High value of controlled attenuation parameter predicts a poor antiviral response in patients with chronic hepatits B
2017-08-16JingChenMengLanWangQinLongLangBaiandHongTang
Jing Chen, Meng-Lan Wang, Qin Long, Lang Bai and Hong Tang
Chengdu, China
High value of controlled attenuation parameter predicts a poor antiviral response in patients with chronic hepatits B
Jing Chen, Meng-Lan Wang, Qin Long, Lang Bai and Hong Tang
Chengdu, China
BACKGROUND:Controlled attenuation parameter (CAP) is a non-invasive method for diagnosing hepatic steatosis based on vibration-controlled transient elastography. The objective of this study was to investigate the effect of high value of CAP on antiviral therapy in patients with chronic hepatitis B (CHB).
METHODS:Patients with CHB receiving enticavir for initial antiviral therapy were studied; they were divided into the high CAP group and normal CAP group at baseline according to the CAP values. The effect of the antiviral therapy between the two groups were compared at week 12, 24 and 48. Patients with high CAP value at baseline were divided into three subgroups, mild, moderate and severe elevation; the therapeutic response were compared among patients with normal CAP and subgroups of patients with elevated CAP.
RESULTS:A total of 153 patients were enrolled. Among them, 63 were in the high CAP group and 90 in the normal CAP group. Patients with high CAP had lower rates of ALT normalization and HBV DNA clearance in response to antiviral therapy compared with those with normal CAP at week 12, 24 and 48. Further analysis showed that the rate of ALT normalization in patients with mildly and moderately elevated CAP were signif i cant lower than those with normal CAP at week 12 and 24; while the difference was not signif i cant between the patients with normal CAP and those with severely elevated CAP. The rate of HBV DNA clearance was signif i cantly lower in patients with severely elevated CAP compared with those with normal CAP at week 12, 24 and 48.
CONCLUSION:CHB patients with high CAP had poor response to antiviral therapy.
(Hepatobiliary Pancreat Dis Int 2017;16:370-374)
controlled attenuation parameter; chronic hepatitis B; treatment
Introduction
Hepatitis B virus (HBV) infection has been a serious global health problem.[1]China is a country with a high incidence of chronic hepatitis B (CHB), which becomes a major cause of liver disease. The current treatments for CHB are interferon and nucleoside analogues.[2]Though the eff i cacy of antiviral treatment in CHB has been improved in recent years, the sustained responses are still poor.[3]Therefore, it is important to explore the factors associated with treatment failure in patients with CHB.
Hepatic steatosis is the accumulation of fat in the liver, which has been a common pathological feature of various liver diseases. Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries. With the development of society and the change of life style, NAFLD has become a common cause of liver related morbidity, with the incidence around 15% in China.[4,5]The estimated frequency of hepatic steatosis in CHB ranges from 27% to 51%, higher than that in the general population, which means hepatic steatosis may have potential effect on CHB.[6]
Hepatic steatosis is considered as a predictive factor in treatment outcome, but the effect of hepatic steatosis on treatment response in patients with CHB is not clear. Previous studies have investigated the relationship between hepatic steatosis and treatment response in patients with CHB.[7-9]However, hepatic steatosis was determined by liver biopsy or abdominal sonographic examination in these studies. As well known, liver biopsy is invasive and abdominal sonographic examination cannot detect early hepatic steatosis. Recently, a parameter called controlled attenuation parameter (CAP) has beenused to evaluate hepatic steatosis. CAP can diagnose patients with >5% steatosis, and previous studies have shown its good performance.[10-14]Therefore, we studied a cohort of patients with CHB receiving entecavir as initial therapy, to investigate the impact of high CAP value on the response to antiviral treatment.
Methods
Study population
One hundred and fi fty-three consecutive naïve patients with CHB receiving entecavir as initial antiviral treatment at West China Hospital between March 2013 and March 2014 were enrolled in the study. The dose of entecavir was 0.5 mg/dayper os. The inclusion criteria were as follows: (1) the diagnosis of CHB consistent with the guideline for the prevention and treatment of CHB;[15](2) age ≥18 years; (3) the hepatitis B surface antigen (HBsAg) positive for more than 6 months, and alanine aminotransferase (ALT) level ≥2 ULN (upper limit of normal range, 50 IU/L). The exclusion criteria included:(1) co-infected with other viruses, such as hepatitis A, C, D and E; (2) co-existed with autoimmune hepatitis, primary biliary cirrhosis and other liver diseases; (3) with severe liver damage, hepatocellular carcinoma or hepatic encephalopathy; (4) pregnancy or on breast feeding; (5) without successful CAP measurement.
The study conformed to the ethical guidelines of the 1975Declaration of Helsinki. Study approval was obtained from the Ethics Committee of West China Hospital of Sichuan University, and informed consent was obtained from each patient.
Clinical and biochemical parameters
Demographic and biochemical parameters at baseline were collected on enrollment, including age, gender, ALT, aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), HBsAg, HBsAb, HBeAg, anti-HBe, anti-HBc, HBV DNA and CAP value. ALT, HBsAg, HBsAb, HBeAg, anti-HBe, anti-HBc and HBV DNA were also collected at week 12, 24 and 48. All blood sample analyses were performed at West China Hospital, China.
CAP measurement
CAP measurements were performed using the Fibroscan 502 (Echosens, Paris, France) by certif i ed experienced operators.[16]All patients were measured using the 3.5 MHz M probe. The principles of CAP measurement have been described elsewhere.[17]The fi nal CAP result was the median of individual CAP values and was expressed in dB/m ranging from 100 to 400. All patients included in the study had 10 valid measurements.
Def i nition of response
ALT normalization is def i ned as ALT level decreases to the normal range. HBeAg seroconversion is def i ned as that HBeAg changes from positive into negative. At week 12 of treatment, <1 log10IU/mL decrease in HBV DNA level is considered as primary non-response. HBV DNA clearance is def i ned as undetectable HBV DNA in both HBeAg positive and negative patients.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0). Continuous variables were expressed as mean±standard deviation (SD). Comparisons between the two groups were evaluated using Studentttest. For qualitative variant, percentages were used and Chi-square test was chosen for comparison. The statistical results associated with a two-sidedP<0.05 were considered signif i cant.
Results
Patient characteristics
A total of 153 patients were enrolled in the study with average age of 42.1±10.3 years. The percentage of male was 80.4%. Since steatosis <5% was considered as normal, we chose 224 dB/m as a cut-off line and divided the patients into two groups:[14]high CAP group (n=63) and normal CAP group (n=90). There were no differences between the age, gender, ALT, AST, GGT, the status of HBeAg positive distribution and HBV DNA level between the two groups (Table 1).
ALT normalization
We compared the rates of ALT normalization between the high CAP group and normal CAP group at different time point after antiviral treatment, as shown in Table 2. The rate of ALT normalization in patients with elevated CAP at week 12, 24 and 48 was 60.3%, 63.5% and 69.8% respectively, this rate in normal CAP group was 80.0%, 87.8% and 91.1%, respectively (P<0.05).
HBV DNA clearance
As shown in Table 3, both groups had obtained high-er rate of HBV DNA clearance with entecavir treatment for 24 and 48 weeks compared with that with 12 weeks treatment. The rates of HBV DNA clearance in patients with normal CAP value were 71.1%, 88.9% and 97.8% at week 12, 24 and 48, respectively, which was signif i cantly higher than that in patients with elevated CAP (P<0.05).
HBeAg seroconversion
Twenty-eight patients in the high CAP group and 46 in the normal CAP group were HBeAg positive. The rates of HBeAg seroconversion in the high CAP group were 3.6%, 7.1% and 14.3% at week 12, 24 and 48, respectively, and these were 8.7%, 13.0% and 17.4% respectively in patients with normal CAP value (Fig. 1,P>0.05). In addition, there was only 1 patient having HBsAg seroconversion in the high CAP group at week 48, while there were 2 patients in the normal CAP group.

Table 1. Baseline characteristics of patients in the two groups
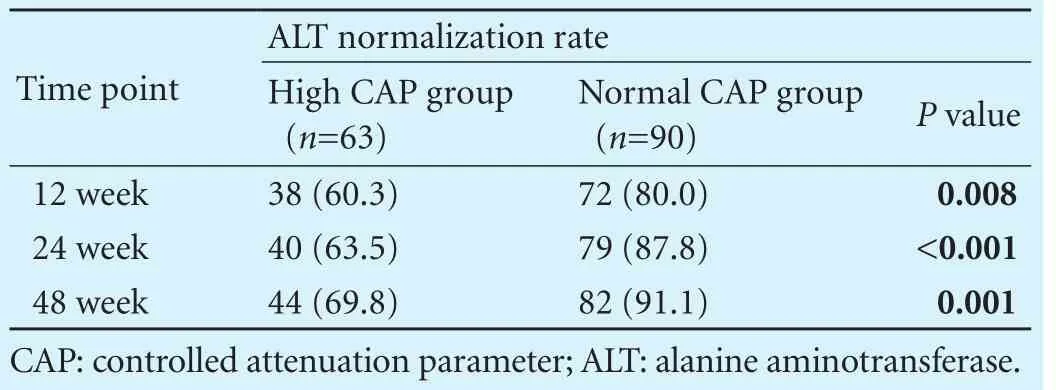
Table 2. Comparison of the ALT normalization rates between two groups (n, %)
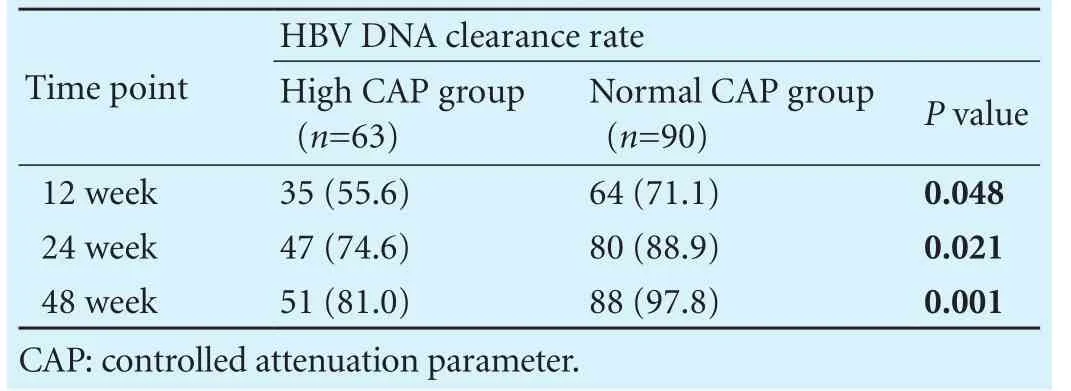
Table 3. Comparison of the HBV DNA clearance rates between two groups (n, %)
The effect of antiviral therapy on patients with different CAP value
Since patients with high CAP had lower rate of ALT normalization and HBV DNA clearance in this study, we further compare the curative effect of antiviral therapy on patients with different degree of elevated CAP and those with normal CAP. According to the reference value provided by Echosens, we def i ned CAP value 224-235 dB/m, 236-285 dB/m and >285 dB/m as mild, moderate and severe elevations respectively. In the 63 patients with elevated CAP value, 26 were mild, 24 moderate and 13 severe elevations.

Fig. 1. Comparison of the rates of HBeAg seroconversion between the two groups. There were no significant differences in HBeAg seroconversion between the high CAP group and normal CAP group at week 12, 24 and 48 (P>0.05).
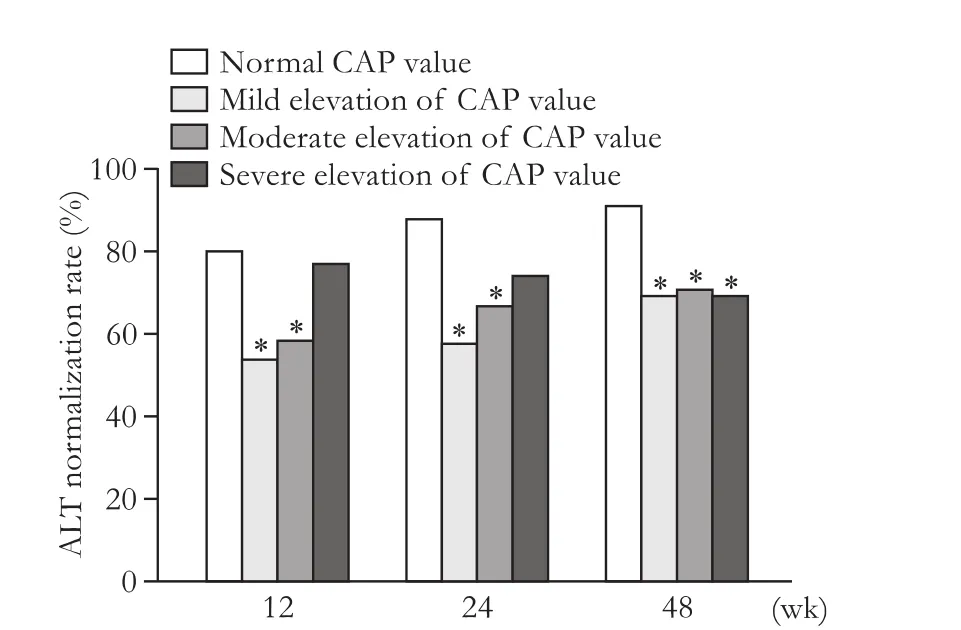
Fig. 2. Comparison of the rates of ALT normalization between normal CAP and different degree of CAP elevation. At week 12 and 24, there were significant differences between normal CAP and mild, moderate elevated CAP (P<0.05), while the differences were not so signif i cant between normal and severe elevated CAP (P>0.05). At week 48, the rate of ALT normalization in patients with normal CAP was significantly higher than that in patients with mild, moderate and severe elevation of CAP (P<0.05). *:P<0.05, compared with the normal CAP group.
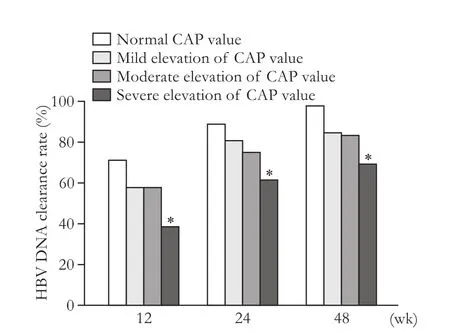
Fig. 3. Comparison of the rates of HBV DNA clearance between normal CAP and different degree of CAP elevation. The rate of HBV DNA clearance in patients with normal CAP was higher than that in patients with mild, moderate and severe elevation of CAP at week 12, 24 and 48, but only the difference between normal CAP and severe elevated CAP had statistical significance (P<0.05). *:P<0.05, compared with the normal CAP group.
Patients with normal CAP and different degree of elevated CAP values were compared at week 12, 24 and 48 (Fig. 2). We found that at week 12 and 24, the rate of ALT normalization in the mild and moderated elevated CAP groups were signif i cant different with that in the normal CAP group (P<0.05), while the difference was not signif i cant between normal and severe elevated CAP. But at week 48, the signif i cant differences existed when patients with normal CAP at baseline were compared with mild, moderate and severe elevated CAP (P<0.05). The rate of HBV DNA clearance in patients with normal CAP was higher than that in those with mild, moderate and severe elevation of CAP throughout the whole time point, but only the difference between normal CAP and severe elevated CAP had statistical signif i cance (P<0.05) (Fig. 3).
Discussion
In recent years, hepatic steatosis has become a common feature in Chinese patients with CHB.[13]In this study, we found that the prevalence of hepatic steatosis was 41.2% in patients with CHB, higher than that in normal population, which was consistent with Sass’s report.[18]For CHB patients, the presence of hepatic steatosis may accelerate the progression of disease, inf l uence the effect of antiviral treatment and even increase the risk of cirrhosis and hepatocellular carcinoma.[9,19]Therefore, prominent attention should be paid to the patients with CHB and steatosis.
A few previous studies have demonstrated the association between hepatic steatosis and antiviral therapy in patients with CHB. But in those studies hepatic steatosis was diagnosed by liver biopsy or abdominal ultrasound.[8,9]As well known, liver biopsy is the gold standard to evaluate hepatic steatosis, but it has some pitfalls, such as invasiveness, high cost, sample error and so on.[20]Though ultrasound is the most common method, it cannot diagnose <30% steatosis or quantitatively measure steatosis.[21,22]A method called CAP has been developed for assessment of hepatic steatosis recently. CAP is noninvasive, easily accepted and can detect <10% or even <5% steatosis which has shown good performance in early studies.[12-14,17,23,24]Therefore, in this study, we investigated the inf l uence of CAP value on the antiviral treatment in patients with CHB. To our knowledge, this is the fi rst study to explore the association between CAP value and antiviral treatment.
Nowadays, entecavir has been considered as fi rst line monotherapy of CHB, but study of the effect of entecavir on CHB patients with hepatic steatosis is paucity.[25]This study investigated the curative effect of entecavir on patients with different CAP value. We found that the rates of ALT normalization and HBV DNA clearance at week 12, 24 and 48 in the high CAP group were signif icantly lower than those in the normal CAP group. This indicated that the CAP value of more than 224 dB/m at baseline may affect the recovery of liver function and the clearance of HBV DNA in patients treated with entecavir and thereby reduce the antiviral eff i cacy. CAP examination is therefore very important for patients with CHB before treatment. In addition, after 48 weeks’ treatment, the rate of ALT normalization reached 91.1% in patients with normal CAP value, while this rate is 69.8% in patients with high CAP value. These data suggested that the poor effect of antiviral therapy on CHB patients was mainly due to hepatic steatosis. This fi nding provided an evidence to change the current pattern of antiviral treatment for patients with CHB and high CAP value.
Since CAP could quantitatively detect the severity of hepatic steatosis, we further investigated the effect of different degree of CAP on antiviral therapy. We found that the ALT normalization and HBV DNA clearance were not always in parallel; the difference of ALT normalization between patients with normal CAP and mild or moderate elevated CAP at week 12 and 24 was signif i cant while the HBV DNA clearance was not signif i cant. To our surprise, the difference of ALT normalization between normal and severe elevated CAP was not signif i cant while the difference of HBV DNA clearance was signif i cant at week 12, 24 and 48. All of these data suggested that mild or moderate elevation of CAP value may mainly affect the ALT normalization, while severe elevation of CAP value may mainly affect the HBV DNA clearance. A previous study had shown no impact of steatosis on the response of pegylated interferon therapy in patients with CHB.[9]Another study had reported a negative effect of hepatic steatosis on entecavir treatment in patients with CHB. Therefore, this study fi rstly suggested the different effect of entecavir on CHB patients with different elevation of CAP value, which represented the severity of hepatic steatosis. This result might be applicable in clinical practice.
Treatment of CHB still faces big challenge. The rate of response to antiviral treatment is still not satisfactory,not only because of viral factors, but also some comorbidities like hepatic steatosis. As our study indicated, if one’s CAP value is ≥224 dB/m, his/her response to entecavir treatment will be impaired. Our data need to be validated in a larger cohort.
In conclusion, the response to entecavir treatment was decreased in CHB patients with CAP ≥224 dB/m which implied that the value of CAP plays an important role in clinical practice. These results provide a new basis of developing special treatment strategy in patients with CHB and steatosis such as drugs for hepatic steatosis. CAP examination before antiviral therapy and treating hepatic steatosis simultaneously may increase the rate of response in patients with CHB.
Contributors: TH proposed the study. CJ performed the research and wrote the fi rst draft. CJ, WML and LQ collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. TH is the guarantor.
Funding: This study was supported by grants from the National Science and Technology Major Project of China (2012ZX10002007-001-003 and 2013ZX10002005-002-003) and the WBE Liver Fibrosis Foundation (XJS20120204).
Ethical approval: This study was approved by the Ethics Committee of the West China Hospital of Sichuan University.
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008;359:1486-1500.
2 Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004;2:87-106.
3 European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009;50:227-242.
4 Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202-1219.
5 Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol 2009;50:204-210.
6 Minakari M, Molaei M, Shalmani HM, Mohammad Alizadeh AH, Jazi AH, Naderi N, et al. Liver steatosis in patients with chronic hepatitis B infection: host and viral risk factors. Eur J Gastroenterol Hepatol 2009;21:512-516.
7 Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol 2007;41:513-517.
8 Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One 2012;7:e34198.
9 Ateş F, Yalnız M, Alan S. Impact of liver steatosis on response to pegylated interferon therapy in patients with chronic hepatitis B. World J Gastroenterol 2011;17:4517-4522.
10 Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantif i cation of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2014;29:1470-1476.
11 Jung KS, Kim BK, Kim SU, Chon YE, Chun KH, Kim SB, et al. Factors affecting the accuracy of controlled attenuation parameter (CAP) in assessing hepatic steatosis in patients with chronic liver disease. PLoS One 2014;9:e98689.
12 Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int 2014;34:102-109.
13 Shen F, Zheng RD, Mi YQ, Wang XY, Pan Q, Chen GY, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol 2014;20:4702-4711.
14 Mi YQ, Shi QY, Xu L, Shi RF, Liu YG, Li P, et al. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan®: validation in chronic hepatitis B. Dig Dis Sci 2015;60:243-251.
15 Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Liu Xing Bing Xue Za Zhi 2011;32:405-415.
16 de Lédinghen V, Vergniol J. Transient elastography for the diagnosis of liver fi brosis. Expert Rev Med Devices 2010;7:811-823.
17 Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825-1835.
18 Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease:a clinical review. Dig Dis Sci 2005;50:171-180.
19 Negro F, Clément S. Impact of obesity, steatosis and insulin resistance on progression and response to therapy of hepatitis C. J Viral Hepat 2009;16:681-688.
20 Tobkes AI, Nord HJ. Liver biopsy: review of methodology and complications. Dig Dis 1995;13:267-274.
21 Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantif i cation of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009;51:433-445.
22 Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol 2008;14:3476-3483.
23 de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911-918.
24 Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan®: validation in chronic hepatitis C. J Viral Hepat 2012;19:244-253.
25 Shepherd J, Gospodarevskaya E, Frampton G, Cooper K. Entecavir for the treatment of chronic hepatitis B infection. Health Technol Assess 2009;13:31-36.
Received December 13, 2015
Accepted after revision September 5, 2016
nts
Fibroscan-CAP before treatment and were followed up for 48 weeks. Mi et al[14]showed that the optimal cut-off value to predict steatosis ≥5% was 224 dB/m in 340 patients with CHB in their study; we therefore def i ned CAP ≥224 dB/m as a rise in CAP value. We divided our patients into two groups (high CAP group and normal CAP group) based on their CAP at baseline. Finally, the ALT normalization, HBeAg seroconversion in HBeAg (+) patients and HBV DNA clearance of the two groups were compared at week 12, 24 and 48.
Author Aff i liations: Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu 610041, China (Chen J, Wang ML, Long Q, Bai L and Tang H)
Hong Tang, MD, PhD, Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu 610041, China (Tel/Fax: +86-28-85422650; Email: htang6198@hotmail.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60144-3
Published online October 17, 2016.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- The International Study Group of Pancreatic Surgery def i nition of delayed gastric emptying and the effects of various surgical modif i cations on the occurrence of delayed gastric emptying after pancreatoduodenectomy
- Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Effects of multimodal fast-track surgery on liver transplantation outcomes
