Emodin alleviates intestinal mucosal injury in rats with severe acute pancreatitis via the caspase-1 inhibition
2017-08-16JianWenNingYanZhangMoSangYuMengLiGuJiaXuAliUsmanandFengJi
Jian-Wen Ning, Yan Zhang, Mo-Sang Yu, Meng-Li Gu, Jia Xu, Ali Usman and Feng Ji
Hangzhou, China
Emodin alleviates intestinal mucosal injury in rats with severe acute pancreatitis via the caspase-1 inhibition
Jian-Wen Ning, Yan Zhang, Mo-Sang Yu, Meng-Li Gu, Jia Xu, Ali Usman and Feng Ji
Hangzhou, China
BACKGROUND:Emodin, a traditional Chinese medicine, has a therapeutic effect on severe acute pancreatitis (SAP), whereas the underlying mechanism is still unclear. Studies showed that the intestinal mucosa impairment, and subsequent release of endotoxin and proinf l ammatory cytokines such as IL-1β, which further leads to the dysfunction of multiple organs, is the potentially lethal mechanism of SAP. Caspase-1, an IL-1βconverting enzyme, plays an important role in this cytokine cascade process. Investigation of the effect of emodin on regulating the caspase-1 expression and the release proinf l ammatory cytokines will help to reveal mechanism of emodin in treating SAP.
METHODS:Eighty Sprague-Dawley rats were randomly divided into four groups (n=20 each group): SAP, sham-operated (SO), emodin-treated (EM) and caspase-1 inhibitor-treated (ICE-I) groups. SAP was induced by retrograde infusion of 3.5% sodium taurocholate into the pancreatic duct. Emodin and caspase-1 inhibitor were given 30 minutes before and 12 hours after SAP induction. Serum levels of IL-1β, IL-18 and endotoxin, histopathological alteration of pancreas tissues, intestinal mucosa, and the intestinal caspase-1 mRNA and protein expressions were assessed 24 hours after SAP induction.
RESULTS:Rats in the SAP group had higher serum levels of IL-1β and IL-18 (P<0.05), pancreatic and gut pathological scores (P<0.05), and caspase-1 mRNA and protein expressions (P<0.05) compared with the SO group. Compared with the SAP group, rats in the EM and ICE-I groups had lower IL-1β and IL-18 levels (P<0.05), lower pancreatic and gut pathological scores (P<0.05), and decreased expression of intestine caspase-1 mRNA (P<0.05). Ultrastructural analysis by transmission electron microscopy found that rats in the SAP group had vaguer epithelial junctions, more disappeared intercellular joints, and more damaged intracellular organelles compared with those in the SO group or the EM and ICE-I groups.
CONCLUSIONS:Emodin alleviated pancreatic and intestinal mucosa injury in experimental SAP. Its mechanism may partly be mediated by the inhibition of caspase-1 and its downstream inf l ammatory cytokines, including IL-1β and IL-18. Our animal data may be applicable in clinical practice.
(Hepatobiliary Pancreat Dis Int 2017;16:431-436)
severe acute pancreatitis; intestinal mucosa; emodin; caspase-1 inhibitor
Introduction
Severe acute pancreatitis (SAP) is a potentially lethal disease characterized by sudden onset, rapid progression, multiple organ failure and high mortality rate.[1-3]The underlying pathogenesis remains incompletely elucidated. As main anatomic and functional barrier, gut mucosa impairment is considered one of the major pathologies in SAP,[4,5]which will increase intestinal permeability, bacteria translocation as well as release of endotoxin, inf l ammatory mediators and cytokines (IL-1β, IL-18).[6]
Caspase-1 (IL-1β-converting enzyme/ICE) is a common intracellular cysteine protease that converts the precursors of IL-1β and IL-18 into active cytokines.[7,8]Blockade of caspase-1 suppresses inf l ammatory mediator expressions, such as IL-1β and IL-18, which will alleviate inf l ammation cascade reaction and ameliorate the overall severity and mortality of patients with SAP.[9,10]Thestudy by Wang et al[11]demonstrated that therapeutic effect of emodin (1, 3, 8-trihydroxy-6-methyl-anthraquinone) is partly mediated by IL-1β suppression, whereas the underlying mechanism is still unclear.
In this study, we hypothesized that emodin may have similar mechanism as caspase-1 inhibitor and investigated whether emodin showed analog inf l ammation cascade blockade with caspase-1 inhibitor in preventing intestinal mucosa injury in SAP.
Methods
Ethical approval
The study was approved by the Animal Care and Use Committee of Zhejiang University and conformed with the regulations of the Chinese guidelines for the care and use of laboratory animals.
Animals
Eighty adult male Sprague-Dawley rats weighing 250±50 g were supplied by Animal Center of Zhejiang Chinese Medical University. The rats were caged in a controlled-temperature of 25 ℃ and 12 hours light-dark cycles with free access to a standard rat chow.
Induction of pancreatitis and treatment
The rats were randomly divided into four groups (n=20 for each group): SAP, sham-operated (SO), emodin-treated (EM) and caspase-1 inhibitor-treated (ICE-I) groups. The rats were fasted for 12 hours before experiment with water access. Anesthesia was induced with 4% chloral hydrate intraperitoneal injection (1 mL/100 g, Solarbio Science & Technology Co, Ltd., Beijing, China). Rats in the SAP group had retrograde infusion of 3.5% sodium taurocholate (0.1 mL/100 g body weight, Sigma-Aldrich, St. Louis, MO, USA) into the pancreatic duct. Normal saline (0.2 mL) replaced sodium taurocholate in rats of the SO group. Rats in the EM and ICE-I groups were administered with emodin (0.5 mg/100 g) and ICE-I (0.1 mg/100 g) intra-gastric 30 minutes before and 12 hours after the induction of SAP. Meanwhile, rats in the SO and SAP groups were treated with normal saline solution of equivalent volume. The survived animals were sacrif i ced 24 hours after SAP induction with prolonged anesthesia and the obtained specimens from venous blood, pancreas body and gut tissues (5 cm from terminal ileac segments) were cut and stored in liquid nitrogen.
Histology
The specimens of pancreas and ileum were fi xed with formalin solution, embedded in paraff i n, stained with hematoxylin & eosin (HE), and analyzed under light microscope. The severity of acute pancreatitis and intestinal mucosal damage was assessed semi-quantitatively based on the scoring method def i ned by previous literatures.[12,13]Two experienced pathologists, who were blinded with the treatment, assessed each tissue sample in 5 microscopic fi elds randomly and totalled those fi elds to calculate the fi nal scores.
Transmission electron microscopy (TEM)
Terminal ileac segments specimens (5 mm×5 mm in size) were conventional fi xed by 2.5% glutaraldehyde and 2% osmium tetroxide, dehydrated by ethanol, and subjected to isoamyl acetate transition. AHCP-2 type critical point drying apparatus was used for critical point drying. The specimens were gilded target alloy using an IB-5 ion sputter coater. The cell morphology of the intestinal mucosa, the junctions of the intestinal mucosa epithelial cells, the ultrastructural changes of the intracellular mitochondria, the Golgi apparatus and other organelles were observed under TEM.
Serum IL-1β and IL-18 levels
Blood samples were collected from the inferior vena cava and centrifuged at 800 g for 10 minutes at 4 ℃. Serum levels of IL-1β and IL-18 were measured by enzymelinked immunosorbent assays (ELISA) according to the manufacturer’s instructions (IL-1β: e-Bioscience Company, Vienna, Austria; IL-18: Abnova Company, Heidelberg, Germany).
Caspase-1 mRNA levels
Total RNA of ileum tissues was extracted with chloroform and TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Two μg of RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Dalian, China). cDNA was stored at -80 ℃ until use. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Specif i c primers were as follows: caspase-1 forward 5’-CCA GAG CAC AAG ACT TCT GAC-3’ and reverse 5’-TGG TGT TGA AGA GCA GAA AGC-3’; GAPDH forward 5’-ACA GCA ACA GGG TGG TGG AC-3’ and reverse 5’-TTT GAG GGT GCA GCG AAC TT-3’. DNA products were amplif i ed with SYBR® Premix Ex Taq™ (Tli RNaseH Plus) (Takara). The amplif i cations were done with an initial denaturation at 95 ℃ for 10 minutes, followed by 40 thermal cycles at 95 ℃ for 30 seconds and 60 ℃ for 34 seconds. The threshold cycle (Ct) value wasmeasured, and the relative quantitative gene expression was calculated by 2-ΔΔCtmethod described previously.[14]
Caspase-1 protein levels
The ileum tissues from rats were homogenized in lysis buffer on ice for 30 minutes and then centrifuged at 12 000 g for 10 minutes at 4 ℃. The supernatants were then collected and stored at -80 ℃ until use. Protein concentrations were measured by the BCA Protein Assay Kit (Pierce, Rockford, IL, USA), following the manufacturer’s instructions. After separated on SDS-PAGE gel electrophoresis, proteins were transferred to polyvinylidene dif l uoride (PVDF) membranes (Merck Millipore, Darmstadt, Germany) and then blocked with 5% milk for 2 hours at room temperature. The rabbit anti-caspase-1 antibody (ab1872, Abcam, Cambridge, UK, 1:1000 dilution) and rabbit anti-GAPDH (2118, Cell Signaling Technology, Beverly, MA, USA, 1:5000 dilution) were used as primary antibodies, and then followed by the goat polyclonal secondary antibody to rabbit IgG-H& LHRP (ab6721, Abcam, Cambridge, UK, 1:1000 dilution). An enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA) was used for immune complexes detection. The samples were scanned by ChemiDoc TMXRS (Bio-Rad). The images were acquired and analyzed by Quantity One software (Bio-Rad). Protein levels were detected by density and were normalized to GAPDH for statistical comparison.
Statistical analysis
All values were expressed as mean±SD. Statistical signif i cance of differences among the groups was determined by Kruskal-Wallis test for histopathologic scores and factorial design ANOVA test for other continuous variables. Statistical analysis was carried out by SPSS16.0 statistical software. AP<0.05 was considered to be statistically signif i cant.
Results
Animals
There was no mortality in SO group; 18 (90%) rats survived in EM group; 17 (85%) in ICE-I group and 12 (60%) in SAP group which was signif i cantly lower than that in the SO group (P<0.05).
Histological changes and pathological score of pancreas and intestinal mucosa under light microscopy
The pancreas showed normal morphology in SO rats (Fig. 1A). The SAP group had remarkable damaged on pancreas, which were characterized by prominent edema, extensive neutrophil inf i ltration, acinar cell necrosis and saponif i cation spot (Fig. 1B), with high pathological score and statistically signif i cant difference compared with the SO group (P<0.05, Fig. 2). For the EM and ICE-I groups, pancreas showed slight edema, diffuse inf l ammatory inf i ltration without obvious pancreatic necrosis and hemorrhage (Fig. 1 C&D), and their pathological severity scores were lower than that in the SAP group (P<0.05, Fig. 2).
Intestine mucosa was severely damaged with visible hyperemia and edema, inf i ltration of neutrophil cells and bleeding in the intestinal lacunae in the SAP group (Fig. 1F) compared with that in the SO group (Fig. 1E). The EM and ICE-I groups showed milder damage with less inf l ammatory inf i ltration and microvilli exfoliation(Fig. 1 G&H). The mean pathological score of intestinal mucosa in the SAP group was signif i cantly higher than that in the SO (P<0.05, Fig. 2), EM and ICE-I groups (P<0.05, Fig. 2). However, there was no signif i cant difference between the EM and ICE-I groups (P>0.05, Fig. 2).
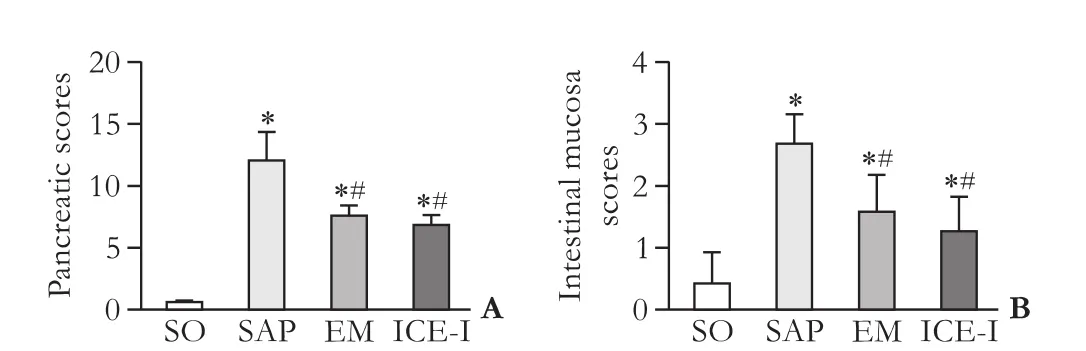
Fig. 2. Pathological score of pancreas (A) and intestinal mucosa (B) in different groups. *:P<0.05, compared with the SO group; #:P<0.05, compared with the SAP group.
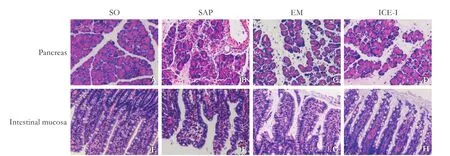
Fig. 1. Histological changes of pancreatic and intestinal tissues from rats at 24 hours after SAP induction (HE staining, original magnif i cation ×200). The SAP group had remarkable edema, neutrophil inf i ltration, necrosis and saponif i cation spot on pancreas mucosa and severely hyperemia and edema on intestine mucosa. The histological changes are milder in the EM and ICE-I groups.
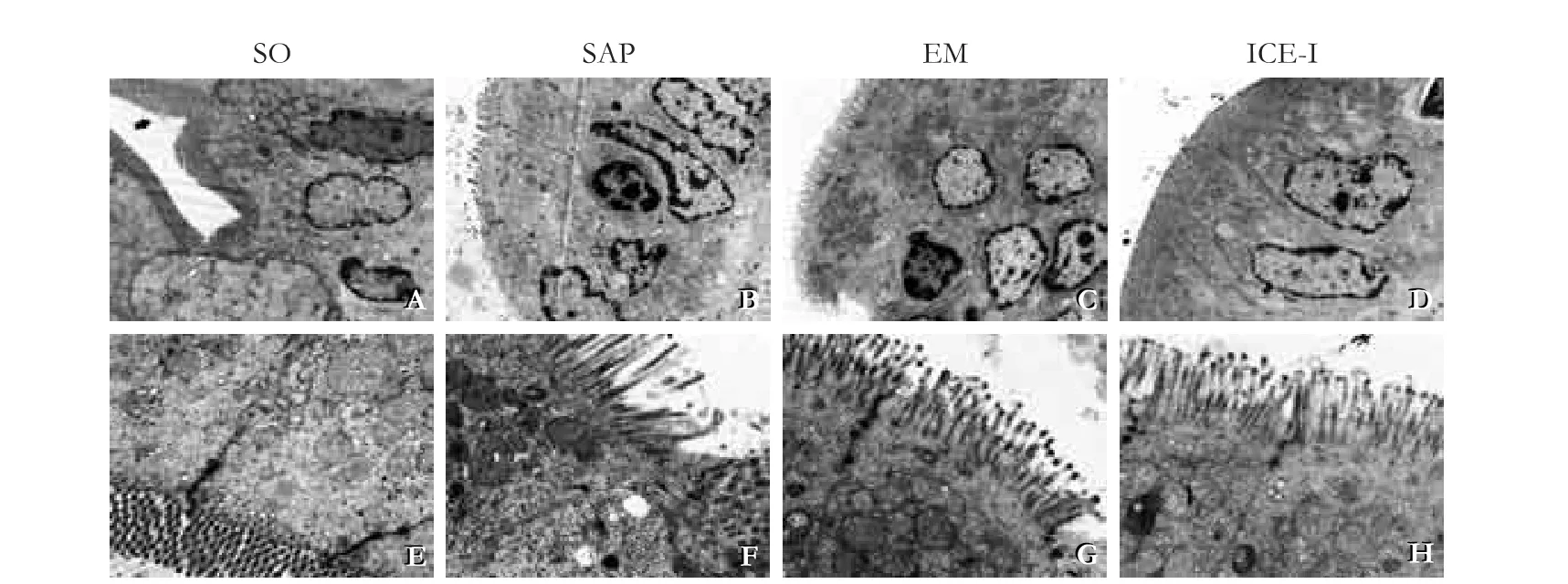
Fig. 3. Changes in the ultrastructure of rat terminal ileum (TEM, A-D, ×3700; E-H, ×12500). The SAP group showed sparse and atrophic epithelial microvilli, condensation and margination of nuclear chromatin, swollen crista mitochondria and endoplasmic reticulum, dissolved organelle, and obscured cellular tight junctions. The changes are milder in the EM and ICE-I groups.
Ultrastructure changes under TEM
In the SO group, the terminal ileum showed arranged microvilli, clear cellular junction, integrated structure of the nuclei and cellular organelles (Fig. 3 A&E). In the SAP group, the sparse and atrophic epithelial microvilli grovelled in the basement membrane. Obscured cellular tight junctions, dissolved organelle, swollen crista mitochondria and endoplasmic reticulum, evidenced nuclear chromatin condensation and margination were observed (Fig. 3 B&F). Both in the EM and ICE-I groups, microvilli structure was relatively clear and still normal, the mitochondrial crista were mild swollen, and the nuclear chromatin was less condensation and margination compared with that of the SAP group (Fig. 3 C&D; G&H).
Comparison of serum IL-1β and IL-18 concentrations
As shown in Fig. 4, the serum concentrations of IL-1β and IL-18 were signif i cantly increased in the SAP group compared to those in the SO group (P<0.01). The levels of IL-1β and IL-18 in both the EM and ICE-I groups were decreased signif i cantly compared to those in the SAP group (P<0.05). There was no signif i cant difference between the EM and ICE-I groups (P>0.05).
Up-regulation of caspase-1 mRNA and protein expressions in rats with SAP
The expressions of caspase-1 mRNA and protein showed a signif i cant increase in the SAP group compared to those in the SO group (P<0.05) and the EM and ICE-I groups (P<0.05) (Fig. 5&6). However, there was no signif i cant difference between the EM and ICE-I groups (P>0.05).
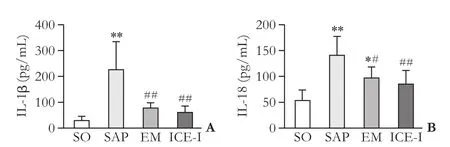
Fig. 4. Serum levels of IL-1β and IL-18 in different groups. *:P<0.05, **:P<0.01, compare with the SO group; #:P<0.05, ##:P<0.01, compare with the SAP group.
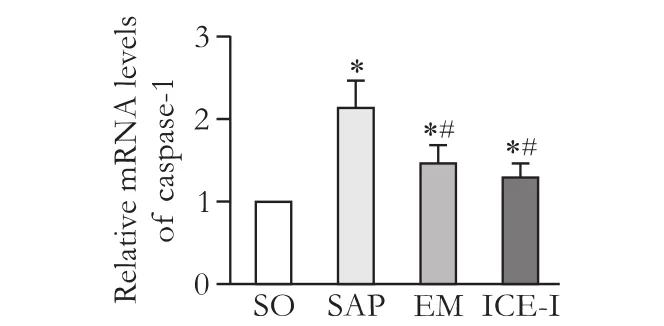
Fig. 5. mRNA expression levels of caspase-1 in ileum tissues of different groups. *:P<0.05, compared with SO group; #:P<0.05, compared with the SAP group.
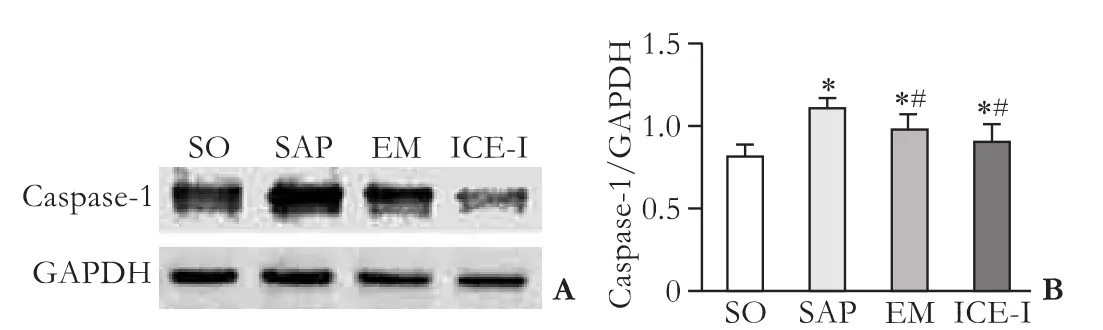
Fig. 6. Changes in protein expression levels of caspase-1 in ileum tissues of different groups. *:P<0.05, compared with the SO group; #:P<0.05, compared with the SAP group.
Discussion
The intestinal epithelium barrier protects the gut from the ingested pathogens and maintains the stability of internal environment. It is widely believed that the in-jury of intestinal mucosa barrier occurs in one-third of patients at an early stage of SAP.[15-17]Excessive release of in fl ammatory cytokines has a pivotal role in the pathogenesis of the barrier dysfunction,[18]which may provoke translocation of enterobacteria,[19,20]increase intestinal permeability,[21]disturb microcirculation,[22,23]and furthermore, result in apoptosis[24]of intestinal mucosal cells. Therefore, suppression of the in fl ammatory cytokines that cause gut barrier dysfunction in SAP may improve gut barrier function and the prognosis of patients with SAP.[25,26]
IL-1β is a central downstream effector of different inlf ammatory bowel diseases.[27]Similar to IL-1β, IL-18 is synthesized as an inactivate precursor and processed by caspase-1 into an active type. The pro-inf l ammatory cytokines play a major role in inf l ammation and immune responses.[28]Research showed that IL-1β and IL-18 play the pivotal role in the pathogenesis of intestinal mucosa injury.[29]In addition, IL-1β and IL-18 blocking agents ameliorate acute intestinal injury and inf l ammation and endotoxemia. Norman et al[30]found that IL-1β is required for evoking acute pancreatitis in IL-1 receptor gene knockout mice. A cohort study proposed a high correlation between IL-18 levels and pancreatic necrosis and systemic complications.[31]Our study showed that the levels of IL-1β and IL-18 were higher in the SAP group compared with those in the SO, EM and ICE-I groups. This phenomenon was in accordance with those of previous studies. It is indicated that IL-1β and IL-18 play a pivotal role in the pathogenesis of intestinal mucosa injury. A previous study[32]showed that caspase-1 inhibition inactivates pro-inf l ammatory cytokines (IL-1β and IL-18) and indirectly regulates cell apoptosis and inhibits inf l ammatory cell inf i ltration. In our study, caspase-1 inhibition signif i cantly down-regulated the serum levels of IL-1β and IL-18 and improved pancreatic and intestinal mucosa damage in the SAP rats. These fi ndings provided evidence again that caspase-1 signaling pathway plays an important role in the procession of SAP and treatment with caspase-1 inhibition signif i cantly alleviates the inf l ammation of the pancreas and intestinal mucosa in rats with SAP.
Emodin, a Chinese traditional medicine, has been validated clinically as an important therapy for SAP.[12,33,34]However, the underling mechanism is still unclear. Yao et al[34]proposed that emodin has protective effect on SAP animals via the inhibition of nuclear factor-κB. In our study, emodin treated rats showed signif i cantly decreased serum levels of IL-1β and IL-18, and down-regulated intestinal caspase-1 mRNA and protein expressions. The EM group also showed less pancreatic and intestinal mucosa damage, renovated cellular junctions and receded swelling level of mitochondria and endoplasmic reticulum. The administrations of emodin and caspase-1 inhibition showed similar therapeutic effects on SAP rats. Combining with the previous studies, we proposed that the therapeutic effects of emodin may be via the inhibition of caspase-1 expression and subsequently decreasing the release of pro-inf l ammatory cytokines such as IL-1β and IL-18, thus alleviating the pancreatic and intestinal mucosa damage. However, further studies are needed to reveal the molecular mechanism between emodin and caspase-1.
In conclusions, our study suggested that emodin signif i cantly ameliorated intestinal mucosa injury, alleviated inf l ammatory reaction, improved the intestinal immunity and maintained the stability of internal environment. These effects may be partly via inhibiting caspase-1 and its downstream inf l ammatory cytokines. Our data may be applicable in clinical practice.
Acknowledgement: We thank Dr. Da Yu for the help of rat model building.
Contributors: JF proposed the study. NJW, ZY, YMS and GML performed the research and wrote the fi rst draft. XJ and UA collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. JF is the guarantor.
Funding: This study was supported by grants from Zhejiang Province Traditional Chinese Medicine Scientif i c Research Fund (2011-ky1-001-164 and 2016ZB066), and Public Welfare Projects of Ministry of Science of Zhejiang Province (20130101120016).
Ethical approval: This study was approved by the Ethics Committee of the First Aff i liated Hospital of Zhejiang University School of Medicine (2013-0022).
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Buddingh KT, Koudstaal LG, van Santvoort HC, Besselink MG, Timmer R, Rosman C, et al. Early angiopoietin-2 levels after onset predict the advent of severe pancreatitis, multiple organ failure, and infectious complications in patients with acute pancreatitis. J Am Coll Surg 2014;218:26-32.
2 Vaz J, Akbarshahi H, Andersson R. Controversial role of tolllike receptors in acute pancreatitis. World J Gastroenterol 2013;19:616-630.
3 Kiss L, Sarbu G, Bereanu A, Kiss R. Surgical strategies in severe acute pancreatitis (SAP): indications, complications and surgical approaches. Chirurgia (Bucur) 2014;109:774-782.
4 Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol 2012;46:S12-17.
5 Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inf l ammation, autoimmunity, and cancer. Physiol Rev 2011;91:151-175.
6 Zhong Y, Cai D, Cai W, Geng S, Chen L, Han T. Protective ef-fect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin Nutr 2009;28:575-580.
7 Zhang XH, Li ML, Wang B, Guo MX, Zhu RM. Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J Gastroenterol 2014;20:10457-10463.
8 Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg 2002;235:68-76.
9 Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol 2007;13:623-627.
10 Giamarellos-Bourboulis EJ, van de Veerdonk FL, Mouktaroudi M, Raftogiannis M, Antonopoulou A, Joosten LA, et al. Inhibition of caspase-1 activation in Gram-negative sepsis and experimental endotoxemia. Crit Care 2011;15:R27.
11 Wang G, Sun B, Zhu H, Gao Y, Li X, Xue D, et al. Protective effects of emodin combined with danshensu on experimental severe acute pancreatitis. Inf l amm Res 2010;59:479-488.
12 Wu L, Cai B, Zheng S, Liu X, Cai H, Li H. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inf l ammation 2013;36:1020-1029.
13 Zhang XP, Jiang J, Yu YP, Cheng QH, Chen B. Effect of Danshen on apoptosis and NF-κB protein expression of the intestinal mucosa of rats with severe acute pancreatitis or obstructive jaundice. Hepatobiliary Pancreat Dis Int 2010;9:537-546.
14 Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-428.
15 Zhang JX, Dang SC, Yin K, Jiang DL. Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int 2011;10:544-551.
16 Ji L, Lv JC, Song ZF, Jiang MT, Li L, Sun B. Risk factors of infected pancreatic necrosis secondary to severe acute pancreatitis. Hepatobiliary Pancreat Dis Int 2016;15:428-433.
17 Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci 2013;17:349-355.
18 Li J, Luo Y, Li Z, Liu Y, Liu Z. Effects of erythropoietin pretreatment on pro-and anti-inf l ammatory balance in rats with severe acute pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao 2012;32:93-96.
19 Huang J, Qu HP, Zheng YF, Song XW, Li L, Xu ZW, et al. The revised Atlanta criteria 2012 altered the classif i cation, severity assessment and management of acute pancreatitis. Hepatobiliary Pancreat Dis Int 2016;15:310-315.
20 Sakorafas GH, Sampanis D, Lappas C, Kokoropoulos P, Mastoraki A, Smyrniotis V. Necrotizing acute pancreatitis current status - emerging new strategies in surgical management. Infect Disord Drug Targets 2012;12:138-143.
21 Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, et al. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery 2009;145:157-167.
22 Sha H, Ma Q, Jha RK, Wu Z, Qingyuan Z, Wang Z, et al. Resveratrol suppresses microcirculatory disturbance in a rat model of severe acute pancreatitis. Cell Biochem Biophys 2013;67:1059-1065.
23 Chen P, Hu B, Tan Q, Liu L, Li D, Jiang C, et al. Role of neurocrine somatostatin on sphincter of Oddi contractility and intestinal ischemia reperfusion-induced acute pancreatitis in macaques. Neurogastroenterol Motil 2010;22:935-941, e240.
24 Bai X, Song Z, Zhou Y, Pan S, Wang F, Guo Z, et al. The apoptosis of peripheral blood lymphocytes promoted by hyperbaric oxygen treatment contributes to attenuate the severity of early stage acute pancreatitis in rats. Apoptosis 2014;19:58-75.
25 Wang YL, Zheng YJ, Zhang ZP, Su JY, Lei RQ, Tang YQ, et al. Effects of gut barrier dysfunction and NF-kappaB activation on aggravating mechanism of severe acute pancreatitis. J Dig Dis 2009;10:30-40.
26 Dang S, Shen Y, Yin K, Zhang J. TREM-1 promotes pancreatitis-associated intestinal barrier dysfunction. Gastroenterol Res Pract 2012;2012:720865.
27 Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, et al. IL-1β mediates chronic intestinal inf l ammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012;209:1595-1609.
28 Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol 2013;4:289.
29 Shaker ME, Ashamallah SA, Houssen ME. Celastrol ameliorates murine colitis via modulating oxidative stress, inf l ammatory cytokines and intestinal homeostasis. Chem Biol Interact 2014;210:26-33.
30 Norman JG, Fink GW, Sexton C, Carter G. Transgenic animals demonstrate a role for the IL-1 receptor in regulating IL-1beta gene expression at steady-state and during the systemic stress induced by acute pancreatitis. J Surg Res 1996;63:231-236.
31 Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg 2002;9:401-410.
32 Lipinska K, Malone KE, Moerland M, Kluft C. Applying caspase-1 inhibitors for inf l ammasome assays in human whole blood. J Immunol Methods 2014;411:66-69.
33 Wang GJ, Wang Y, Teng YS, Sun FL, Xiang H, Liu JJ, et al. Protective effects of emodin-induced neutrophil apoptosis via the Ca2+-Caspase 12 pathway against SIRS in rats with severe acute pancreatitis. Biomed Res Int 2016;2016:1736024.
34 Yao WY, Zhou YF, Qian AH, Zhang YP, Qiao MM, Zhai ZK, et al. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factor-κB activation resulting in antioxidation. Mol Med Rep 2015;11:1416-1420.
December 3, 2016
Accepted after revision June 28, 2017
Author Aff i liations: Division of Emergency (Ning JW and Xu J) and Division of Gastroenterology (Yu MS, Gu ML, Usman A and Ji F), First Aff i liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China; Division of Gastroenterology, Yixing People’s Hospital, Yixing 214200, China (Zhang Y)
Feng Ji, MD, Division of Gastroenterology, First Aff i liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: +86-571-87236522; Fax: +86-571-87236863; Email:jifeng@zju.edu.cn)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60041-9
Published online July 13, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- The International Study Group of Pancreatic Surgery def i nition of delayed gastric emptying and the effects of various surgical modif i cations on the occurrence of delayed gastric emptying after pancreatoduodenectomy
- Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Effects of multimodal fast-track surgery on liver transplantation outcomes
