基于呋喃-3-酮配体的铜(II)配合物的合成及其晶体结构
2017-06-19赵明霞熊丽琴蔡永乐张华北齐传民
赵明霞, 熊丽琴, 蔡永乐, 张华北, 齐传民*
(1. 山西工程技术学院 采矿工程系,山西 阳泉 045000; 2. 上海交通大学 生物医学工程学院,上海 200025;3. 北京师范大学 化学学院,教育部放射性药物重点实验室,北京 100875)
·快递论文·
基于呋喃-3-酮配体的铜(II)配合物的合成及其晶体结构
赵明霞1, 熊丽琴2, 蔡永乐1, 张华北3, 齐传民3*
(1. 山西工程技术学院 采矿工程系,山西 阳泉 045000; 2. 上海交通大学 生物医学工程学院,上海 200025;3. 北京师范大学 化学学院,教育部放射性药物重点实验室,北京 100875)
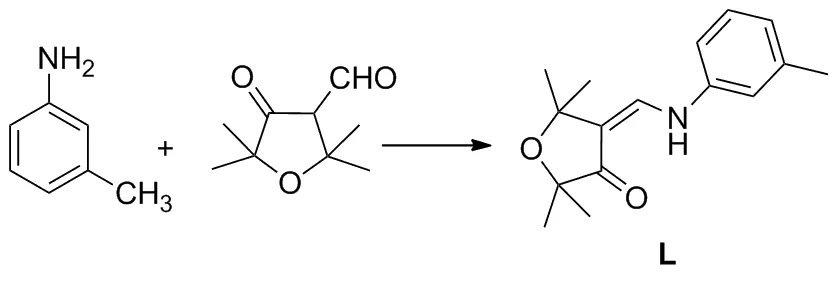
以4-甲酰基-2,2,5,5-四甲基四氢呋喃-3-酮和间甲基苯胺为原料,经缩合反应制得配体(Z)-4-[(间甲苯胺基)亚甲基]-2,2,5,5-四甲基二氢呋喃-3(2H)-酮(L);以Cu(CH3COO)2为铜源,L经配位反应合成了一个新型的铜(II)配合物[Cu(L)2]n(I),其结构经FT-IR, X-射线单晶衍射和元素分析表征。I属单斜晶系,P2(1)/c空间群,晶胞参数a=11.361 3(19) Å,b=24.564(4) Å,c=12.026(2) Å,α=γ=90,β=110.452(3),V=3 144.6(9) Å3,Dc=1.226 g·cm-3,Z=4,R1=0.051 5,wR2=0.113 2。
呋喃-3-酮; 铜(II)配合物; 晶体结构; 弱相互作用; 合成
各种分子间相互作用力在超分子化学和晶体工程中有着重要作用[1-5]。由各种作用产生的结构基元的知识可用于设计新型的具有预期物理和化学性质的材料,在磁性、荧光、气体吸附与分离等领域引起广泛关注[6-9]。这些相互作用被用于控制固态中的分子组装体的构象和形态特点[10-14]。近年来,这些研究延伸到非常规氢键,即H┈H作用距离非常短,典型的键长范围是1.7~2.4 Å[15-20]。包括X—Hδ+┈Hδ-—Y相互作用,如B—H┈H—N相互作用[15]和Ga—H┈H—N相互作用[17]; X—Hδ+┈Hδ+—Y相互作用,如C—H┈H—C相互作用[20]。
3(2H)-呋喃酮类衍生物是一类重要的杂环化合物,具有较高的药理活性。如从植物Jatrolha gossypi Ifolia中提取的Jatrophones具有抗癌活性[21-22],从植物Geilera parviflora的树叶中提取分离并己完成人工合成的抗肿瘤作用物Genparvarin[23-24],及一些生物合成中重要的前体Patulin[25-26]等,在这些分子中,呋喃酮为关键骨架。然而有关含3(2H)-呋喃酮配体的金属配合物的报道很少[27-28]。
本文以4-甲酰基-2,2,5,5-四甲基四氢呋喃-3-酮和间甲基苯胺为原料,经缩合反应制得配体(Z)-4-[(间甲苯胺基)亚甲基]-2,2,5,5-四甲基二氢呋喃-3(2H)-酮(L, Scheme 1);以Cu(CH3COO)2为铜源,L经配位反应合成了一个新型的铜(II)配合物[Cu(L)2]n(I),其结构经FT-IR, X-射线单晶衍射和元素分析表征。
Scheme 1
1 实验部分
1.1 仪器与试剂
RY-1型熔点仪(温度未校正); Varian 500 型核磁共振仪(CDCl3为溶剂,TMS为内标); Nicolet-AVATAR 360 FT-IR 型红外光谱仪(KBr压片); Elementar vario EL 型元素分析仪; Bruker Smart 1000 CCD型X-射线单晶衍射仪。
所用试剂均为分析纯。
1.2 合成
(1) L的合成[29]
将4-甲酰基-2,2,5,5-四甲基四氢呋喃-3-酮0.171 g(1.0 mmol)和间甲基苯胺0.107 g(1.0 mmol)加至50 mL茄型瓶中,搅拌使其混匀,加热30 min。冷却至室温,抽滤得固体物质,依次用1 mol·L-1盐酸和蒸馏水洗涤得粗品,用乙醇和水重结晶得L, m.p.81~83 ℃;1H NMRδ: 1.39(s, 6H, CCH3), 1.49(s, 6H, CCH3), 2.38(s, 3H, ArCH3), 6.90(m, 3H, ArH), 7.18(d,J=12.3 Hz, 1H,CH), 7.25(t,J=7.6 Hz, 1H, ArH), 10.96(d,J=11.7 Hz, 1H, NH);13C NMRδ: 139.35, 137.52, 129.12, 124.16, 116.09, 112.63, 110.93, 80.76, 78.36, 31.77, 26.08, 21.00; FT-IRν: 3 431, 2 971, 2 924, 1 693, 1 669, 1 612, 1 579, 1 492, 1 459, 1 415, 1 356, 1 292, 1 270, 1 242, 1 164, 1 118, 1 037, 993, 969, 773 cm-1。
(2) I的合成
将Cu(CH3COO)20.018 1 g(0.1 mmol)的THF(4 mL)溶液滴至L 0.025 8 g(0.1 mmol)的THF(4 mL)溶液中,滴毕,加热15 min;加入CH3CN 4 mL,回流反应1.5 h。冷却至室温,反应液过滤,滤液于室温放置5 d后得红色块状晶体I; FT-IRν: 2 972, 2 923, 2 859, 1 607, 1 516, 1 498, 1 482, 1 456, 1 429, 1 354, 1 305, 1 216, 1 186, 1 138, 1 125, 988 cm-1; Anal.calcd for C32H40N2O4Cu: C 66.01, H 6.92, N 4.87; found C 66.24, H 6.99, N 4.73。
1.3 晶体结构测定
将I置单晶衍射仪上,用经石墨单色化的Mo-Kα射线(λ=0.710 73 Å),于294(2) K以ω-2θ扫描方式收集衍射点15 662个(独立衍射点5 538个)。I的结构由直接法解出(SHELXTL-97程序)[30],用全矩阵最小二乘法精修,氢原子位置用理论加氢法确定。
2 结果与讨论
2.1 表征
L和I的IR谱图分析表明: L在3 431 cm-1处的一个宽吸收带为N—H伸缩振动峰,I中此带消失说明配体中氮上脱氢。L在1 693 cm-1、 1 669 cm-1、 1 612 cm-1和1 579 cm-1处的强吸收带为C=C—C=O的伸缩振动峰。I的1 607 cm-1处的吸收峰是由氧和氮原子与CuII的配位所引起,并且显示N—C—C—C—O六元螯合环的电子离域状态。这种现象与X-射线分析的结果一致。
2.2 晶体结构
I(CCDC: 602560)的晶体学数据见表1,部分键长和键角数据见表2,氢键键长和键角见表3。

表1 I的晶体学数据Table 1 Crystal data and refinement details of I
I的不对称单元由一个CuII和两个L配体分子组成。CuII离子与两个配体L通过反式-N2O2单元连接形成了变形四面体的配位环境(图1)。CuII与配位原子之间的距离分别为:
Cu1—O3: 1.900(3), Cu1—O1: 1.905(3), Cu1—N2: 1.960(3)以及Cu1—N1: 1.962(3) Å。这些值都在文献报道的四-配位的含氧、氮配体的CuII化合物范围内[31]。六元鳌合环中,O3—Cu1—N2的键角为94.33(13), O1—Cu1—N1的键角为94.43(13)。呋喃环与甲基C19/C31/C32之间的二面角为89.84(3);与甲基C18/C29/C30之间的二面角为89.62(4)。苯环上的甲基显示无序状态。

表2 I的部分键长和键角Table 2 Selected bond lengths and angles of I

图1 I的晶体结构图Figure 1 The crystal structure of I表3 I的氢键键长和键角*Table 3 Hydrogen bond lengths and bond angles of I

DHAD—H/ÅH┈A/ÅD…A/ÅD—H┈A/(°)C29H29AC21#10.9602.8343.684148C28H28BO1#20.9602.6743.619168C12H12BC5#10.9612.8223.611140
*Symmetry code:#1x, 3/2-y, -1/2+z;#21-x, 2-y, -z。
图2 I的三维网络结构图Figure 2 Three-dimensional framework stabilized by the weak interactions in I
呋喃氧原子与苯环甲基之间存在C—H┈O氢键(C28—H28B┈O1=3.619 Å),连接两个分子形成了二聚体。此外,呋喃环上的甲基与苯环上的甲基之间存在弱的C—H…H—C作用(C12—H12A…H16C—C16,H12A…H16C=2.158 Å),以及弱的C—H…C作用(C29—H29A…C21=3.684 Å和C12—H12B…C5=3.611 Å),这些弱的氢键作用连接分子形成了三维超分子网络结构(图2)。
以4-甲酰基-2,2,5,5-四甲基四氢呋喃-3-酮和间甲基苯胺为原料,经缩合反应制得配体(Z)-4-[(间甲苯胺基)亚甲基]-2,2,5,5-四甲基二氢呋喃-3(2H)-酮(L);以Cu(CH3COO)2为铜源,L经配位反应合成了一个新型的铜(II)化合物[Cu(L)2]n(I), I中的CuII离子中心与两个配体L中反式-N2O2单元的N和O配位,形成变形四面体几何构型。
[1] Dethlefs K M, Hobza P. Noncovalent interactions:A challenge for experiment and theory[J].Chem Rev,2000,100(1):143-168.
[2] Guru Row T N. Hydrogen and fluorine in crystal engineering:Systematics from crystallographic studies of hydrogen bonded tartrate-amine complexes and fluoro-substituted coumarins,styrylcoumarins and butadienes[J].Coord Chem Rev,1999,183(1):81-100.
[3] Lehn J M. Supramolecular chemistry[M].Weinheim:VCH,1995.
[4] Aakeroy C B. Crystal engineering: Strategies and architectures[J].Acta Crystallogr B,1997,B53:569-586.
[5] Nangia A. Supramolecular chemistry and crystal engineering[J].J Chem Sci,2010,122(3):295-310.
[6] Brown A J, Pinkowicz D, Dunbar K R,etal. A trigonalpyramidal Erbium(III) single-molecule magnet[J].Angew Chem Int Edit,2015,54(20):5864-5868.
[7] 徐衡,朱昌海,薛晨,等. 新型8-氨基喹啉锌配合物的合成及其荧光性能[J].合成化学,2015,23(7):619-622.
[8] 梁姗姗,张慧,郭京京,等. 双联咪唑和对苯二甲酸构筑的二重穿插锌(II)配位聚合物{[Zn(bbi)(tpa)n}的合成及其荧光性能[J].合成化学,2016,24(5):384-388.
[9] Yang F, Liu Q K, Dong Y B,etal.p-Benzoquinone adsorption-separation,wensing and its photoinduced transformation within a robust Cd(II)-MOF in a SC-SC fashion[J].Chem Commun,2015,51:7443-7446.
[10] Weissbuch I, Popovitz-Biro R, Lahav M,etal. Understanding and control of nucleation,growth,habit,dissolution and structure of two- and three-dimensional crystals using tailor-made auxiliaries [J].Acta Crystallogr B,1995,B51:115-148.
[11] Kadirvelraj R, Umarji A M, Robinson W T,etal. Systematic crystallographic investigation of hydrogen-bonded networks involving monohydrogen tartrate-amine complexes:Potential materials for nonlinear optics[J].Chem Mater,1996,8(9):2313-2323.
[12] Fuji K, Furuta T, Otsubo T,etal. Hydrogen-bonded network with a unique structural unit having zeolite-like properties[J].Tetrahedron Lett,1999,40(15):3001-3004.
[13] Dai C, Nguyen P, Marder T B,etal. Control of single crystal structure and liquid crystal phase behaviour via arene-perfluoroarene interactions[J].Chem Commun,1999,24(24):2493-2494.
[14] Coates G W, Dunn A R, Henling L M,etal. Phenyl-perfluorophenyl stacking interactions:A new strategy for supermolecule construction[J].Angew Chem Int Ed Engl,1997,36(3):248-251.
[15] Richardson T B, Gala S D, Crabtree R H. Unconventional hydrogen bonds:Intermolecular B—H┈H—N interactions[J].J Am Chem Soc,1995,117(51):12875-12876.
[16] Georg S F, Laurent P, Aline M F,etal. The cluster dication [H6Ru4(C6H6)4]2+revisited:The first cluster complex containing an intact dihydrogen ligand?[J].J Organomet Chem,2000,609(1-2):196-203.
[17] Campbell J P, Hwang J W, Young V G,etal. Crystal engineering using the unconventional hydrogen bond:Synthesis,structure,and theoretical investigation of cyclotrigallazane [J].J Am Chem Soc,1998,120(3):521-531.
[18] Yilmaz V T, Degirmencioglu I, Andac O,etal. Synthesis,spectra and crystal structure of 2-({[3-(methyl{3-[(2-hydroxybenzylidene)amino]propyl} amino)propyl]imino}methyl)phenol copper(II) complex[J].J Mol Struct,2003,654(1-3):125-129.
[19] Nimz O, Gessler K, Usón I,etal. Inclusion complexes of V-amylose with undecanoic acid and dodecanol at atomic resolution:X-ray structures with cycloamylose containing 26 d-glucoses(cyclohexaicosaose) as host[J].Carbohy Res,2004,339(8):1427-1437.
[20] Wolstenholme D J, Cameron T S. Comparative study of weak interactions in molecular crystals:H—H bondsvshydrogen bonds[J].J Phys Chem A,2006,110(28):8970-8978.
[21] Kupchan S M, Sigel C W, Matz M J,etal. Tumor inhibitors. 111. Structure and stereochemistry of jatrophone,a novel macrocyclic diterpenoid tumor inhibitor[J].J Am Chem Soc,1976,98(8):2295-2300.
[22] Smithlll A B, Guaciaro M A, Schow S R,etal. A strategy for the total synthesis of jatrophone:Synthesis of normethyljatrophone[J].J Am Chem Soc,1981,103(1):219-222.
[23] Dreyer D L, Lee A. Extractives of Geijera parviflora[J].Phtochemistry,1972,11(2):763-767.
[24] Quesne P W L, Levery S B, Menachery M D,etal. Antitumor plants. Part 6. Novel modified germacranolides and other constituents of Eremanthus elaeagnus Schultz-bip (compositae)[J].ChemInform,1979,12(9):1572-1580.
[25] Goss R J M, Fuchser J, O′Hagan D. Biosynthesis of longianone from Xylaria longiana:A metabolite with a biosynthetic relationship to patulin[J].Cheminform,2000,31(20):2255-2256.
[26] Scott A I, Yalpani M. A mass-spectrometric study of biosynthesis:Conversion of deutero-m-cresol into patulin[J].Chem Commun,1967,1967(18):945-946.
[27] Marmion M E, Woulfe S R, Neumann W L,etal. Preparation and characterization of technetium complexes with Schiff base and phosphine coordination. 1. complexes of technetium-99g and -99m with substituted acacen and trialkyl phosphines (where acacen=N,N-ethylenebis[acetylacetone iminato])[J].Nucl Med Biol,1999,26(7):755-770.
[28] Crankshaw C L, Marmion M, Luker G D,etal. Novel technetium (III)-Q complexes for functional imaging of multidrug resistance(MDR1) P-glycoprotein[J].J Nucl Med,1998,39(1):77-86.
[29] 于振华,尹承烈. 4-甲酰基-2,2,5,5-四甲基四氢呋喃-3-酮与胺的缩合产物及其性质[J].高等学校化学学报,1982,3(2):210-216.
[30] Sheldrick G M. SHELXS-97 and SHELEXL-97, Program for the solution and refinement of crystal structures[K].Germany:University of Göttingen,1997.
[31] 杨艳,巴召静,龚俐,等. 新型铜配合物{[Cu4(N3)6(mpm)2]n}的合成及其晶体结构[J].合成化学,2015,23(9):806-810.
Synthesis and Crystal Structure of Cu(II) Complex Containing Furan-3-one Ligand
ZHAO Ming-xia1, XIONG Li-qin2, CAI Yong-le1,ZHANG Hua-bei3, QI Chuan-min3*
(1. Department of Mining Engineering, Shanxi Institute of Technology, Yangquan 045000, China;2. Department of Nuclear Medicine, Shanghai Jiao Tong University, Shanghai 200025, China; 3. Key Laboratory of Radiopharmaceuticals, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, China)
(Z)-4-((m-toluidino)methylene)-2,2,5,5-tetramethyl-dihydrofuran-3(2H)-one(L) was prepared from 2,2,5,5-tetramethyl-4-oxo-tetrahydrofuran-3-carbaldehyde andm-toluidine by condensation reaction. A novel 3D supramolecular architecture [Cu(L)2]n(I) was obtained through self-assembly of Cu(CH3COO)2with L. The structure was characterized by FT-IR, single-crystal X-ray diffraction and elemental analysis. I belongs to monoclinic system, space groupP2(1)/cwitha=11.3613(19) Å,b=24.564(4) Å,c=12.026(2) Å,α=γ=90,β=110.452(3),V=3 144.6(9) Å3,Dc=1.226 g·cm-3,Z=4,R1=0.051 5,wR2=0.113 2.
furan-3-one; Cu(II) complex; crystal structure; weak interaction; synthesis
2016-11-28;
2017-04-14
国家自然科学基金资助项目(230100075); 国家科技支撑资助项目(2014BAA03B03); 山西工程技术学院引进研究生启动基金资助项目(201605002)
赵明霞(1980-),女,汉族,河北行唐人,博士研究生,主要从事金属配合物和药物合成研究。
齐传民,教授,博士生导师, E-mail: qicmin@sohu.com
O626.11
A
10.15952/j.cnki.cjsc.1005-1511.2017.06.16296
