones and other reasons, Ranson criteria were calculated at 0 h and 48 h separately and a total Ranson score was achieved. All laboratory fi ndings of the patients were obtained at the time of admission.
Statistical analysis
Statistical Package for Social Sciences (SPSS) for Windows 20 (IBM SPSS Inc., Chicago, USA) and Med-Calc 11.4.2 (MedCalc Software, Mariakerke, Belgium) softwares were used for statistical assessments. The normal distribution of the data was evaluated with the Shapiro-Wilk test. Values with normal distribution were presented as mean±standard deviation and values without normal distribution were presented as median (range). Categorical variables were presented as numbers and percentages. Numerical values in the deceased patient group and the survival patient group were compared using the Student’sttest and the Mann-WhitneyUtest. Chi-square test and Fisher’s exact test were used in comparison of categorical data. Univariable Cox regression analysis was utilized in order to determine the effects of potential prognostic factors on mortality. Signif icant factors were included in the stepwise multivariate Cox regression model and independent predictors were identif i ed. The diagnostic discrimination of independent predictors in mortality was examined with ROC curve analysis, area under the curve (AUC).[20]The Youden index method was used in order to determine the prediction point of the CRP/albumin ratio for mortality. The Kaplan-Meier analysis was used to show the effect of the determined prediction value on survival. In statistical analysis, aP<0.05 with 95% conf i dence interval and 5% margin of error was considered statistically signif i cant.
Results
Entire population fi ndings
The study population consisted of 192 patients, 120 females (62.5%) and 72 males (37.5%). The mean age of the patients was 61.9±18.0 years. One hundred eightyif ve (96.4%) of the patients had abdominal pain, 15 (7.8%) had jaundice, 32 (16.7%) had fever, 39 (20.3%) had nausea, and 1 (0.5%) was asymptomatic. The distribution of the patients in terms of etiology was as follows: 154 (80.2%) gallstone, 5 (2.6%) alcohol, 3 (1.6%)hypertriglyceridemia, 18 (9.4%) hereditary, 3 (1.6%) endoscopic retrograde cholangiopancreatography (ERCP), and 9 (4.7%) other etiologies. With regard to AP complications, 17 (8.9%) of the patients had acute renal failure, 8 (4.2%) had abscess, 10 (5.2%) had sepsis, 9 (4.7%) had pseudocyst, 3 (1.6%) had ascites, 5 (2.6%) had hematoma, and 6 (3.1%) had cholangitis. 153 (79.7%) of the patients had edematous and 39 (20.3%) had necrotizing pancreatitis. 38 (19.8%) patients died. The median hospitalization length of the patients was 9 days (2-100) and the median follow-up length was 63 months (1-126).
Twenty-nine (15.1%) of the patients had Ranson score of “0”, 36 (18.8%) had Ranson score of “1”, 44 (22.9%) had Ranson score of “2”, 31 (16.1%) had Ranson score of “3”, 17 (8.9%) had Ranson score of “4”, 25 (13.0%) had Ranson score of “5”, and 10 (5.2%) had Ranson score of “6”. One hundred twenty-seven (66.1%) patients had a “mild” Atlanta classif i cation, 36 (18.8%) had a “moderately severe” Atlanta classif i cation, and 29 (15.1%) had a “severe” Atlanta classif i cation.
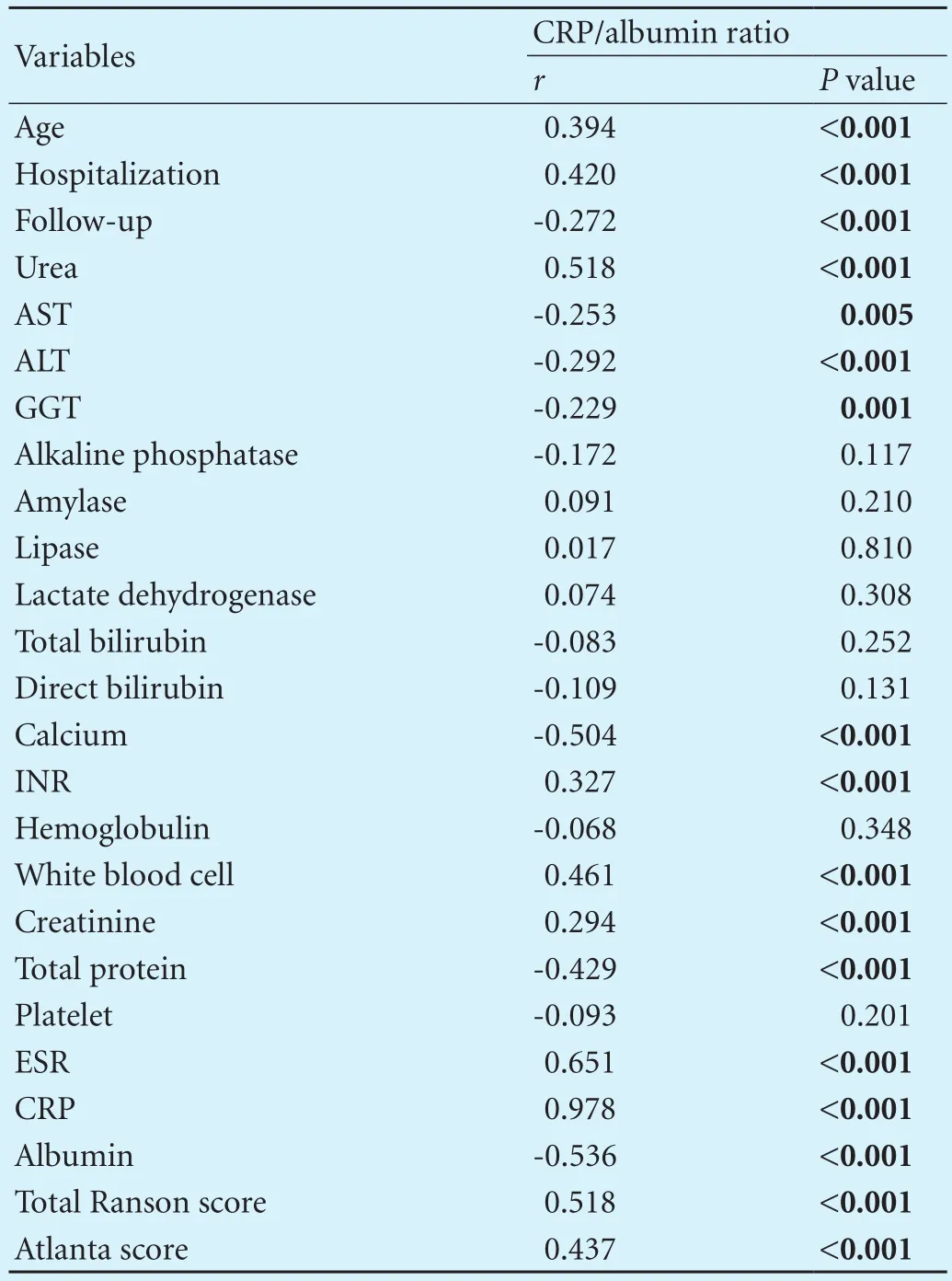
Table 1. The relationships between CRP/albumin ratio and other clinical characteristics
Correlation
The CRP/albumin ratio had a positive correlation with age (r=0.394,P<0.001), hospitalization (r=0.420,P<0.001), urea (r=0.518,P<0.001), INR (r=0.327;P<0.001), leukocyte (r=0.461;P<0.001), creatinine (r=0.294;P<0.001), ESR (r=0.651;P<0.001), CRP (r=0.978;P<0.001), Atlanta classif i cation (r=0.437;P<0.001), total Ranson score (r=0.518;P<0.001) and a negative correlation with follow-up (r=-0.272;P<0.001), AST (r=-0.253,P=0.005), ALT (r=-0.292;P<0.001), GGT (r=-0.229;P<0.001), calcium (r=-0.504;P<0.001), total protein (r=-0.429;P<0.001) and albumin (r=-0.536,P<0.001) levels (Table 1).
Independent risk factors
Signif i cant prognostic factors were included in the backward stepwise regression analysis (Tables 2-4). In the Cox regression model and multivariable regression anal-ysis, the CRP/albumin ratio (HR=1.52, 95% CI: 1.23-1.89,P<0.001), necrotizing pancreatitis type (HR=4.44, 95% CI:3.56-5.32,P<0.001), moderately severe and severe Atlanta classif i cation (HR=6.66, 95% CI: 4.96-8.96,P<0.001;HR=11.47, 95% CI: 6.58-20.0,P<0.001, respectively), and total Ranson score (HR=1.50, 95% CI: 1.27-1.78,P<0.001) were independent risk factors of mortality. An increase of 1 unit in the CRP/albumin ratio resulted in an increase of 1.52 times in mortality risk.
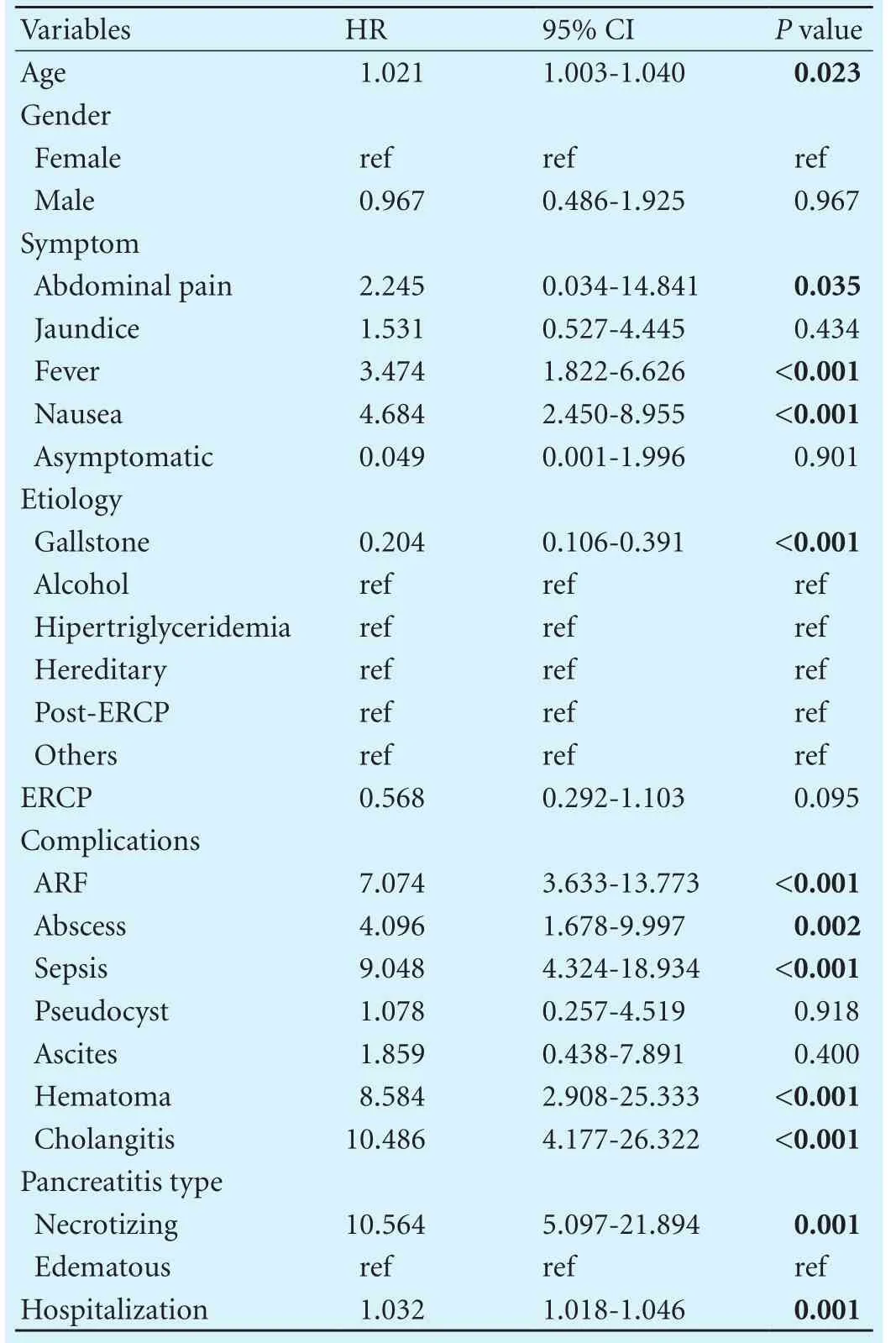
Table 2. Demographic charecteristics and clinical findings of study population

Table 3. Distribution of laboratory fi ndings by risk groups
Fig. 1 shows the diagnostic assessment of independent predictors of mortality. Accordingly, independent predictors with high AUC values did not show a diagnostic difference in terms of predicting mortality. A prediction value of CRP/albumin ratio >16.28 was found to be a signif i cant marker in predicting mortality (sensitivity:92.1%; specif i city: 58.0%; AUC: 0.835;P<0.001). The mortality risk of the patients whose CRP/albumin ratio were higher than 16.28 (35/99) was 19.271 times higher when compared to the patients with a CRP/albumin ratio ≤16.28 (3/93) (HR=19.271, 95% CI: 5.849-63.491,P<0.001). The median survival length of the patients with a CRP/albumin ratio>16.28 was 55 months (95% CI: 45.6-64.4). The 1-, 3-, and 5-year survival rates were 90.4%±0.03%, 67.5%±0.06%, and 23.5%±0.11% (Fig. 2).
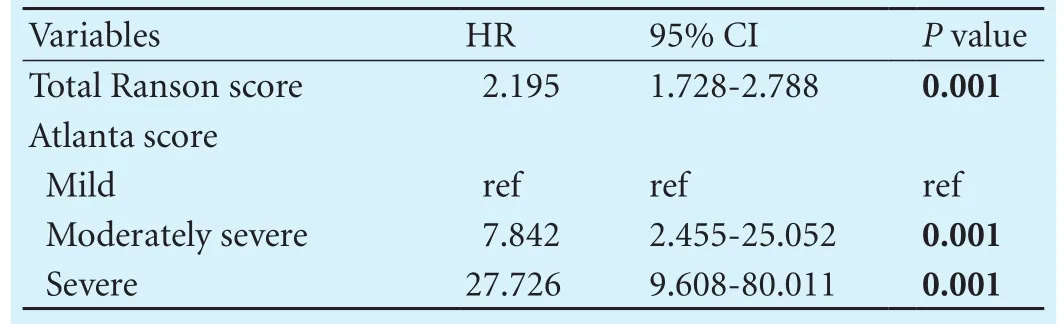
Table 4. Distribution of prognostic scores by risk groups

Fig. 1. Diagnostic assessment of independent predictors of mortality with ROC curve analysis. AUC:area under curve;SE:standard error; 95% CI: 95% conf i dence interval; CRP: C-reacitive protein.
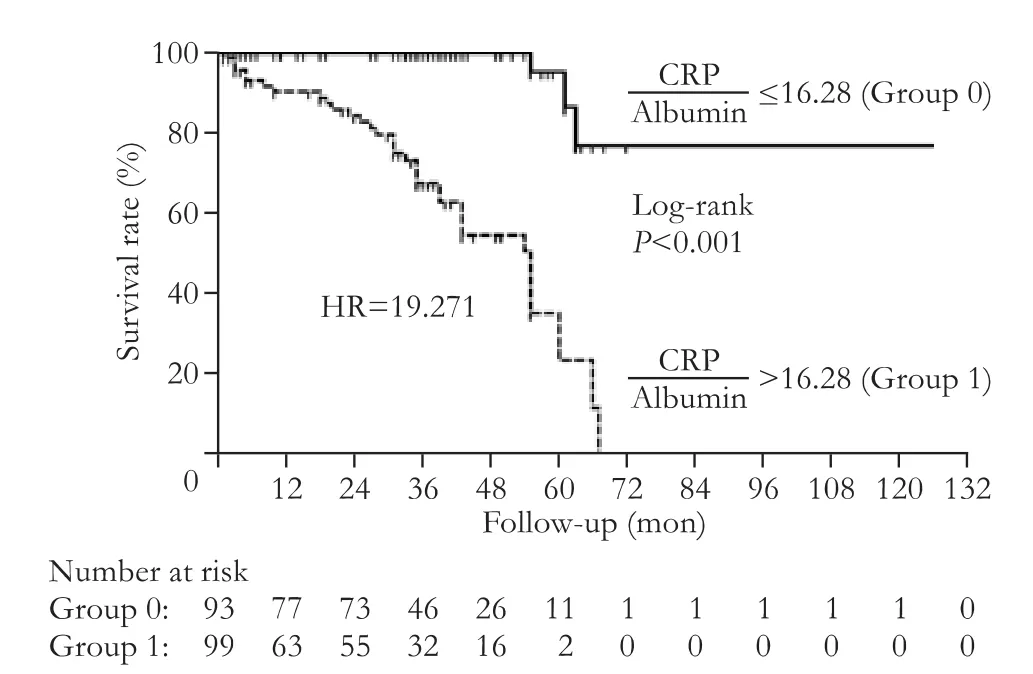
Fig. 2. Survival analysis of acute pancreatitis patients according to CRP/albumin ratio.
Eleven (11.1%) of the patients with a CRP/albumin ratio >16.28 had Ranson score of 4, 22 (22.2%) had Ranson score of 5, 10 (10.1%) had Ranson score of 6. These values were 6.5%, 3.2% and 0 respectively for the patients with a CRP/albumin ratio ≤16.28 (P<0.001). 50 (50.5%) of the patients with a CRP/albumin ratio >16.28 had a mild Atlanta classif i cation, 23 (23.2%) had a moderately severe Atlanta classif i cation, 26 (26.3%) had a severe Atlanta classif i cation. These values were 82.8%, 14.0% and 3.2% respectively for the patients with a CRP/albumin ratio ≤16.28 (P<0.001).
Discussion
The present study demonstrated that the CRP/albumin ratio was higher in deceased AP patients compared to those survived and it was a prognostic factor. The CRP/ albumin ratio was positively correlated with Ranson score, and Atlanta classif i cation in particular and with important prognostic markers such as hospitalization time, CRP and ESR. As well known, necrotizing pancreatitis type, moderately severe and severe Atlanta classif i cation, and total Ranson score were independent risk factors of mortality; the present study showed that CRP/ albumin ratio was another predictor of mortality in AP patients.
AP is a common gastrointestinal emergency and its mortality can reach up to 40%. For this reason, it is of great importance to identify patients to be treated aggressively at the time of admission. There is a need for a simple, repeatable and non-invasive laboratory procedure that does not require additional time and is easy to measure at the time of admission. Thus, we investigated the usability of the CRP/albumin ratio for the determination of prognosis and mortality in patients who are admitted to our clinic with an AP diagnosis. Several studies showed that both markers are used in the diagnosis, treatment follow-up and prognosis determination.[11,13]CRP especially was very valuable in acute response due to its short half-life.[11]CRP levels are frequently used at the time of admission and later during the treatment in AP patients.[21]Wang et al[22]showed that low albumin and high CRP were markers of poor outcome and this support the idea that CRP and albumin could be used for predicting the mortality risk in AP patients.
The CRP/albumin ratio is widely used to predict the patient outcomes in many diseases such as colorectal cancer[23]and sepsis[24]etc. However, the role of CRP/albumin ratio in AP prognosis is not clear. The present study showed that CRP/albumin ratio was higher in deceased patients compared to those who survived which demonstrated the prognostic value in AP patients.
Median age, acute renal failure, abscess, sepsis, cholangitis rate, hospitalization length and necrotizing pancreatitis type were higher in the deceased group compared with survivors, which were consistent with the literature.[25-27]]We also found that the CRP/albumin ratio, Atlanta and Ranson scores, presence of necrosis were independent risk factors of mortality, which were mostly consistent with Ferreira’s study.[25]Our data implied that the CRP/ albumin ratio may be useful for prognostic purposes in AP. Although the Ranson score has been used in AP prognosis for more than three decades, its biggest disadvantage is that it requires 48 hours for assessment. Atlanta classif i cation, another global standard tool for assessment of AP severity, maintains its complexity due to confusing terms related to disease severity.[28]Because of this complexity, we investigated the prognostic signif i cance of the CRP/albumin ratio in AP and found that the CRP/albumin ratio was directly related with AP prognosis.
Kim et al showed that the CRP/albumin ratio was superior to the CRP level in predicting mortality in patients with septic shock; if the cut-off value is 5.09, the sensitivity and specif i city of this ratio were 61% and the Kaplan-Meier curve analysis showed a signif i cantly higher 180-day mortality rate in patients with a CRP/albumin ratio>5.09, compared to patients with a lower CRP/albumin ratio.[24]In our study, compared with patients who had a CRP/albumin ratio <16.28, those with a CRP/albumin ratio ≥16.28 had a 19.3 times higher risk of death. Also, the fact that 1-, 3- and 5-year survival rates were found to be lower in the patients with a CRP/albumin ratio >16.28 supports the fi ndings of the above mentioned study. Similarly, Kawashima et al[29]found that the 5-year survival rate decreased in patients diagnosed with lung carcinoma as CRP/albumin ratio increased.
Presence of gallstones in etiology was a marker of lower mortality. This might be due to low prevalence ofcomplications and patients with pancreatitis associated with gallstones were treated with ERCP urgently in our clinic.
The limitations of our study include the retrospective nature, single-center, low population study, which may limit the prognostic value of the CRP/albumin ratio. The other limitation of our study is biliary pancreatitis ratio.
Mortality rate in AP is found 19.8% during the hospitalization in our study. This might be due to most of AP patients that admitted to our emergency room took medical treatment in emergency and discharged and excluded from the study because of non-hospitalization. Additionally because our hospital is third stage education hospital, more complicated patients admit to our clinic. In literature there are studies that mortality rate reached to 30%.[30-32]Possible bias was likely. However, we think our patients count is enough for statistical analysis. Additional prospective studies with larger populations involving multiple centers are necessary to accurately evaluate the CRP/albumin ratio as a predictor of mortality.
In summary, this study demonstrated that as a cheap, repeatable, non-invasive systemic inf l ammation-based marker, the CRP/albumin ratio is an independent predictor of overall survival for patients with AP. The CRP/ albumin ratio could be used to predict prognosis in patients with AP like other prognostic scores or laboratory parameters.
Contributors: KM and AI were responsible for the study design, analysis and interpretation of the data, drafting of the manuscript. AMY performed the statistical analysis. YM and CO interpreted the data. KS and KE revised the manuscript for important intellectual content. All authors contributed to the design and interpretation of the study and to further drafts. KM and AI contributed equally to this study. KM is the guarantor.
Funding: None.
Ethical approval: The study was approved by the Institutional Review Board of the Turkey Yuksek Ihtisas Training and Research Hospital (2015-0625). All participants provided written informed consent.
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Gurusamy KS, Nagendran M, Davidson BR. Early versus delayed laparoscopic cholecystectomy for acute gallstone pancreatitis. Cochrane Database Syst Rev 2013:CD010326.
2 Testoni PA. Acute recurrent pancreatitis: etiopathogenesis, diagnosis and treatment. World J Gastroenterol 2014;20:16891-16901
3 Kota SK, Krishna SV, Lakhtakia S, Modi KD. Metabolic pancreatitis: etiopathogenesis and management. Indian J Endocrinol Metab 2013;17:799-805.
4 Tiscornia OM, Negri GA, Otero G, López Mingorance FN, Waisman H, Tiscornia-Wasserman PG. Pancreatic polypeptide: a review of its involvement in neuro-endocrine ref l exes, islet-acinar interactions and ethanol-evoked physiopatologic pancreatic gland changes. Acta Gastroenterol Latinoam 2015;45:155-164.
5 Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010;24:219-231.
6 Matowicka-Karna J. Markers of inf l ammation, activation of blood platelets and coagulation disorders in inf l ammatory bowel diseases. Postepy Hig Med Dosw (Online) 2016;70:305-312.
7 Kinoshita A, Onoda H, Imai N, Nishino H, Tajiri H. C-reactive protein as a prognostic marker in patients with hepatocellular carcinoma. Hepatogastroenterology 2015;62:966-970.
8 Rhodes B, Fürnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol 2011;7:282-289.
9 Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-Inf l ammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS One 2016;11:e0147484.
10 Ateş H, Ateş і, Bozkurt B, Çelik HT, Özol D, Yldrm Z. What is the most reliable marker in the differential diagnosis of pulmonary embolism and community-acquired pneumonia? Blood Coagul Fibrinolysis 2016;27:252-258.
11 Lelubre C, Anselin S, Zouaoui Boudjeltia K, Biston P, Piagnerelli M. Interpretation of C-reactive protein concentrations in critically ill patients. Biomed Res Int 2013;2013:124021.
12 Lee KJ, Kim HM, Choi JS, Kim YJ, Kim YS, Cho JH. Comparison of predictive systems in severe acute pancreatitis according to the revised atlanta classif i cation. Pancreas 2016;45:46-50.
13 Goh SL, De Silva RP, Dhital K, Gett RM. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact Cardiovasc Thorac Surg 2015;20:107-113.
14 Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol 2013;19:105-115.
15 Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69.
16 Zhou T, Zhan J, Hong S, Hu Z, Fang W, Qin T, et al. Ratio of C-reactive protein/albumin is an inf l ammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep 2015;5:10481.
17 Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One 2013;8:e59321.
18 Bradley EL 3rd. A clinically based classif i cation system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-590.
19 Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69-81.
20 Arampatzis S, Frauchiger B, Fiedler GM, Leichtle AB, Buhl D, Schwarz C, et al. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med 2012;125:1125.e1-1125.
21 Staubli SM, Oertli D, Nebiker CA. Laboratory markers pre-dicting severity of acute pancreatitis. Crit Rev Clin Lab Sci 2015;52:273-283.
22 Wang X, Cui Z, Li H, Saleen AF, Zhang D, Miao B, et al. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol 2010;25:1386-1393.
23 Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical Signif i cance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann Surg Oncol 2016;23:900-907.
24 Kim MH, Ahn JY, Song JE, Choi H, Ann HW, Kim JK, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One 2015;10:e0132109.
25 Ferreira Ade F, Bartelega JA, Urbano HC, de Souza IK. Acute pancreatitis gravity predictive factors: which and when to use them? Arq Bras Cir Dig 2015;28:207-211.
26 Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, et al. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton) 2015;20:485-491.
27 Gomatos IP, Xiaodong X, Ghaneh P, Halloran C, Raraty M, Lane B, et al. Prognostic markers in acute pancreatitis. Expert Rev Mol Diagn 2014;14:333-346.
28 Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015;21:2387-2394.
29 Kawashima M, Murakawa T, Shinozaki T, Ichinose J, Hino H, Konoeda C, et al. Signif i cance of the Glasgow Prognostic Score as a prognostic indicator for lung cancer surgery. Interact Cardiovasc Thorac Surg 2015;21:637-643.
30 Carroll JK, Herrick B, Gipson T, Lee SP. Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician 2007;75:1513-1520.
31 Fu CY, Yeh CN, Hsu JT, Jan YY, Hwang TL. Timing of mortality in severe acute pancreatitis: experience from 643 patients. World J Gastroenterol 2007;13:1966-1969.
32 Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am 2008;92:889-923, ix-x.
Accepted after revision December 21, 2016
Adversity reveals genius; fortune conceals it.
—Horace
March 30, 2016
Author Aff i liations: Department of Gastroenterology, Turkey Yuksek Ihtisas Training and Research Hospital, Ankara 06100, Turkey (Kaplan M, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, Coskun O and Kayacetin E); Department of Internal Medicine, Ankara Numune Training and Research Hospital, Ankara 06100, Turkey (Ates I)
Ihsan Ates, MD, PhD, Department of Internal Medicine, Ankara Numune Training and Research Hospital, Ankara 06100, Turkey (Tel: +0903125084666; Fax: +0903123113958; Email:dr.ihsanates@hotmail.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60007-9
Published online April 24, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Indocyanine green fl uoroscopy and liver transplantation: a new technique for the intraoperative assessment of bile duct vascularization
- Effects of multimodal fast-track surgery on liver transplantation outcomes
- Characteristics of recipients with complete immunosuppressant withdrawal after adult liver transplantation
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
- Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience
