Remote ischemic preconditioning protects liver ischemia-reperfusion injury by regulating eNOS-NO pathway and liver microRNA expressions in fatty liver rats
2017-08-16YunFeiDuanYongAnFengZhuandYongJiang
Yun-Fei Duan, Yong An, Feng Zhu and Yong Jiang
Changzhou, China
Remote ischemic preconditioning protects liver ischemia-reperfusion injury by regulating eNOS-NO pathway and liver microRNA expressions in fatty liver rats
Yun-Fei Duan, Yong An, Feng Zhu and Yong Jiang
Changzhou, China
BACKGROUND:Ischemic preconditioning (IPC) is a strategy to reduce ischemia-reperfusion (I/R) injury. The protective effect of remote ischemic preconditioning (RIPC) on liver I/R injury is not clear. This study aimed to investigate the roles of RIPC in liver I/R in fatty liver rats and the involvement of endothelial nitric oxide synthase-nitric oxide (eNOS-NO) pathway and microRNA expressions in this process.
METHODS:A total of 32 fatty rats were randomly divided into the sham group, I/R group, RIPC group and RIPC+I/R group. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and nitric oxide (NO) were measured. Hematoxylin-eosin staining was used to observe histological changes of liver tissues, TUNEL to detect hepatocyte apoptosis, and immunohistochemistry assay to detect heat shock protein 70 (HSP70) expression. Western blotting was used to detect liver inducible NOS (iNOS) and eNOS protein levels and realtime quantitative polymerase chain reaction to detect miR-34a, miR-122 and miR-27b expressions.
RESULTS:Compared with the sham and RIPC groups, serum ALT, AST and iNOS in liver tissue were signi fi cantly higher in other two groups, while serum NO and eNOS in liver tissue were lower, and varying degrees of edema, degeneration and infl ammatory cell in fi ltration were found. Cell apoptosis number was slightly lower in the RIPC+I/R group than that in I/R group. Compared with the sham group, HSP70 expressions were signi fi cantly increased in other three groups (allP<0.05). Compared with the sham and RIPC groups, elevated miR-34a expressions were found in I/R and RIPC+I/R groups (P<0.05). MiR-122 and miR-27b were found signif i cantly decreased in I/R and RIPC+I/R groups compared with the sham and RIPC groups (allP<0.05).
CONCLUSION:RIPC can reduce fatty liver I/R injury by affecting the eNOS-NO pathway and liver microRNA expressions.
(Hepatobiliary Pancreat Dis Int 2017;16:387-394)
fatty liver; ischemia-reperfusion; remote ischemic preconditioning; nitric oxide; heat shock protein 70; endothelial nitric oxide synthase; inducible nitric oxide synthase; liver microRNA
Introduction
Fatty liver disease was primarily caused by obesity and alcohol consumption and might increase the risk of progressive liver injury, such as nonalcoholic steatohepatitis, fi brosis and cirrhosis.[1]Ischemia-reperfusion (I/R) injury is an essential cause of liver damage occurring during hepatic resections or liver transplantation, and is an important reason for post-operative liver dysfunction.[2]Hepatic steatosis contributes to liver damage because fatty livers vulnerable to I/R injury.[3]Fatty inf i ltration can exacerbate mitochondrial oxidative injury and reduce tolerance to I/R injury.[4]
Ischemic preconditioning (IPC) is a strategy to reduce I/R injury, which submits tissues to controlled periods of ischemia and reperfusion prior to the longer periods of I/R injury and is initially proven to be benef i cial when applied to a different tissue (remote).[5,6]Remote ischemic preconditioning (RIPC) has recently emerged and been investigated mainly on myocardium, and has alsobeen shown to have some protection against liver I/R injury.[7]Circulating mediators and/or signals mediate the effect of RIPC on liver I/R injury by modulating oxidative stress, inf l ammatory cells, and cytokines.[6]Heat shock protein 70 (HSP70) is highly expressed in cells under a large variety of stress and plays a pivotal role in maintaining and repairing cellular homeostasis under I/R stress.[8]Overexpression of HSP70 could protect the brain from ischemic damage and thus HSP70 expression could be a potential marker for the treatment of I/R.[9]
IPC can increase the content of nitric oxide (NO) and attenuate the I/R induced inf l ammation, and can protect liver I/R injury via Akt-eNOS-NO-HIF pathway.[10]Endothelial nitric oxide synthase-nitric oxide (eNOS-NO) pathway is important in the pathogenesis of myocardial I/R injury, and IPC, via activating eNOS, increases NO which protects cells from the insults.[11]
MicroRNA (miR)-34a regulates the expression of sirtuin 1 which protects heart and brain from I/R injury, and ameliorates inf l ammation and apoptosis through NF-κB/p65 and p53 deacetylation in primary hepatocytes.[12]MiR-122 is a potent marker of hepatocyte apoptosis and liver injury.[13]MiR-27b overexpression regulated by transforming growth factor-β involves in the cardiac hypertrophy and dysfunction and thus miR-27b can be a therapeutic target for heart diseases.[14]
The present study aimed to investigate the roles of RIPC in liver I/R of fatty liver rats and the effects of RIPC on eNOS-NO pathway and microRNA expressions.
Methods
Fatty liver rat model
Specif i c-pathogen free male Sprague-Dawley rats (n=32; weighted 190-210 g) were purchased from Changzhou CAVENS Laboratory Animals, Co., Ltd. All the animal experiments were approved by the Ethics Committee of the Third Aff i liated Hospital of Soochow University. A stable fatty liver rat model was established according to Kim et al’s method.[15]Brief l y, animals were fed under a temperature-controlled environment with a 12-hour diurnal cycle and had free access to a high fat feed (basic feed 82.3%, lard 10%, sucrose 5%, cholesterol 2%, sodium cholate 0.5%, and propylthiouracil 0.2%; provided by the Soochow University Experimental Animal Center) for 5 weeks. The fatty liver model was successfully established at the end of 5 weeks.
Animal grouping and I/R treatment
The 32 fatty liver rats were divided into four groups. Animals fasted for 12-hour preoperatively and had free access to water, were anaesthetized with 2% sodium pentobarbital (0.3 mL/100 g, Merck, Germany) by intraperitoneally injection. Sham group: rats were subjected to only laparotomy and liver fl apping; RIPC group: the hind legs were tied tightly by a tourniquet until the color of the hind legs turned purple and cannot touch the femoral pulse. Blood fl ow was blocked for 5 minutes, and then was restored after the tourniquet was released for 5 minutes, which repeated six times, followed by reperfusion of the hind legs for 160 minutes; I/R group: an electric blanket was used to keep the body temperature at 37.0±0.5 ℃. After laparotomy incision, the periphery liver ligament was isolated, and a microvascular clip was used to block the blood supply of left and central hepatic lobes for 40 minutes to cause ischemia, resulting in approximately 70% hepatic ischemia, and liver reperfusion was conducted for 2 hours; RIPC+I/R group: rats were subjected to I/R treatment immediately after RIPC, and the reperfusion was the same as I/R rats.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measurement
The rats were cultured for one week after successful modeling, and blood (2 mL) was taken by puncture from hepatic inferior cava vena to nearly hepatic vein, stewed at 4 ℃ for 1 hour, followed by centrifugation at 3000 r/min for 10 minutes. After that, the serum was collected, preserved in a -20 ℃ refrigerator, and measured within one month. HITACHI 7600-100 automatic biochemical analyzer was used to measure serum ALT and AST contents.
Serum NO content measured by nitrate reductase
Serum was collected, the standards and samples were prepared at 85 μL/well according to the manual operating procedures of NO detection kit (Biovision, California, Paroo, USA). Nitrate reductase mixture (5 μL) and enzyme cofactors (5 μL) were added, and the whole system was incubated at room temperature for 1 hour to enable nitrate in each well to be converted to nitrite; enhancers (5 μL) were added into each well, and the whole system was incubated for 10 minutes; Griess Reagent R1 (50 μL) and Griess Reagent R2 (50 μL) were added into each well, and the whole system was colored at room temperature for 10 minutes; and the absorbance at 540 nm was detected within 1 hour using a microplate. The formula was as follows: NO content (μmol/L)=(ODsample-ODblank) × standard concentration (100 μmol/L)×dilution factor of samples before test/ (ODstandard-ODblank).
Rat liver histology
The rats were sacrif i ced after the establishment of model and observation for one week. Liver tissues were rapidly removed, fi xed with 10% formalin, dehydratedconventionally, embedded, sliced, dewaxed, stained with hematoxylin-eosin (HE) (Solarbio, Beijing, China), clariif ed, sealed and observed under an optical microscope.
Rat hepatocyte apoptosis detected by TUNEL assay
Liver tissues were subjected to paraff i n sections and sequentially immersed in xylene I, xylene II, 100% ethanol, 90% ethanol, 70% ethanol, and distilled water for 8 minutes×2, and then treated with DNase-free proteinase K (Merck) at 37 ℃ for 30 minutes, washed with phosphate buffered saline (PBS), incubated with 3% H2O2at room temperature for 10 minutes, and washed with PBS. Mixed TdT enzyme (Roche, Indianapolis, IN, USA) and Biotin-dUTP (Roche) was added in the samples, and the mixture was incubated at 37 ℃ in dark for 60 minutes, and washed with PBS. The labeled reaction-terminated liquid was added, and the mixture was incubated at room temperature for 10 minutes and washed with PBS. Mixed streptavidin-HRP enzyme (10 μL, Roche) and Biotin-dUTP (490 μL) were added into the samples, and the mixture was incubated at 37 ℃ in dark for 60 minutes, and washed with PBS. The mixture was colored with diaminobenzidine (DAB), re-stained with HE staining solution (Solarbio), washed with PBS, dehydrated, sealed with neutral resin, and photographed. Five random fi elds (×400 magnif i cation) were selected from each slice, the average number of apoptotic cells per 100 cells were calculated, and the apoptotic index was expressed as a percentage.[16]Apoptotic cell morphology was consistent with that single cells presented shrinkage of cell membranes, no inf l ammation around, and dense nuclei presenting deep stained brown particles or debris.
HSP70 in rat liver tissue detected by immunohistochemistry
Strept avidin-biotin complex (SABC) method was used for immunohistochemical staining. All specimens were fi xed in 10% neutral formalin, dehydrated conventially, embedded in paraff i n, and sliced into 4 μm thick slices. The sliced specimens were dewaxed, hydrated by graded alcohol, antigen was retrieved by microwave and endogenous peroxidase was blocked using 3% hydrogen peroxide. Primary antibody (rabbit anti-mouse HSP70, 1:500 dilution) (R&D Systems, Minneapolis, MN, USA) was added and the slices were put into a -4 ℃ refrigerator overnight. The slices were washed with PBS for 5 minutes×3, biotinylated goat anti-rabbit secondary antibody (Santa Cruz, Carlsbad, CA, USA) was added, and the slices were incubated at room temperature for 30 minutes. The slices were washed with PBS for 5 minutes ×3 and SABC reagent (Solarbio) was added to conduct reaction at room temperature for 30 minutes. The slices were washed with PBS for 10 minutes×3, stained with DAB (Sigma, Englewood Cliffs, NJ, USA), re-stained with HE, sealed, and photographed. HSP70 positive expressions were referred as cells stained with brown particles. Positive cell percentage and staining intensity were combined to conduct scoring:[17]A, the percentage of positive cells (0: no positive cells; 1: 1%-10% of positive cells; 2:11%-50% of positive cells; 3: 51%-80% of positive cells; and 4: 81%-100% of positive cells); and B, staining intensity of positive cells (0: negative; 1: weak positive; 2: moderate positive; and 3: strong positive); and the fi nal scores were obtained by multiplying the indexes of A and B.
Inducible NOS (iNOS) and eNOS levels detected by Western blotting
Liver tissues stored in liquid nitrogen were taken out and crushed by a mortar, and radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China) was used to extract protein, and the proteins were quantif i ed by Coomassie blue method. The samples were heated at 100 ℃ for 5 minutes, and 40 μg of samples were subjected to electrophoresis. Proteins were separated by 10% Tris-glycine gel (Solarbio), transferred to a nitrocellulose membrane, sealed with 5% skim milk for 2 hours, and washed with 1×Tris-buffered saline and tween 20 (TBST). Primary antibodies (rabbit anti-mouse iNOS; rabbit anti-mouse eNOS; 1:500 dilution; Biovision) were added and reacted at 4 ℃ overnight, β-actin mouse monoclonal antibody was used as an internal reference, and the samples were washed with 1×TBST. Peroxidaselabeled goat anti-rabbit secondary antibody (1:2000; Santa Cruz) was added and the whole system was incubated at room temperature for 1 hour, and washed with TBST, and incubated with chemiluminescence reagent (Thermo, Cleveland, OH, USA) for 1 minute, and developed by electro-generated chemiluminescence.
MiRNA expression in liver tissue detected by real-time quantitative polymerase chain reaction (qPCR)
MiR-34a, miR-122 and miR-27b were measured by qPCR. Liver tissues stored in liquid nitrogen were crushed by a mortar, and Trizol (Invitrogen, USA) was used to extract total RNA. The reverse transcription was performed according to a kit instruction (Fermentas, Richmond, VA, USA) and the reaction conditions were as follows: 70 ℃ for 10 minutes, ice bath for 2 minutes, 42 ℃ for 60 minutes, and 70 ℃ for 10 minutes. The cDNA was amplif i ed by qPCR using TaqMan probe method and was conducted according to a kit instruction (Fermentas); the primer sequences were shown in Table 1. qPCR instrument (Bio-Rad iQ5, Bio-Rad, Her-cules, CA, USA) platform was used for measurement and the PCR conditions were as follows: 94 ℃ for 5 minutes, and 40 cycles of 94 ℃ denaturation for 15 seconds, 55 ℃annealing for 30 seconds, and 72 ℃ extension for 1 minute. U6 was used as internal control. Relative quantitation is the 2-ΔΔCtmethod. Each experiment was repeated for three times.

Table 1. Primer sequences of miR-34a, miR-122 and miR-27b
Statistical analysis
Data were expressed as mean±standard deviation. SPSS20.0 (IBM Corporation, New York, NY, USA) was used for data analysis. Comparisons among groups were analyzed by using analysis of variance (ANOVA), and comparisons between groups were analyzed by Student’sttest. AP<0.05 indicated signif i cant difference.
Result
Analysis of fatty liver model
In morphology, the fatty liver presented light yellow color, was smooth in surface and soft in texture, and had no nodules or tumor-like changes (Fig. 1A). Histopathological tissues were stained with HE and observed under a light microscope: rat fatty liver presented steatosis which was obvious around leaf l ets, the hepatocytes presented enlarged round shape, and the cytoplasm was full of fat empty bubble of varying sizes and showed loose-like changes (Fig. 1B), indicating the successful establishment of fatty liver model. There was no mortality.
Serum ALT, AST and NO levels
Serum ALT and AST levels were signif i cantly increased and NO levels were signif i cantly decreased in the RIPC+I/R group and the I/R group compared with the sham group and the RIPC group (allP<0.05). There were no signif i cant differences between the sham group and the RIPC group (P>0.05). Serum ALT and AST levels were signif i cantly lower while NO levels were higher in the RIPC+I/R group than those in the I/R group (allP<0.05) (Table 2).
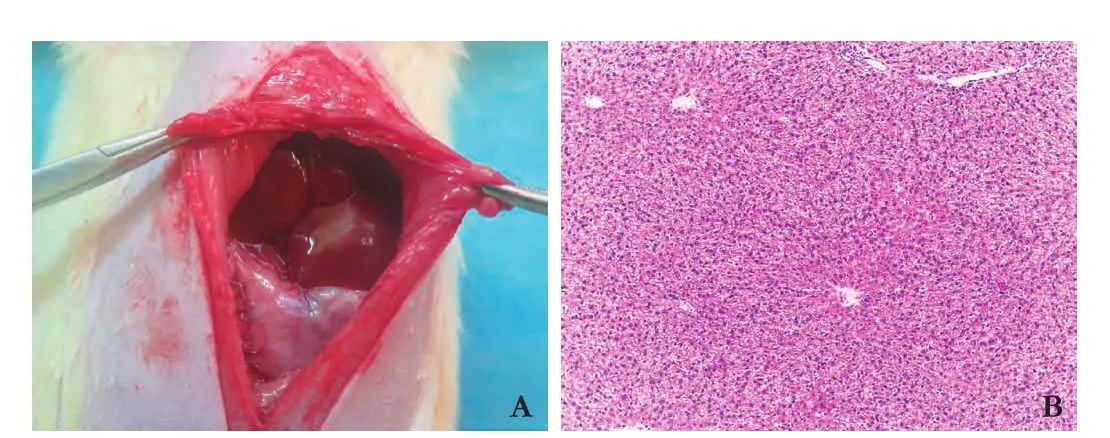
Fig. 1. Liver pathology observation of rat fatty liver model. A: rat fatty liver presented light yellow and red color, grainy surface, blunt edges, soft texture, no nodular or focal changes and normal liver volume size; B: tissue sections (HE, original magnif i cation × 400): rat fatty liver presented steatosis which was obvious around leaf l ets, the hepatocytes presented enlarged round shape, and the cytoplasm was full of fat empty bubble of varying sizes and showed loose-like changes.
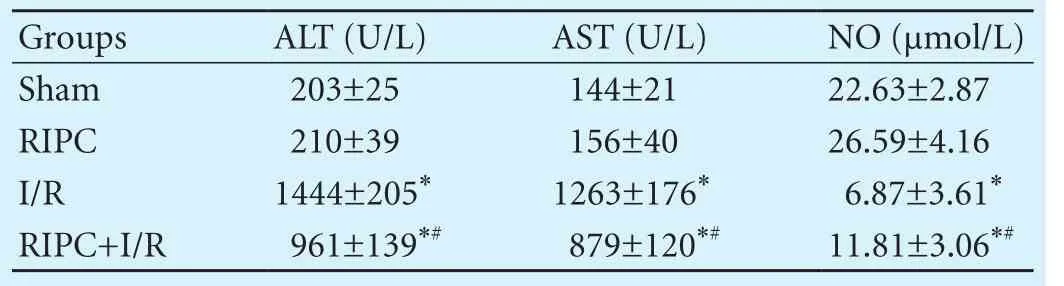
Table 2. Serum ALT, AST and NO levels (n=8)
Liver histology
Hepatocyte steatosis was found in the sham group and the RIPC group; hepatocyte steatosis, hepatic lobular disorder, severe swelling cells, and hepatocyte degeneration and necrosis accompanied by massive neutrophil inf i ltration were found in the I/R group; hepatocyte steatosis in hepatic lobules, less disorder, mild swelling cells, and a small amount of neutrophil inf i ltration and liver cell necrosis were found in the RIPC+I/R group. Compared with the sham group and the RIPC group, the pathological changes were severer in the RIPC+I/R group and the I/R group, while the pathological changes in the RIPC+I/R group were milder than those in the I/R group (Fig. 2).
Hepatocyte apoptosis
TUNEL was used to detect hepatocyte apoptosis, and the result showed that the apoptosis index was 4.25%± 0.42% in the sham group, 4.07%±0.28% in the RIPC group, 41.43%±1.73% in the I/R group and 35.30% ± 1.62% in the RIPC+I/R group (Fig. 3,P<0.05). The number of apoptotic cells was signif i cantly less in the RIPC+I/ R group than that in the I/R group (P<0.05). There was no signif i cant difference between the sham group and the RIPC group (P>0.05).
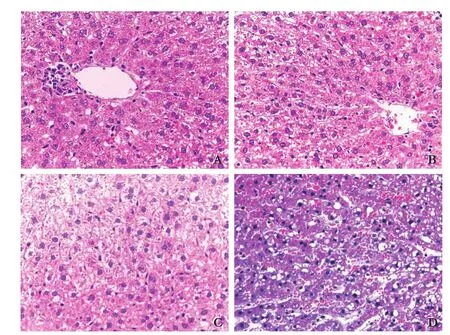
Fig. 2. Rat liver histopathology changes (HE, original magnification ×400). A: sham group; B: RIPC group; C: I/R group; D:RIPC+I/R group.
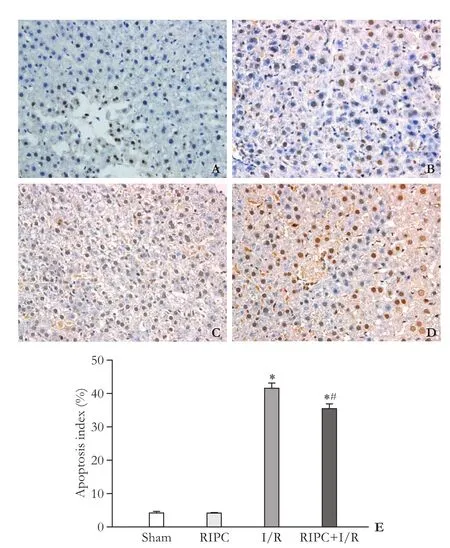
Fig. 3. TUNEL assay of rat hepatocyte apoptosis (Original magnification ×400). A: sham group; B: RIPC group; C: I/R group; D: RIPC+I/R group; E: apoptotic index histogram of each group. *:P<0.05, compared with the sham group; #:P<0.05, compared with the I/R group.
HSP70 expression in rat liver tissue
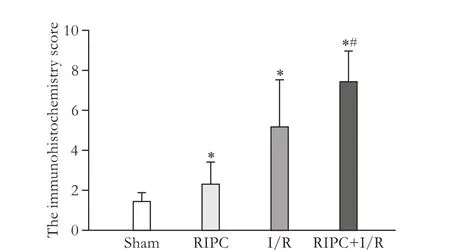
Fig. 4. Immunohistochemistry score of HSP70 in rat liver tissue. *:P<0.05, compared with the sham group; #:P<0.05, compared with the I/R group.
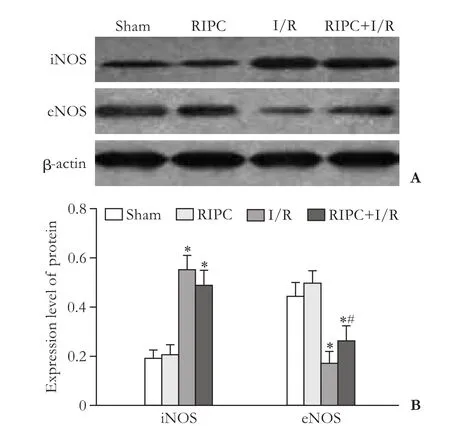
Fig. 5. iNOS and eNOS expressions in rat liver tissue. *:P<0.05, compared with the sham group; #:P<0.05, compared with the I/R group.
HSP70 occasionally showed weak positive expressions in the sham group, weak to moderate positive expressions in the RIPC group with the ratio of positive cells less than 50%, mostly moderate positive expressions in the I/R group with the ratio of positive cells greater than 50%, while showed moderate to strong positive expression in the RIPC+I/R group with the ratio of positive cells greater than 50%. Compared to the sham group, the immunohistochemistry scores were higher in the other three groups; while the immunohistochemistry score was signif i cantly higher in the RIPC+I/R group than that in the I/R group (P<0.05) (Fig. 4).
iNOS and eNOS expressions in rat liver tissue
Western blotting showed that iNOS levels were signif i cant higher, while the eNOS levels were signif icantly lower in the RIPC+I/R group and the I/R group than those in the sham group and the RIPC group (allP<0.05); and there was no statistical difference between the sham group and the RIPC group (bothP>0.05). eNOS level was signif i cantly higher in the RIPC+I/R group than that in the I/R group (P<0.05) (Fig. 5).
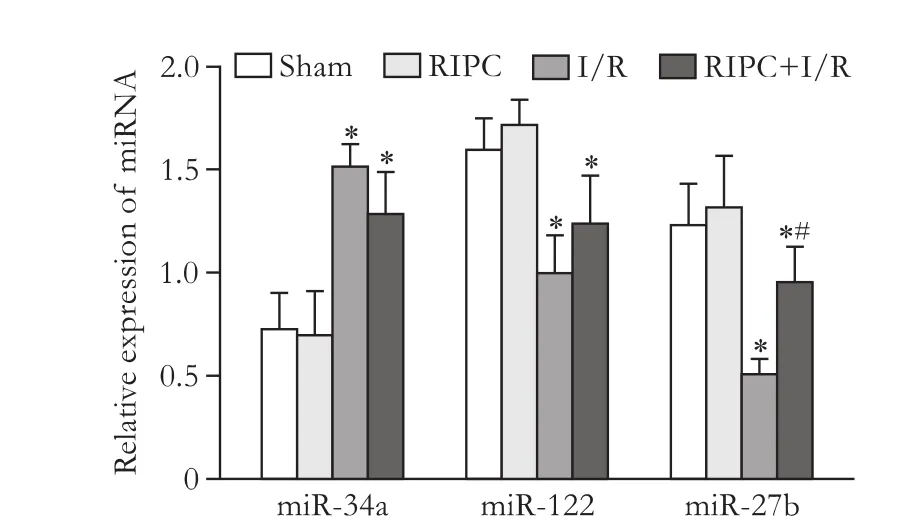
Fig. 6. MiR-34a, miR-122 and miR-27b expressions in rat liver tissue. *:P<0.05, compared with the sham group and RIPC group; #:P<0.05, compared with the I/R group.
MiR-34a, miR-122 and miR-27b expressions in rat liver tissue
Elevated miR-34a expressions were found in I/R group and RIPC+I/R group compared with the sham group and RIPC group (P<0.05). No such signif i cant difference was found in miR-34a expression between I/R group and RIPC+I/R group. MiR-122 and miR-27b were found signif i cantly decreased in I/R group and RIPC+I/R group compared with the sham group and RIPC group (Fig. 6,P<0.05).
Discussion
In the present study, we demonstrated that RIPC can reduce fatty liver I/R injury by regulating the eNOS-NO pathway and liver microRNA expressions. The pathological changes were severer in the I/R group, RIPC signi fi cantly attenuated I/R injury. Furthermore, RIPC mitigated I/R-induced cell apoptosis. I/R injury is deif ned as cellular damage secondary to oxygen deprivation which is aggravated by the sudden blood reperfusion.[18]RIPC involves a brief period of ischemia followed by reperfusion and can protect organs or tissues from ischemic injury.[19]RIPC has been reported to protect against I/R injury by inducing HO-1/p38-MAPK-dependent autophagy, a vital cell signaling pathway in cellular injury or stress.[20]RIPC could also alleviate patients with contrast-induced acute kidney injury by decreasing oxidative stress and plasma asymmetrical dimethy larginine level.[21]
One of our results showed that I/R signif i cantly increased serum ALT and AST levels and RIPC signif i cantly decreased these enzymes. Compared with the sham group, HSP70 expressions were signif i cantly increased in other three groups and RIPC could increase the HSP70 expression in the I/R group. The expression changes of ALT, AST and HSP70 indicated that RIPC could protect against I/R injury. Serum ALT and AST levels are used to predict the severity of liver disease and liver-related mortality.[22]HSP70 might function as a chaperone during periods of cellular stress, and could induce expression of several inf l ammatory cytokines which are key players during early liver regeneration, and might protect against the denaturing of folded intracellular proteins, formation of disordered protein aggregates, and compromised cell viability.[23]HSP70 plays a signif i cant role in protecting cells against various cellular stressors including heat, hypoxia, acidosis, ultraviolet irradiation, starvation, and oxidative stress; and in the absence of induction of HSPs expression, the cells may be subjected to apoptosis or necrosis when exposed to acute stressors.[24]
In order to investigate the mechanism of RIPC in protecting against I/R injury, we focused on pathway and miR studies. One important result showed that NO level was lower after I/R treatment, RIPC signif i cantly increased NO level in the following I/R procedure. iNOS was remarkably higher, while eNOS was signif i cantly lower in I/R treated rats. NO can reduce vascular crisis incidence by inf l uencing vascular permeability, regulating platelet function, inhibiting adhesion and aggregating leukocytes, and improving graft microcirculation.[25]NO could also alleviate the degree of damage and necrosis of hepatocytes, reduce the level of inf l ammatory mediators and decrease mortality, and also improve most liver function parameters.[26]In addition, NO could attenuate liver I/R injury by reducing hepatocyte apoptosis, oxidative stress and leukocyte adhesion, and increasing microcirculatory fl ow and enhancing mitochondrial function.[27,28]NO is synthesized by the following three constitutive NOS, including eNOS, neuronal NOS (nNOS), and iNOS.[29]iNOS is up-regulated during I/R injury, but not expressed in normal circumstances, while eNOS is expressed in liver endothelial cells, hepatocytes and other cells.[30]eNOS provides NO basal production to maintain normal vascular tone; and conversely iNOS provides a higher level of NO in the inf l ammatory process to promote I/R injury.[31,32]
The present study also showed that elevated miR-34a expressions were found in I/R group and RIPC+I/R group. MiR-122 and miR-27b were found signif i cantly decreased in I/R group and RIPC+I/R group compared with the sham and RIPC groups. Increased expression of miR-34a was involved in age-dependent loss of oxidative defense in the liver and presented in a partial hepatectomy model, resulting in inhibition of hepatocyte proliferation.[33]Increased expression of miR-34a was found byin situhybridization during liver regeneration and renal injury.[34]MiR-122, as a liver-specif i c microRNA, has recently shown potential for predicting liver injury as well as a liver injury biomarker for cholestasis-inducedliver injury.[35,36]MiR-27b is down-regulated in preconditioned astrocytes of ischemic brain through the NF-κB pathway which upregulates the expression of stromal cell-derived factor 1 and the increased stromal cell-cellderived factor 1 might be a possible mechanism underlying therapeutic application of IPC.[37]
Taken together, our study showed the mechanisms of RIPC on fatty liver I/R injury by regulating the expressions of iNOS and eNOS and the expressions of miR-34a, miR-122 and miR-27b injury related miRs. Thus RIPC might be a useful tool to reduce fatty liver I/R injury.
Contributors: DYF proposed the study. DYF and AY performed the research and wrote the fi rst draft. ZF and JY collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. DYF is the guarantor.
Funding: This study was supported by a grant from 2013 Applied Basic Research of Changzhou Bureau of Science and Technology (CJ20130044).
Ethical approval: This study was approved by the Ethics Committee of the Third Aff i liated Hospital of Soochow University.
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 2004;40:933-941.
2 Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol 2002;161:587-601.
3 Jiang Y, Tang JJ, Wu BQ, Yuan B, Qu Z. The protective effects of different-time-ischemic preconditioning on the reperfusion injury in fatty livers in rats. PLoS One 2013;8:e58086.
4 Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, et al. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res 2005;124:160-168.
5 Erling Junior N, Montero EF, Sannomiya P, Poli-de-Figueiredo LF. Local and remote ischemic preconditioning protect against intestinal ischemic/reperfusion injury after supraceliac aortic clamping. Clinics (Sao Paulo) 2013;68:1548-1554.
6 Camara-Lemarroy CR. Remote ischemic preconditioning as treatment for non-ischemic gastrointestinal disorders: beyond ischemia-reperfusion injury. World J Gastroenterol 2014;20:3572-3581.
7 Björnsson B, Winbladh A, Bojmar L, Sundqvist T, Gullstrand P, Sandström P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J Gastroenterol 2014;20:9506-9512.
8 Sun L, Fan H, Yang L, Shi L, Liu Y. Tyrosol prevents ischemia/ reperfusion-induced cardiac injury in H9c2 cells: involvement of ROS, Hsp70, JNK and ERK, and apoptosis. Molecules 2015;20:3758-3775.
9 Dai J, Chen L, Qiu YM, Li SQ, Xiong WH, Yin YH, et al. Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin’s neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull 2013;90:1-9.
10 Guo JY, Yang T, Sun XG, Zhou NY, Li FS, Long D, et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci 2011;18:79.
11 Qian GQ, Peng X, Cai C, Zhao GP. Effect on eNOS/NO pathway in MIRI rats with preconditioning of GFPC from Dang Gui Si Ni decoction. Pharmacognosy Res 2014;6:133-137.
12 Kim HJ, Joe Y, Yu JK, Chen Y, Jeong SO, Mani N, et al. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim Biophys Acta 2015;1852:1550-1559.
13 Roderburg C, Benz F, Vargas Cardenas D, Koch A, Janssen J, et al. Elevated miR-122 serum levels are an independent marker of liver injury in inf l ammatory diseases. Liver Int 2015;35:1172-1184.
14 Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res 2012;22:516-527.
15 Kim G, Lee YH, Park YM, Kim J, Kim H, Lee BW, et al. Use of a diabetes self-assessment score to predict nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Medicine (Baltimore) 2015;94:e1103.
16 Brownhill S, Cohen D, Burchill S. Proliferation index: a continuous model to predict prognosis in patients with tumours of the Ewing’s sarcoma family. PLoS One 2014;9:e104106.
17 Specht E, Kaemmerer D, Sänger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015;67:368-377.
18 Guimarães Filho MA, Cortez E, Garcia-Souza éP, Soares Vde M, Moura AS, Carvalho L, et al. Effect of remote ischemic preconditioning in the expression of IL-6 and IL-10 in a rat model of liver ischemia-reperfusion injury. Acta Cir Bras 2015;30:452-460.
19 Abu-Amara M, Yang SY, Quaglia A, Rowley P, Tapuria N, Seifalian AM, et al. Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transpl 2011;17:70-82.
20 Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, et al. Remote ischemic preconditioning protects against liver ischemiareperfusion injury via heme oxygenase-1-induced autophagy. PLoS One 2014;9:e98834.
21 Igarashi G, Iino K, Watanabe H, Ito H. Remote ischemic preconditioning alleviates contrast-induced acute kidney injury in patients with moderate chronic kidney disease. Circ J 2013;77:3037-3044.
22 van Beek JH, de Moor MH, de Geus EJ, Lubke GH, Vink JM, Willemsen G, et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet 2013;43:329-339.
23 Wolf JH, Bhatti TR, Fouraschen S, Chakravorty S, Wang L, Kurian S, et al. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transpl 2014;20:376-385.
24 Zhang M, Yue Z, Liu Z, Islam A, Rehana B, Tang S, et al. Hsp70 and HSF-1 expression is altered in the tissues of pigs transported for various periods of times. J Vet Sci 2012;13:253-259.
25 Diao TJ, Chen X, Deng LH, Chen HX, Liang Y, Zhao XD, etal. Protective effect of nitric oxide on hepatopulmonary syndrome from ischemia-reperfusion injury. World J Gastroenterol 2012;18:3310-3316.
26 Saracyn M, Brytan M, Zdanowski R, Ząbkowski T, Dyrla P, Patera J, et al. Hepatoprotective effect of nitric oxide in experimental model of acute hepatic failure. World J Gastroenterol 2014;20:17407-17415.
27 Lang JD Jr, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, et al. A randomized clinical trial testing the anti-inf l ammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One 2014;9:e86053.
28 Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, et al. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg 2014;6:122-128.
29 Kim JI, Jang HS, Park KM. Endotoxin-induced renal tolerance against ischemia and reperfusion injury is removed by iNOS, but not eNOS, gene-deletion. BMB Rep 2010;43:629-634.
30 Abu-Amara M, Yang SY, Quaglia A, Rowley P, Fuller B, Seifalian A, et al. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver. Liver Transpl 2011;17:610-619.
31 Trocha M, Merwid-Lad A, Szuba A, Chlebda E, Pieśniewska M, Sozański T, et al. Effect of simvastatin on nitric oxide synthases (eNOS, iNOS) and arginine and its derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat liver. Pharmacol Rep 2010;62:343-351.
32 Betz B, Schneider R, Kress T, Schick MA, Wanner C, Sauvant C. Rosiglitazone affects nitric oxide synthases and improves renal outcome in a rat model of severe ischemia/reperfusion injury. PPAR Res 2012;2012:219319.
33 Huang X, Gao Y, Qin J, Lu S. The role of miR-34a in the hepatoprotective effect of hydrogen sulf i de on ischemia/reperfusion injury in young and old rats. PLoS One 2014;9:e113305.
34 Meng F, Glaser SS, Francis H, Yang F, Han Y, Stokes A, et al. Epigenetic regulation of miR-34a expression in alcoholic liver injury. Am J Pathol 2012;181:804-817.
35 Starckx S, Batheja A, Verheyen GR, Jonghe SD, Steemans K, Dijck BV, et al. Evaluation of miR-122 and other biomarkers in distinct acute liver injury in rats. Toxicol Pathol 2013;41:795-804.
36 Shifeng H, Danni W, Pu C, Ping Y, Ju C, Liping Z. Circulating liver-specif i c miR-122 as a novel potential biomarker for diagnosis of cholestatic liver injury. PLoS One 2013;8:e73133.
37 Shin JH, Park YM, Kim DH, Moon GJ, Bang OY, Ohn T, et al. Ischemic brain extract increases SDF-1 expression in astrocytes through the CXCR2/miR-223/miR-27b pathway. Biochim Biophys Acta 2014;1839:826-836.
November 18, 2015
Accepted after revision May 23, 2016
Author Af fi liations: Department of Hepatobiliary Surgery, The Third Affi liated Hospital of Soochow University, Changzhou 213003, China (Duan YF, An Y, Zhu F and Jiang Y)
Yong Jiang, PhD, Department of Hepatobiliary Surgery, The Third Af fi liated Hospital of Soochow University, Changzhou 213003, China (Tel/Fax: +86-519-68870000; Email: jy_sz3h@126.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60006-7
Published online April 24, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- The International Study Group of Pancreatic Surgery def i nition of delayed gastric emptying and the effects of various surgical modif i cations on the occurrence of delayed gastric emptying after pancreatoduodenectomy
- Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Effects of multimodal fast-track surgery on liver transplantation outcomes
