出生体重与慢性肾脏病关联性的系统评价和Meta分析
2017-05-16张若林帅兰军李晓燕陈海霞何庆南
张若林 帅兰军 李晓燕 陈海霞 王 英 何庆南
·论著·
出生体重与慢性肾脏病关联性的系统评价和Meta分析
张若林 帅兰军 李晓燕 陈海霞 王 英 何庆南
目的 系统评价出生体重与慢性肾脏病风险的关联性。方法 电子检索The Cochrane图书馆、MEDLINE、OVID数据库、Springer数据库、维普数据库、万方数据库和中国知网,检索时间为建库截止至2016年6月,纳入以BW为暴露因素、评估生后发生CKD或其相关结局变量的观察性研究,文献需对CKD或其他结局变量的诊断标准做出描述并能提取CKD或其相关结局变量的OR值及其95%CI,语种限定为中、英文;排除文献:研究对象生后12个月内肾功能衰竭和死亡、有宫内感染病史或患先天遗传性疾病,母孕期暴露因素有潜在毒性物质接触史,研究人群包括成人和儿童但无法单独提取儿童数据。由两位作者独立检索关于出生体重(BW)对CKD风险相关的观察性研究,病例对照和队列研究采用NOS量表、横断面研究采用AHRQ量表对文献进行偏倚风险评价,提取OR值和95%CI。应用Stata 12.0对文献进行Meta分析,均选用DerSimonian & Laird随机效应模型分析。结果 10篇观察性研究纳入本文分析,其中病例对照4篇(3篇8分,1篇7分),队列研究4篇(3篇7分,1篇8分),横断面研究2篇(1篇7分,1篇8分)。慢性肾脏病风险低出生体重(LBW)较正常BW人群高约80%,OR=1.80,95%CI:1.37~2.35;其中蛋白尿、终末期肾脏病和低eGFR在LBW较正常BW人群的OR及其95%CI分别为2.58(1.49~4.46)、1.42(1.22~1.66)和1.87(1.19~2.94)。终末期肾脏病、低eGFR在高出生体重(HBW)中较正常BW人群的OR及其95% CI分别为1.19(0.94~1.49)和1.09(0.93~1.27),HBW与生后慢性肾脏病之间无明显关联;LBW人群作为慢性肾脏病的高危人群仅见于男性人群中,OR=1.83,95%CI:1.10~3.05)。Egger回归提示纳入文献不存在发表偏倚。结论 男性LBW是CKD的高危因素,HBW与生后CKD的发生无明显关联。
出生体重; 慢性肾脏病; 蛋白尿; 观察性研究; Meta分析
新生儿重症监护技术在过去50年中取得了重大突破,牛津大学最近数据显示,超过90%的低出生体重(LBW)儿得以挽救,其中60%以上无严重并发症[1]。然而, LBW是否为某些慢性疾病的前驱因素,目前仍不明朗。胎儿起源学说推测宫内营养不良将导致胎儿永久性的生理及代谢异常,从而增加成年期慢性疾病的风险[2],例如高血压、糖耐量异常、血脂异常、心血管疾病以及神经系统认知异常等[2~5]。同时“营养过剩学说”指出,高出生体重(HBW)是肥胖和代谢异常的高危因素[6, 7],并可能通过此途径而增加慢性肾脏病(CKD)的患病风险。近年来,CKD发病率呈逐渐上升趋势[8~10]。在美国约11.5%的成年人患有CKD,为终末期肾脏病(ESRD)所支出的医疗费用占总医疗预算的6.7%[11]。迄今为止,CKD仍无法治愈[12]。对CKD的高危人群进行早期监测和筛选非常重要。
出生体重(BW)是胎儿宫内发育的重要指标,可用于评价器官发育的程度。Hughson和Manalich等[13, 14]研究指出,BW与生后肾小球滤过率(GFR)正相关,与尿蛋白定量负相关。目前关于BW与肾脏病潜在相关机制的观点各异[15]。基于流行病学和动物模型的研究[16~18]认为,BW和肾单位数目变化的关联是最直接的机制[19];也有学者认为[20],心血管疾病的高危因素和CKD有明显的家族聚集性,如何区分遗传因素和环境因素的影响目前尚无定论。
本研究旨在通过Meta分析方法评价BW与CKD的关联性,为临床对高危人群CKD监测的选择提供参考。
1 方法
1.1 文献纳入标准 ①观察性研究(病例对照研究、队列研究和横断面研究);②以BW为暴露因素,评估生后CKD或其相关结局变量;③文献中描述了CKD或其他结局变量(蛋白尿、低eGFR)的诊断标准;④能提取CKD或其相关结局变量的OR值及其95%CI;⑤重复发表的文献,取样本量较大的文献,同一研究不同观察时间发表的文献,取观察时间最长的文献;⑥语种限定为中、英文。
1.2 文献排除标准 ①研究对象生后12个月内发生肾功能衰竭或死亡的文献;②研究对象有宫内感染病史;③母孕期暴露因素有潜在毒性物质;④研究对象患先天遗传性疾病;⑤无法单独提取儿童数据的文献。
1.3 结局指标 BW与生后CKD发病风险关联强度的OR及其95% CI。
1.4 文献检索 以修订版的观察性研究Meta分析指南(MOOSE)[21]为指导。检索数据库包括The Cochrane Library、MEDLINE、 OVID 、Springer和维普数据库、万方数据库、中国知网。中文检索词为:出生体重、慢性肾脏病、肾衰竭、蛋白尿、肾小球滤过率、观察性研究;英文数据库以MEDLINE为例,检索检索词和检索式如下:(AB birth weight OR AB low birth weight OR AB high birth weight OR AB very low birth weight)AND ((AB ( kidney disease or renal disease ) OR AB ( chronic kidney disease or chronic renal failure ) OR AB ( kidney failure or renal failure ) OR AB glomerular filtration function OR AB glomerular filtration rate OR AB albuminuria ;回溯纳入文献的参考文献。检索时间均为建库至 2016年6月24日。
1.5 文献筛选、资料提取和偏倚风险评估 由张若林和帅兰军各自独立完成,如遇分歧由李晓燕决定。采用自制资料登记表提取纳入文献数据,包括题目、发表年份、研究设计、受试对象、样本量、结局指标和暴露因素等。队列、病例对照研究采用NOS量表[22,23]行偏倚风险评价,总分为 9 分,评分≥7分为高质量研究。横断面研究采用AHRQ量表[24]行偏倚风险评价,共包含11个条目,各条目均评为“是”(1分)、“否”和“不清楚”(0分);评分0~3分为低质量文献,~7分为中等质量文献,~11分为高质量文献。
1.6 统计学方法 采用Stata 12.0软件进行 Meta 分析,效应量以OR及其 95%CI表示。纳入文献由于样本量不同、年龄段不同以及结局变量测量的时间不定,不可避免地造成偏倚,均选择DerSimonian & Laird随机效应模型分析。应用Egger回归对文献进行发表偏倚分析。
2 结果
2.1 一般情况 初步检索到919篇相关文献,符合本文纳入和排除标准的10篇文献进入本文Meta分析,文献筛选流程见图1。表1显示纳入文献的基本特征,其中病例对照研究4篇[26, 28, 30, 34],队列研究4篇[25, 31~33],横断面研究2篇[27, 29]。
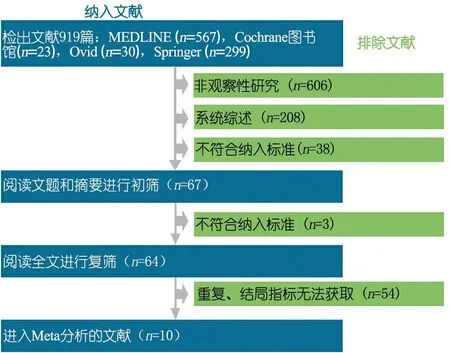
图1 文献筛选流程图

2.2 文献偏倚评价结果 ①4篇队列研究的NOS量表评价:3篇[25,31,33]总分8分,文献[32]为7分。条目8“观察到结局发生随访是否充分”和条目9“随访的完整性”,4篇文献均为0分。文献[32]条目6“研究控制了其他重要的混杂因素”为0分。②4篇病例对照研究的NOS量表评价:3篇[26,28,30]总分为7分,1篇[34]为8分。条目1~5、7和8,4篇文献均为1分,条目6“研究控制了重要的混杂因素”除文献[34]外均为0分,条目9“无应答率”均被评为0分。 ③ 2篇横断面研究的AHRQ量表评价:条目4“如果不是人群来源的话,研究对象是否连续?”和条目11“如果有随访,查明预期的患者不完整数据所占的百分比或随访结果”不适用;条目10“总结了患者的应答率及收集的完整性”,2篇文献均为“否”;条目2、3、5~9均为“是”;条目1 “是否明确了资料的来源”,文献[27]为“不清楚”。
2.3 Meta分析结果
2.3.1 LBW与CKD的定量分析 10篇文献均具体描述了以CKD为结局变量的相应指标,且均提供了完整的Logistic回归的OR值以及95%CI。图2显示,LBW人群较正常BW人群CKD的发生率差异有统计学意义(P<0.001),OR=1.79,95%CI:1.37~2.34。4篇文献[25~27,29]报道了LBW与蛋白尿的关联性,LBW人群较正常BW人群CKD的发生率差异有统计学意义(P<0.001),OR=2.58,95%CI:1.49~4.46。3篇文献[28,30,31]以ESRD为结局变量并报道了LBW与ESRD的关联性,LBW人群较正常BW人群CKD的发生率差异有统计学意义(P<0.001),OR=1.42,95%CI:1.22~1.66。3篇文献[32~34]报道LBW与低eGFR和其他类型CKD关联性,LBW人群较正常BW人群CKD的发生率差异有统计学意义(P<0.05),OR=1.87,95% CI:1.19~2.94。

图2 LBW与CKD关联的Meta分析

图3 敏感性分析

图4 HBW与CKD关联的Meta分析
2.3.2 敏感性分析 剔除2篇横断面研究,行LBW与CKD的关系亚组分析,图3显示,病例对照研究和队列研究中LBW人群较正常BW人群CKD的风险均增高,差异有统计学意义(P<0.05),OR=1.69,95%CI:1.23~2.30;OR=1.79,95%CI:1.18~2.72。
2.3.3 HBW与CKD的定量分析 图4显示,6篇文献[25, 28, 30, 31, 33, 34]同时比较了巨大儿(≥4 000 g)和正常BW人群的CKD发生风险。其中,Nelson等[25]以蛋白尿为结局变量,描述了HBW人群与蛋白尿的关联性,OR=3.2,95% CI:0.75~13.4。3篇文献[28, 30, 31]报道了HBW与ESRD的关联性,HBW人群与正常BW人群差异无统计学意义(P=0.144),OR=1.19,95%CI:0.94~1.49。2篇文献[33, 34]报道了HBW与低eGFR以及其他CKD的关联性,HBW与正常BW人群差异无统计学意义(P=0.311),OR=1.09,95% CI:0.93~1.27。
2.3.4 LBW人群中性别差异与CKD的定量分析 图5显示,3篇文献可以提取到LBW男性或女性人群与CKD之间关联性的数据,经Meta分析,男性中LBW人群较正常BW人群CKD发生率差异有统计学意义(P=0.021),OR=1.83,95%CI:1.10~3.05;女性中差异无统计学意义。
2.3.5 发表偏倚分析 图6显示,应用Egger回归对纳入文献进行发表偏倚风险分析,P>|t|= 0.06,提示不存在明显发表偏倚。

图5 LBW人群性别与CKD关联的Meta分析

图6 Egger回归的漏斗图
3 讨论
20世纪90年代“胎儿起源学说”提出后,Brenner等在1993将这一假说应用于肾脏[35],指出宫内营养不良可能导致肾单位显著减少,正常新生儿与LBW相比其肾单位较多[36];这与高血压、损伤后渐行性肾功能减退以及年龄相关性肾小球硬化密切相关。因此,宫内营养不良可能作为预测生后远期高血压、ESRD和其他肾脏病发生的风险指标。此外,研究显示LBW能引起成人系统性高血压和CKD[37, 38]。
本文纳入10篇文献均为观察性研究,其中队列研究 4 项,病例对照研究4篇,横断面研究2篇。纳入文献存在一定的异质性,如研究设计方法多样、CKD测量指标不同、年龄段不同、人种差异以及纳入人群的性别差异等均不可避免地造成一定偏倚。因此,进一步以CKD相关指标蛋白尿、ESRD、低eGFR、体重和性别进行亚组分析,同时根据文献设计类型的不同行亚组分析。Egger检验显示,文献之间发表偏倚较低。Meta分析结果显示,LBW与CKD关联性密切,可作为CKD的预测指标,与其他系统综述和Meta分析的结果[39]相一致。本文首次就HBW作为CKD的高危因素进行分析,结果显示,HBW与蛋白尿、ESRD、低eGFR及其他CKD指标关联性不大,并非CKD的高危因素,与Das等[39]报道一致,但其机制目前仍无定论。HBW 通常提示巨大儿、糖尿病母亲所生婴儿、胰岛素抵抗以及对CKD的基因易感性。均提示HBW亦可能作为CKD的预测指标之一。为了消除妊娠期糖尿病、高血压以及指标测定时间差异等混杂因素,需要更加严格控制的大样本观察性研究。
本文Meta分析显示,LBW人群中男性与CKD的关联性显著,而女性人群则无明显关联,与Li等[33]的研究结果相一致。有文献指出成年女性的肾单位较男性明显减少,可能解释了为何LBW以及宫内营养不良所致肾单位减少对女性影响较男性小的原因[40]。此外,雌激素作为一种肾脏保护激素,能通过抑制系膜细胞的增生、硬化和减少胶原蛋白的合成来抵消宫内营养不良所致肾脏损害[41]。也有人认为,男性高血压、糖耐量异常较女性出现早,使男性更容易因肾单位减少而出现CKD[42]。由于本文中各文献纳入男女病例差异较大,且未就胎龄、早产以和其他未知因素排除,都不可避免地对研究结果造成偏倚。
本文局限性:①纳入的观察性研究样本量较少;②纳入研究文献的人群年龄段差别较大、划分不明确,以至于无法有效量化各年龄段CKD发生风险的比较;③纳入文献的观察对象大部分为欧美地区人群;④近3年关于宫内发育迟缓相关文献缺乏,可能有文献选择偏倚。
结论:男性LBW是CKD的高危因素。HBW与生后CKD发生无明显关联。
[1]Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics, 2012,129(6):1019-1026
[2]Barker DJP, Bull AR, Osmond C, et al. Fetal and placental size and risk of hypertension in adult life. BMJ, 1990,301(6746):259-262
[3]Phipps K, Barker DJP, Hales CN, et al. Fetal growth and impaired glucose tolerance in men and women. Diabetologia, 1993,36(3):225-228
[4]Barker DJP, Martyn CN, Osmond C, et al. Growth in utero and serum cholesterol concentrations in adult life. BMJ, 1993,307(6918):1524-1527
[5]Chen J, Chen P, Bo T, et al. Cognitive and behavioral outcomes of intrauterine growth restriction school-age children. Pediatrics, 2016,137(4) , pii: e20153868
[6]Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev, 2011,12(7):525-542
[7]Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics, 2005,115(3):e290-e296
[8]Woo KT, Choong HL, Wong KS, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int, 2012,81(10):1044-1045
[9]Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a sy3stematic analysis for the Global Burden of Disease Study 2013. Lancet, 2014,384(9945):766-781
[10]Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet, 2011,377(9765):568-577
[11]Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009,150(9):604-612
[12]Levey AS, Coresh J. Chronic kidney disease. Lancet, 2012,379(9811):165-180
[13]Hughson M, Farris AR, Douglas-Denton R, et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int, 2003,63(6):2113-2122
[15]Hübinette A, Cnattingius S, Ekbom A, et al. Birthweight, early environment, and genetics: a study of twins discordant for acute myocardial infarction. Lancet, 2001,357(9273):1997-2001
[16]Langley-Evans SC. Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr, 2000,51(1):11-17
[17]Kwong WY, Wild AE, Roberts P, et al. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programmingof postnatal hypertension. Development, 2000,127(19):4195-4202
[18]Roseboom TJ, van der Meulen JHP, Ravelli AC, et al. Effects of prenatal exposure to the dutch famine on adult disease in later life: an overview. Twin Res, 2001,4(5):293-298
[19]Giapros V, Drougia A, Hotoura E, et al. Kidney growth in small-for-gestational-age infants: evidence of early accelerated renal growth. Nephrol Dial Transplant, 2006,21(12):3422-3427
[20]La Batide-Alanore A, Trégou⊇t DA, Jaquet D, et al. Familial aggregation of fetal growth restriction in a French cohort of 7,822 term births between 1971 and 1985. Am J Epidemiol, 2002,156(2):180-187
[21]Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 2000,283(15):2008-2012
[22]Doi SA, Thalib L. An alternative quality adjustor for the quality effects model for meta-analysis. Epidemiology, 2009,20(2):314
[23]Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology, 2008,19(1):94-100
[24]Dougherty DM, Shapiro GG, Dougherty DM, et al. Agency for Healthcare Research and Quality: International Conference of the IEEE Engineering in Medicine & Biology Society, 2008
[25]Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol, 1998,148(7):650-656
[26]Rudberg S, Stattin EL, Dahlquist G. Familial and perinatal risk factors for micro- and macroalbuminuria in young IDDM patients. Diabetes,1998,47(7):1121-1126
[27]Hoy WE, Rees M, Kile E, et al. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int, 1999,56(3):1072-1077
[28]Lackland DT, Bendall HE, Osmond C, et al. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med, 2000,160(10):1472-1476
[29]Ramirez SP, Hsu SI, McClellan W. Low body weight is a risk factor for proteinuria in multiracial Southeast Asian pediatric population. Am J Kidney Dis, 2001,38(5):1045-1054
[30]Dyck R, Klomp H, Tan L, et al. An association of maternal age and birth weight with end-stage renal disease in Saskatchewan. Sub-analysis of registered Indians and those with diabetes. Am J Nephrol, 2003,23(6):395-402
[31]Fan ZJ, Lackland DT, Lipsitz SR, et al. The association of low birthweight and chronic renal failure among Medicaid young adults with diabetes and/or hypertension. Public Health Rep, 2006,121(3):239-244
[32]Salmi IA, Hoy WE, Wang Z , et al. Adult chronic kidney disease patients have lower birth weight than the general Australian population. Early Hum Dev, 2007, 83(7):S109-S109
[33]Li S, Chen SC, Shlipak M, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int, 2008,73(5):637-642[34]Hsu CW, Yamamoto KT, Henry RK, et al. Prenatal risk factors for childhood CKD. J Am Soc Nephrol, 2014,25(9):2105-2111
[35]Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens, 1993,2(5):691-695
[36]Hinchliffe SA, Sargent PH, Howard CV, et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest, 1991,64(6):777-784
[37]Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis, 1994,23(2):171-175
[38]Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens, 1988,1(4 Pt 1):335-347
[39]Das SK, Mannan M, Faruque AS, et al. Effect of birth weight on adulthood renal function: A bias-adjusted meta-analytic approach. Nephrology (Carlton), 2016,21(7):547-565
[40]Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in AfricanAmericans and white subjects in the southeastern United States. Kidney Int, 2006,69(4):671-678
[41]Blush J, Lei J, Ju W, et al. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int, 2004,66(6):2148-2154
[42]Reyes D, Lew SQ, Kimmel PL. Gender differences in hypertension and kidney disease. Med Clin North Am, 2005,89(3):613-630.
(本文编辑:张崇凡,孙晋枫)
The relationship between birth weight and chronic kidney disease:a systematic review and meta-analysis
ZHANGRuo-lin,SHUAILan-jun,LIXiao-yan,CHENHai-xia,WANGYing,HEQing-nan
(LaboratoryofPediatricNephrology,InstituteofPediatrics,CentralSouthUniversity,TheSecondXiang-YaHospital,Changsha410011,China)
Corresponding Author: HE Qing-nan, E-mail: heqn2629@163.com
ObjectiveTo systematically review the association between birth weight with risk of the chronic kidney disease.MethodsThe Cochrane Library, MEDLINE, OVID, Springer, VIP, WangFang Data and CNKI up to June 30, 2016 were searched to retrieve the online observation studies about birth weight and chronic kidney disease and other relative outcomes. The articles were selected if they were published in English or Chinese regarding the association between birth weight and CKD, which included the exact information on OR and 95%CI. Excluded studies: Studies that assessed the final outcome among neonates or children less than 1 year old, the participants of studies who were infected in utero, congenital abnormalities, the mothers who were exposed to toxins, as well as the incompletely information literature. Two reviewers inspected the included studies on the association between birth weight and chronic kidney disease independently. Quality assessment was performed based on the Newcastle Ottawa Scale( case-control study and cohort study) and AHRQ(cross-sectional study). OR and 95%CI were extracted from the selected articles and then analyzed with Stata 12.0 software by the way of DerSimonian & Laird.ResultsTen observational studies containing four case-control studies(three articles in 8 points and one article in 7 points ), four cohort studies(three articles in 7 points and one article in 8 points ) and two cross-sectional studies(7 points and 8 points for the two articles ) were selected finally. The overall combination of weighted estimates from the selected 10 studies about low birth weight associated with risk of the chronic kidney disease was 1.80 (1.37-2.35). The risks of albuminuria (OR, 2.58; 95% CI, 1.49-4.46), end-stage renal disease (OR, 1.42; 95% CI, 1.22-1.66), or lower estimated glomerular filtration rate (OR, 1.87; 95% CI, 1.19-2.94) were similar with the chronic kidney disease. However,according to the subgroup analysis that the combined OR of high birth weight between end-stage renal disease and lower estimated glomerular filtration rate was OR, 1.19; 95% CI, 0.94-1.49 and OR, 0.09; 95% CI, 0.93-1.27,which showed no significant impact. In the subgroup analysis by gender,increased risk was only found in men:1.83 (1.10-3.05),unexpectedly.Finally, the Egger regression method showed no publication bias.ConclusionOnly men with low birth weight had a greater CKD risk in later life. And the high birth weight did not show any significant impact on CKD.
Birth weight; Chronic kidney disease; Albuminuria; Observation study; Meta-analysis
中南大学湘雅二医院儿科 长沙,410011
何庆南 ,E-mail: heqn2629@163.com
10.3969/j.issn.1673-5501.2017.02.003
2017-03-07
2017-04-18)
