Synthesis and characterization of 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl grafted polysiloxane as stationary phase for gas chromatography
2017-04-10WANGHuanHANXueHEXinxinWANGBingWUBo
WANG Huan, HAN Xue, HE Xinxin, WANG Bing, WU Bo
(School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China)
Article
Synthesis and characterization of 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl grafted polysiloxane as stationary phase for gas chromatography
WANG Huan, HAN Xue, HE Xinxin, WANG Bing, WU Bo*
(SchoolofChemistryandChemicalEngineering,ShandongUniversity,Jinan250100,China)
A new high-temperature stationary phase called 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl grafted polysiloxane (DPFP) was synthesized and statically coated on fused-silica capillary columns. The chromatogram of the polyethylene pyrolysis products showed that the DPFP column still owned good separation ability until 360 ℃. The column efficiency of the DPFP was 3 324 plates/m (retention factor (k)=4.24, naphthalene, 0.25 mm i. d.). McReynolds constants revealed that the polarity of the stationary phase was moderate. Abraham system constants indicated that DPFP possessed strong dipole-induced dipole interactions and H-bond acceptance with analytes. Analysis of Grob test mixtures showed good selectivity of DPFP and inner-surface inertness of the column. Furthermore, the aromatic isomers, substituted benzenes, polycyclic aromatic hydrocarbons, and fatty acid esters could be well separated on the column, thereby indicating that DPFP had great potential for application.
polysiloxanes; gas chromatography (GC); stationary phases; thermal stability; selectivity
In the past a few decades, capillary gas chromatography (GC) has been widely used in petroleum chemical industry, pesticide analysis and environment monitoring fields [1-4]; different kinds of polysiloxane stationary phases have been synthesized to cater to the demands in GC [5-6]. Peaden et al. [7] synthesized a series of functionalized polysiloxanes containing polarizable biphenyl, naphthyl, and phenoxy phenyl side groups for GC separations. Relying on the “softer” dipole-induced dipole interactions with the analytes, these stationary phases were good candidates for a number of isomer separation. However, the molecular weight of the functionalized polysiloxanes was not high enough, which led to poor thermal-stability below 280 ℃. Takayama et al. [8] synthesized a high-temperature siloxane polymer stationary phase that contained silarylene grafted to the main chain. This phase decomposed at 380 ℃, which indicated that silarylene-containing moieties began to be eliminated and that the stationary phase became highly cross-linked and solidified. Mayer et al. [9] synthesized 75% diphenyl and 25% dimethyl polysiloxane (SOP-75), which was coated on a fused silica capillary for high-temperature GC. Accordingly, the phase offered particular selectivity and high inertness up to 400 ℃. However, the high content of rigid diphenyl weakened its coating efficiency and column efficiency.
According to the aforementioned researches, the high content of aromatic rings introduced into the backbone or side chain of polysiloxanes is extremely important to the thermal stability and selectivity of the polysiloxanes stationary phases; however, their presence may lead to structure stiffness. To avoid this problem, the present study has synthesized a series of multiphenyl rings instead of single benzene rings as side groups. Zhao et al. [10] reported diphenyl-phenyl polysiloxanes as a GC stationary phase. A wide operating temperature range (30-330 ℃) and unique selectivity were obtained by the introduction of the diphenyl-phenyl group. Then a 3,4-di(trifluoromethyl phenyl)-2,5-diphenyl phenyl grafted polysiloxane was also synthesized to strengthen the polarity and selectivity of the stationary phase, thus leading to better separation performance for polar compounds and aromatic isomers [11].
A higher number of conjugate aromatic rings leads to a higher average molecular polarizability of side groups. The average molecular polarizability of 2,3-di(3-phenoxy-4-fluoro phenyl)-1,4-diphenyl phenyl with seven aromatic rings was 6.53×10-2nm3(calculated according to Ref. [12]). This value was much higher than those of benzene (1.04×10-2nm3), biphenyl (2.00×10-2nm3), diphenyl-phenyl (2.97×10-2nm3), and 2,3-di(trifluoromethyl phenyl)-1,4-diphenyl phenyl (5.10×10-2nm3). When 2,3-di(3-phenoxy-4-fluoro phenyl)-1,4-diphenyl phenyl is used as a side chain grafted onto the backbone of polysiloxanes, the increased polarizability will strengthen the dipole-induced dipole interactions between the grafted polysiloxanes and polar compounds. The side chain will also strengthen its dispersive interaction with nonpolar compounds.
In this study, a novel 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl grafed polysiloxane (DPFP) stationary phase was synthesized. Then the phase was coated on the inner of the fused-silica capillary column. The column was evaluated for its thermal stability, column efficiency, polarity, selectivity, and repeatability. After that, disubstituted benzene isomers, polycyclic aromatic mixtures, polycyclic aromatic hydrocarbons (PAHs), fatty acid esters (FAEs), and fatty alcohols were used to test the DPFP column separation capability.
1 Experimental
1.1 Apparatus and reagents
The apparatus used for the column evaluation was a GC-2014C (Shimadzu Scientific Instruments Inc, Kyoto, Japan) equipped with a split/splitless injector and a flame ionization detector. The carrier gas was nitrogen (N2, 99.999%). The column efficiency was evaluated by measuring the number of plates per meter for naphthalene at 120 ℃.1H nuclear magnetic resonance (NMR) spectra in CDCl3were recorded on a DPX 300 spectrometer (Bruker Analytische Messtechnik, Karlsruhe, Germany). FT-IR was evaluated on an FTIR-8400S (Shimadzu Scientific Instruments Inc, Kyoto, Japan). Thermal stability was evaluated on an LCT-2 themogravimetric analysis (TGA) system (Beijing Optical Instrument Factory, Beijing, China).
All the chemicals or reagents used in this study, except 3-phenyoxyl-4-fluoro-benzaldehyde, were of analytical grade, and they were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). 3-Phenyoxyl-4-fluoro-benzaldehyde was industrial grade; it was purchased from Shanghai Lanrun Chemical Co., Ltd. (Shanghai, China). Octamethylcyclotetra-siloxane and 2,4,6,8-tetravinyl-2,4,6,8-tetramethylcyclotet-rasiloxane were purchased from Dow Corning Corporation (Michigan, USA). Grob test mixtures were prepared according to the procedure mentioned in Ref. [13]. Polyethylene pyrolysis samples were prepared according to Ref. [14]. Fused silica capillary tubes (0.25 mm i.d.) were produced in our laboratory from fiber-level (SiO2, 99.999%) raw tubes by using a self-made drawing machine. The commercial DB-17 capillary column (30 m×0.25 mm i. d., film thickness 0.50 μm) was purchased from Agilent Technologies (Palo Alto, USA).
1.2 Polymer synthesis
DPFP was prepared via the Diels-Alder reaction, as shown in Fig. 1.
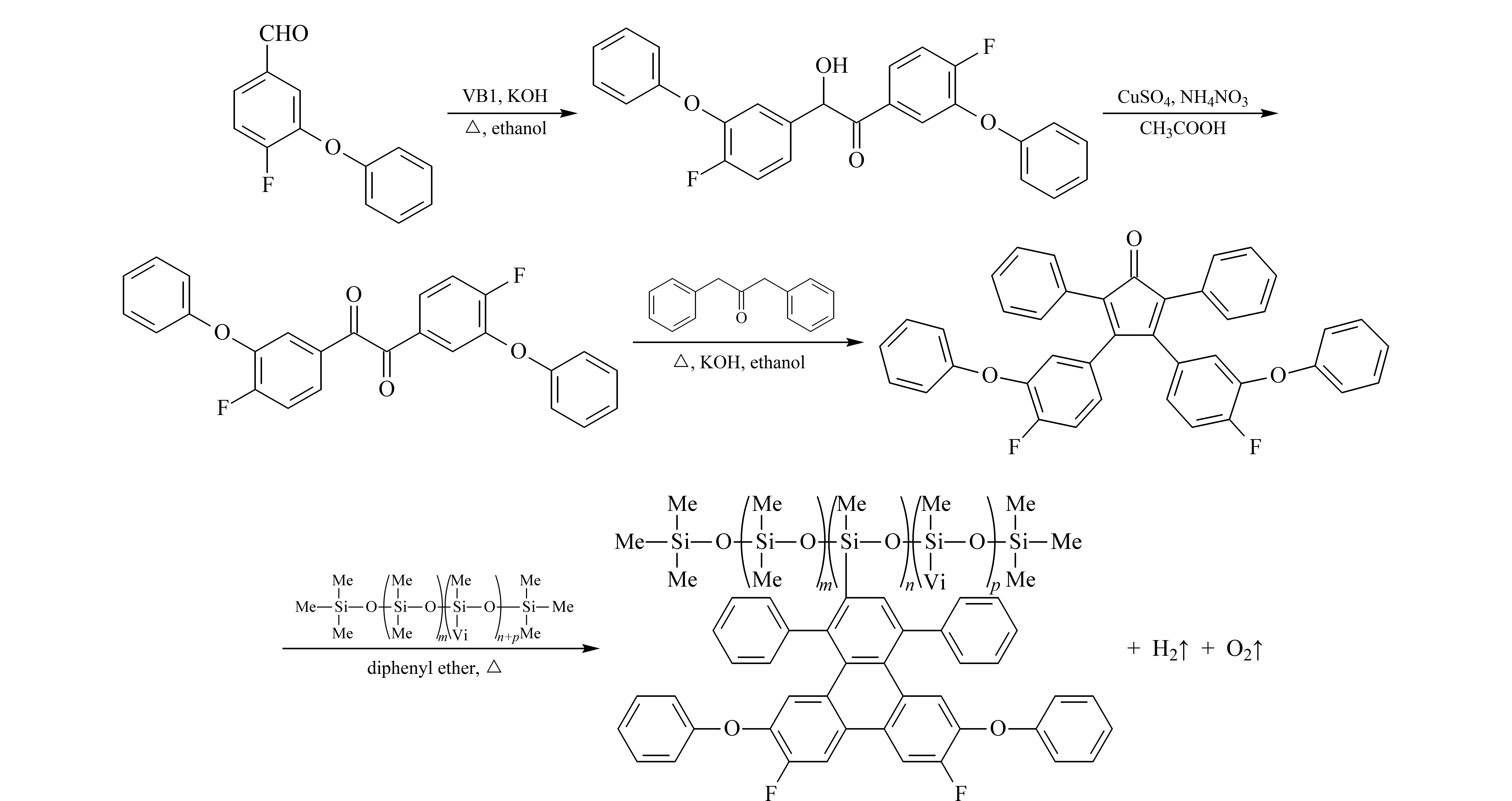
Fig. 1 Synthesis routes of DPFPMe: CH3; Vi: CH=CH2.
1.2.1 Synthesis of 1,2-di(3-phenoxy-4-fluoro phenyl)-2-hydroxyethan-one
3-Phenyoxy-4-fluoro-benzaldehyde of 17.3 g (80 mmol) and thiamine hydrochloride (VB1) of 2.0 g (6.5 mmol) were dissolved in 25.0 mL of KOH ethanol solution (1.0 mol/L) under a nitrogen atmosphere. The reaction was run at 60 ℃ for 2 h and filtered after cooling. The crude product was recrystallized using ethanol. Light yellow crystal product (13.0 g) was obtained, and the yield was about 75% (melting point (m. p.): 132.1-133.4 ℃).
1.2.2 Synthesis of 3,3′-diphenoxy-4,4′-difluorobenzil
1,2-Di(3-phenoxy-4-fluoro phenyl)-2-hydroxy-ethanone at 9.9 g (23 mmol), anhydrous cupric sulfate at 0.8 g (4 mmol), and ammonium nitrate at 2.0 g (25 mmol) were dissolved in 35.0 mL of 80% (v/v) acetic acid. The reaction was refluxed at 140 ℃ for 90 min and filtered after cooling. The crude product was recrystallized using ethanol. Yellow crystal product (7.8 g) was obtained, and the yield was about 79% (m. p.: 165.4-166.7 ℃).
1.2.3 Synthesis of 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenylcyclo-pentadienone
3,3′-Diphenoxy-4,4′-difluorobenzil of 7.0 g (16 mmol) and 1,3-diphenylacetone of 3.7 g (18 mmol) were dissolved in 30.0 mL of ethanol under a nitrogen atmosphere. KOH ethanol solution (3 mol/L) at 2.5 mL was added into the solution. The mixture was stirred at boiling temperature for 45 min and filtered after cooling. The crude product was recrystallized using ethanol/toluene (1∶1, v/v). Bright black crystals (8.2 g) were obtained, and the yield was about 85% (m.p.: 228.5-229.3 ℃). The structure was analyzed by means of1H NMR (300 MHz, CDCl3): 6.49-6.53 (d, 3H, Ar-H), 6.67-6.71 (d, 2H, Ar-H), 7.01-7.10 (m, 3H, Ar-H), 7.13-7.28 (m, 5H, Ar-H).
1.2.4 Synthesis of DPFP
Methyl vinyl polysiloxanes with a 15% vinyl side chain was obtained according to Ref. [15]. Methyl vinyl polysiloxane (3.38 g, containing 6.6 mmol vinyl side chain) and 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenylcyclopentadienone (6.0 g, 9.9 mmol) were dissolved in 50.0 mL of diphenyl ether and heated at 240 ℃ for 48 h under a nitrogen atmosphere. The solution was opaque black purple, and numerous bubbles were formed. The reaction was terminated until the solution turned to transparent burgundy and without bubbles. After cooling, the solution was divided into two layers, and the upper diphenyl ether layer was removed to obtain the polymer. For purification, the product was dissolved in 10.0 mL of toluene and precipitated with 25.0 mL of methanol. This step was repeated thrice. Finally, the solvent was evaporated by vacuum distillation to obtain a clear, pale yellowish highly-viscous gum. The yield of gum (4.7 g) obtained was about 65.8%. The structure of the DPFP was analyzed by FT-IR and1H NMR, FT-IR (KBr): 3 064.3 cm-1[γ(Ar-H)]; 2 962.3, 1 511.4 cm-1[γ(C-H)]; 1 589.4, 1 410.5 cm-1[γ(CH=CH2)]; 1 490.2 cm-1[γ(C-F)]; 1 261.0, 802.4 cm-1[γ(Si-CH3)]; 1 213.8 cm-1[γ(C-O-C)]; 1 106.2, 1 022.3 cm-1[γ(O-Si-O)]; 700.5 cm-1[(δ(Ar-H)].1H NMR (CDCl3, 300 MHz,δ): 6.48-6.52 (m, 12H, Ar-H), 6.85-6.94 (m, 8H, Ar-H), 7.01-7.06 (m, 12H, Ar-H), 7.20-7.25 (m, 20H, Ar-H), 7.54 (s, 2H, Ar-H). Peaks at 5.99 and 0.069 were respectively attributed to vinyl and C-H on Si atoms in the skeleton of polysiloxane. The results of gel permeation chromatography showed the number of average molecular weight (Mn) was 1.20×105with the average molecular weight ratio (Mw/Mn) of 1.51.
1.3 Capillary column preparation
The fused silica capillary column (30 m×0.25 mm i. d.) was treated according to Ref. [16]. Before coating, the capillary tube was rinsed with 5.0 mL of dichloromethane. Then it was purged with nitrogen at 260 ℃ for 1 h. The fused silica capillary column was statically coated with a solution of DPFP (0.02 g) in 2.5 mL dichloromethane (containing 5.0% (wdicumylperoxide/wDPFP) dicumylperoxide as a free radical initiator). After the coating procedure, the column was conditioned from 50 to 160 ℃ (maintained for 2 h) at 1 ℃/min and then to 370 ℃ at 1 ℃/min (maintained for 24 h) under a nitrogen flow. The film thickness of the capillary column used in this work was calculated to be 0.50 μm [17]. The 10 m-long DPFP column was prepared by using a similar method.
2 Results and Discussion
2.1 Polymer characterization by NMR spectroscopy
Based on1H NMR data, the DPFP contained about 12.5% 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl groups and was estimated from the ratio of integral atδ6.43-7.54 (attributed to Ar-H) to that at aboutδ0.069×10-6(0.069 ppm) (attributed to Si-CH3) [18]. The contents of the remaining vinyl groups were determined to be 2.4%, which divided the integral atδ5.99 ppm (attributed to CH=CH2) with that at aboutδ0.069 ppm (attributed to Si-CH3).
2.2 Thermal stability of DPFP
To evaluate the thermal stability of the DPFP polymer, TGA was conducted. As shown in Fig. 2, the DPFP began to decompose slightly at 390 ℃. After reaching 420 ℃, the DPFP began to show more drastic weight loss.
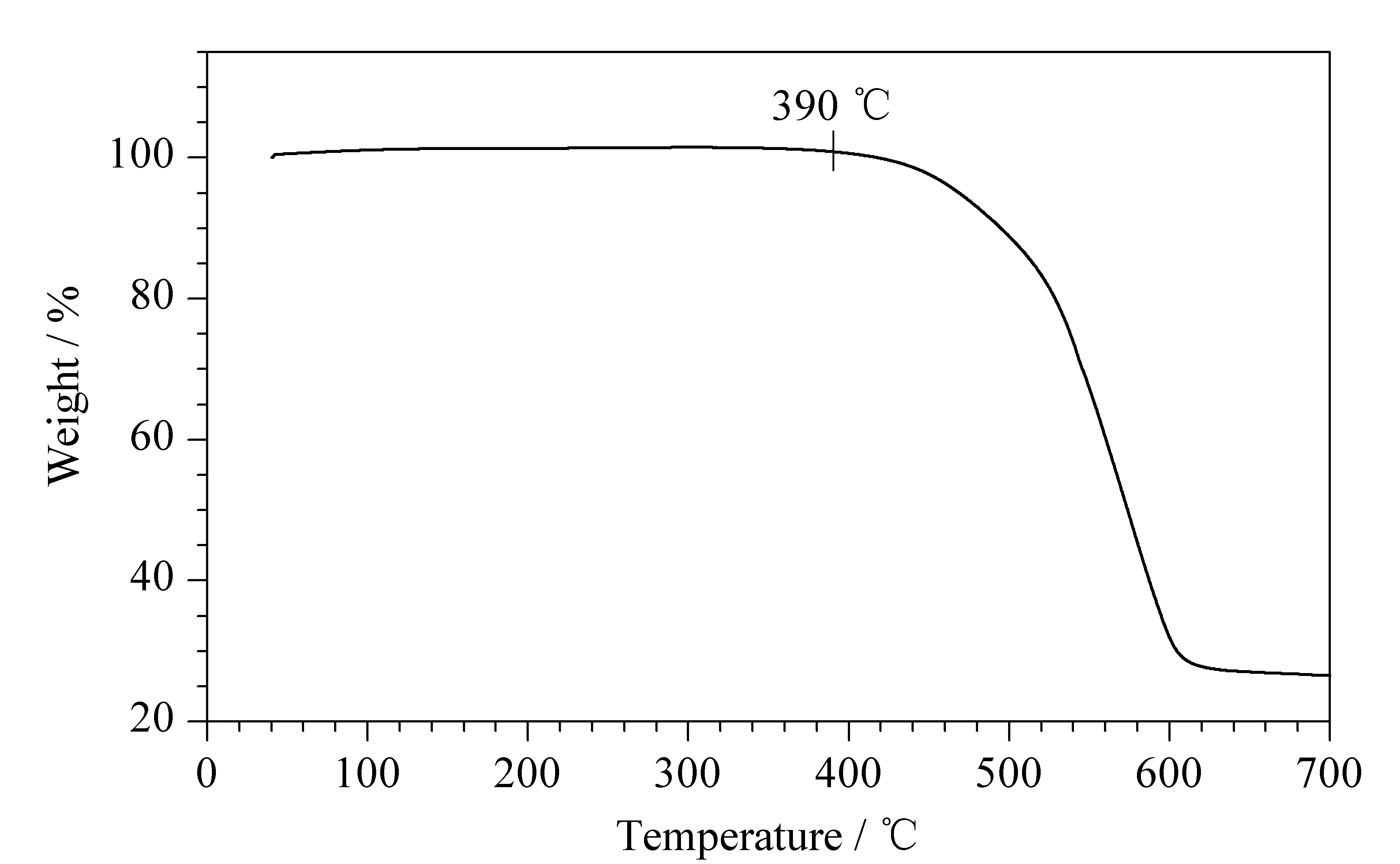
Fig. 2 TGA curve of DPFP polymer The weight of sample was 10 mg and the operating temperature range was from 25 to 700 ℃ at a rate of 10 ℃/min under helium atmosphere.
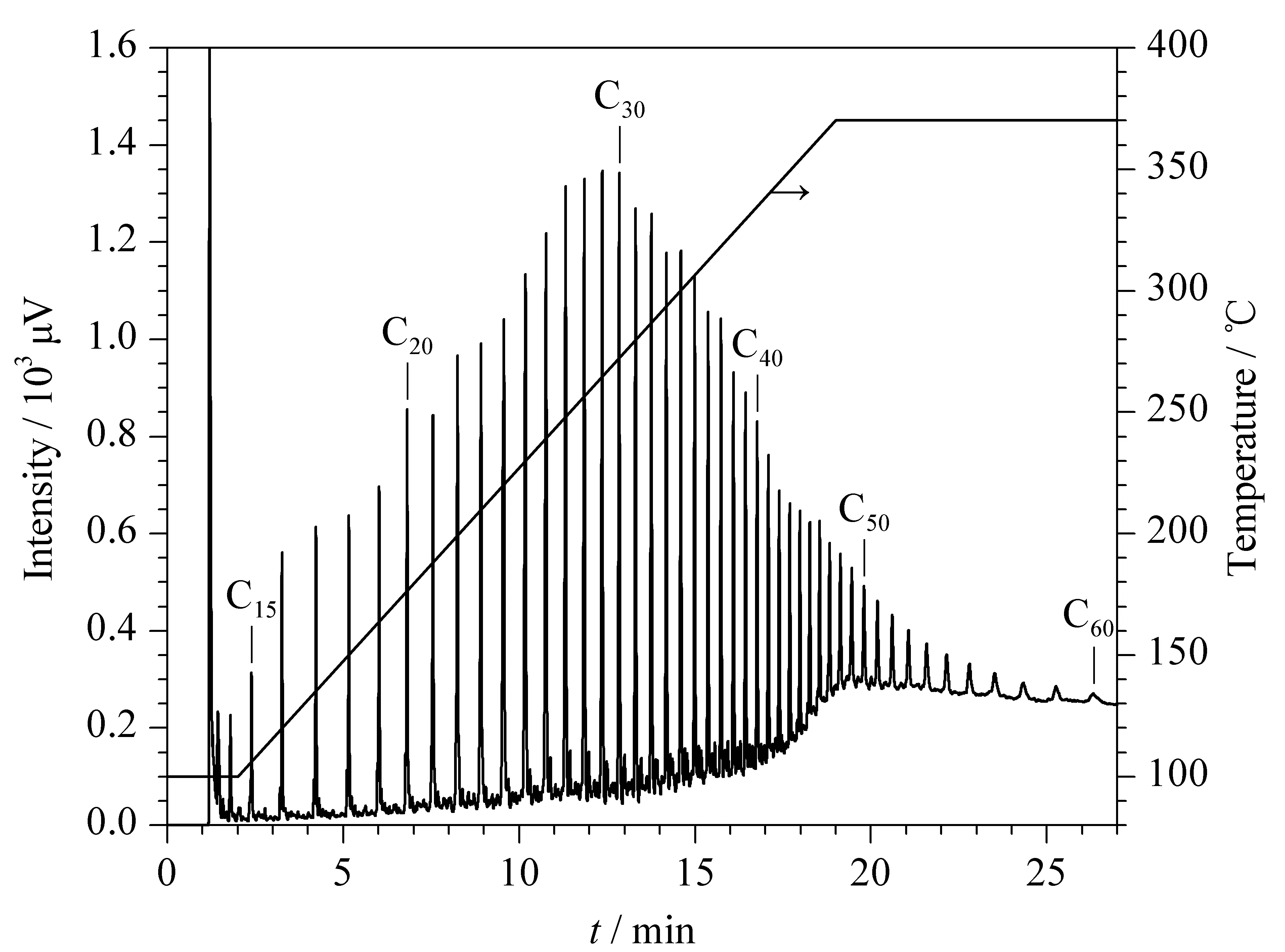
Fig. 3 GC separation of the polyethylene pyrolysis products on the DPFP column Conditions: N2 linear velocity, 14 cm/s; 100 ℃ (maintained for 2 min) increased to 370 ℃ at 15 ℃/min (maintained for 7 min); injection split ratio, 30∶1; both injector and detector temperatures, 400 ℃. Peaks: hydrocarbons, from C13 to C60.
To provide further evidence of the temperature resistance of the DPFP column, the polyethylene pyrolysis products were separated on the DPFP column (10 m×0.25 mm i. d.). As shown in Fig. 3, the polyethylene pyrolysis products were baseline separated. All compounds were well separated with almost symmetrical peak shapes within 27 min. The DPFP column was still effective for separating pyrolysis products when aged at 370 ℃ for 24 h.
2.3 Column efficiency and selectivity of DPFP
The efficiency of the column was evaluated by measuring the number of plates per meter at 120 ℃ for naphthalene at a linear velocity of 10 cm/s. The column efficiency of the DPFP column was 3 324 plates/m (k=4.24, naphthalene, 0.25 mm i. d.), and the coating efficiency of the DPFP column was 68.7% [19]. Degree of cross-linking was 81.2%, and this was calculated from the capacity factor after rinsing with 5.0 mL of dichloromethane divided by that of the column tested previously [10].
The chromatogram of Grob test mixtures is shown in Fig. 4. The Grob test is well-recognized for comprehensive evaluation of separation performance of a column. The figure showed that all compounds were well-separated, indicating that this new stationary phase had good film-forming capability. The elution order of Grob test mixtures on the DPFP column was similar to that on OV-17 (50% diphenyl-50% dimethyl polysiloxane) [20] except for am, which was eluted after E11. Notably, A was eluted after P, this result revealed that the new stationary phase retained alkaline analytes more strongly than acidic ones. The tailing factor of peak ol on the DPFP column was 1.19, thereby indicating that hydrogen bonding adsorption could be formed on the fused-silica capillary in the DPFP column.

Fig. 4 Chromatogram of Grob test mixture on DPFP column Conditions: N2 linear velocity, 12 cm/s; 40 to 160 ℃ at 3 ℃/min; injection split ratio, 30∶1; both injector and detector temperatures, 200 ℃. Peaks: 1. hexane; 2. trichloromethane; D. 2,3-butanediol; C10. decane; C11. undecane; ol. 1-octanol; al. nonanal; S. 2-ethylhexanoic acid; P. 2,6-dimethylphenol; A. 2,6-dimethylaniline; E10. methyl decanoate; E11. methyl undecanoate; am. dicyclohexylamine; E12. methyldodecanoate.
2.4 McReynolds constants and Abraham system constants
Rohrschneider-McReynolds constants were commonly used to characterize the polarity of a GC stationary phase. Each probe molecule interacted with the stationary phase in a particular way:X′, dispersive interactions;Y′, proton donor and acceptor capabilities;Z′, dipolar interactions and weak proton acceptor;U′, dipolar interactions;S′, strong proton acceptor capabilities. Table 1 lists the Rohrschneider-McReynolds constants of DPFP, DB-17, FPP (3,4-bis(4-fluorophenyl)-2,5-diphenyl polysiloxane) and TFPP (3,4-bis(3,4,5-trifluoro phenyl)-2,5-diphenyl polysiloxane) at 120 ℃ [21,22]. The CP-index(which was calculated from the sum of the first 5 Rohrschneider-McReynolds constants divided by the sum of the Rohrschneider-McReynolds constants of OV-275 multiplied by 100) of DPFP was 19.4, thereby indicating that the polarity of DPFP was moderate. The polarity of DPFP was higher than that of FPP and TFPP, but lower than DB-17. Given that the values ofU′,Z′, andS′ were relatively high, the major interactions between analytes and the DPFP stationary phase were dipolar interactions and H-bond acceptor interactions.
The Abraham solvation parameter model can offer a straightforward approach to evaluate the individual inter-molecular interactions between probe compounds and GC stationary phases [23,24]. Table 2 lists the Abraham system constants of DPFP, DB-17 and TFPP at 80, 100, and 120 ℃ [22,25]. The statistical data in Table 2 prove that the DPFP stationary phase promotes acidic non-hydrogen bonding (b=0). Compared with DB-17, DPFP exhibited similare,s, andlvalues but much higheravalue, thereby suggesting its stronger H-bond basicity interactions with analytes. Moreover, the much highere,svalues were obtained on DPFP than TFPP, which may mainly result from the increased polarizability of 3,4-di(3-phenoxy-4-fluoro phenyl)-2,5-diphenyl phenyl group. Thus DPFP is expected to have better separation performance for polar and have easily polarizable compounds than DB-17 and TFPP columns.

Table 1 Rohrschneider-McReynolds constants of DPFP, DB-17, FPP and TFPP stationary phases
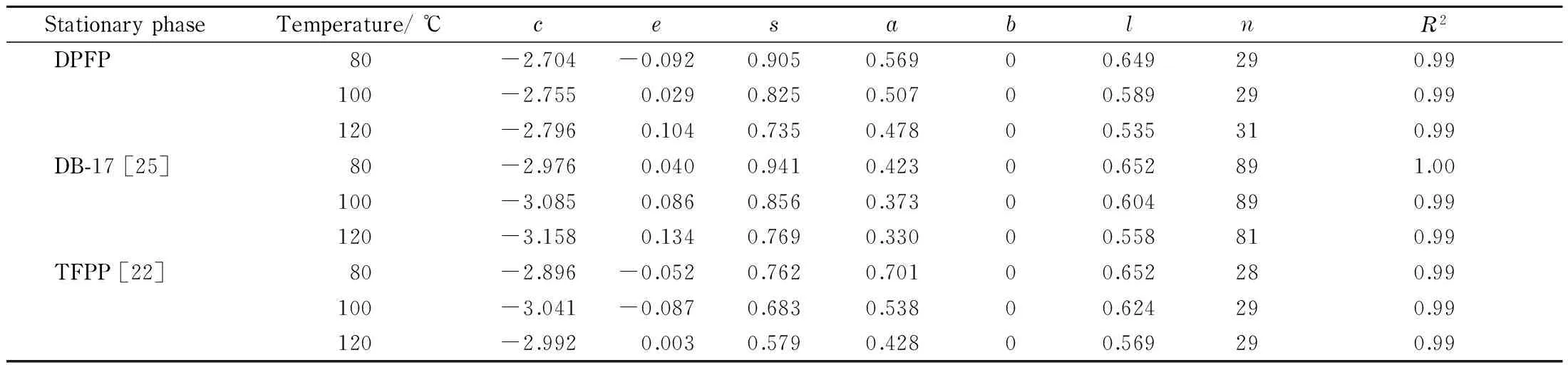
Table 2 Abraham system constants of the DPFP, DB-17 and TFPP stationary phases
cis a system constant without any meaning;e,s,a,b,l,n, andR2stand forπ-π/n-πinteractions, dipole/polarizable interactions, H-bond basicity, H-bond acidity, dispersive interactions, number of solutes used in multiple linear regression, and coefficient of determination, respectively.

Table 3 Separation of aromatic isomers on the DPFP capillary column
α: relative retention.d: 2,4- difluorobenzaldehyde,e: 2,5- difluorobenzaldehyde,f: 3,5-difluorobenzaldehyde.
2.5 Applications
The retention factors and relative retention values of the disubstituted benzene isomers on DPFP columns are shown in Table 3. All positional isomers were eluted according to their boiling points. The tested weak polar isomers, such as xylenes, achieved better separation on the DPFP column than on OV-1701 (7% cyanopropyl-7% phenyl polysiloxane) and SOP-50-Octyl (50%n-octylmethyl-50% diphenyl polysiloxane) [18,26]. Moreover, the tested strong polar isomers, such as nitroaniline, were separated more efficiently than the weak polar isomers, which may be a result of the sensitive dipole-induced dipole interactions between DPFP and polar solutes.
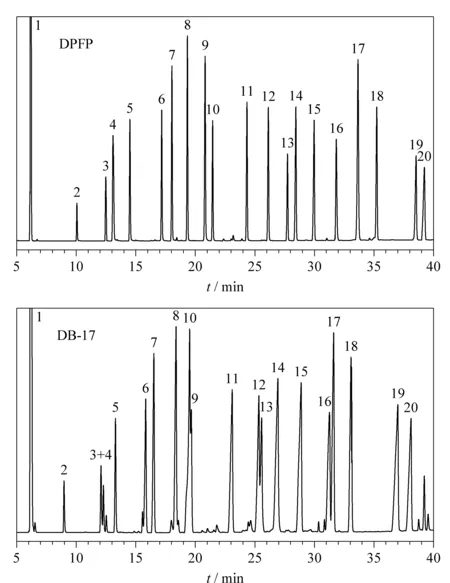
Fig. 5 Chromatograms of substituted benzenes on DPFP column Conditions: N2 linear velocity, 10 cm/s; 70 ℃ (maintained for 5 min) increased to 250 ℃ at 5 ℃/min; injection split ratio, 30∶1; both injector and detector temperatures, 300 ℃. Peaks: 1. benzene; 2. toluene; 3. ethylbenzene; 4. chlorobenzene; 5. m-xylene; 6. p-methylchlorobenzene; 7. m-dichlorobenzene; 8. 1,2,4-trimethylbenzene; 9. nitrobenzene; 10. butylbenzene; 11. o-methylnitrobenzene; 12. o-ethylnitrobenzene; 13. p-dibromobenzene; 14. p-methylnitrobenzene; 15. p-nitrochlorobenzene; 16. p-nitrobromobenzene; 17. biphenyl; 18. diphenylmethane; 19. 1-methyl-2,4-dinitrobenzene; 20. 1-chloro-2,4-dinitrobenzene.
The separations of the substituted benzene mixtures on DPFP and DB-17 are illustrated in Fig. 5. It could be seen that all tested substituted benzene mixtures were well separated with narrow and symmetrical peaks on DPFP, while it could not be done on DB-17. This result is related to the multiphenyl-phenyl group grafted on the side chain of polysiloxane with bulky mobileπ-bonding electrons. The fluorine introduced to the polycyclic phenyl group will strengthen the polarity and selectivity of the stationary phase and provide a more abundant function mechanism. The unique structure of the new stationary phase was able to strengthen both the dipole-induced dipole interaction with polar solutes as well as the dispersive interaction with nonpolar solutes.
Meanwhile, Fig. 6 shows the chromatogram of PAHs on the DPFP column in which baseline separation could be achieved. Even though the boiling points of the substances were nearly the same, they could also be completely separated, as seen in peak 10 (o-dihydroacenaphthylene, boiling point (b. p.): 279.0 ℃) and peak 11 (2-ethoxynaphthalene, b. p.: 282.0 ℃). This phenomenon may be a result of the large aromaticπ-conjugate structure of the DPFP, which made the DPFP column had good behavior in the separation of PAHs. Given that most of the PAHs were environmental contaminants, the DPFP column has significant potential applications in environmental analyses.

Fig. 6 Chromatogram of PAHs on DPFP column Conditions: N2 linear velocity, 10 cm/s; 100 ℃ (maintained for 5 min) increased to 320 ℃ at 6 ℃/min; injection split ratio, 30∶1; both injector and detector temperatures, 350 ℃. Peaks: 1. benzene; 2. decahydronaphthalene; 3. tetralin; 4. naphthalene; 5. 1-benzothiophene; 6. 1-methylnaphthalene; 7. biphenyl; 8. diphenylmethane; 9. 8-hydroxyquinoline; 10. o-dihydroacenaphthylene; 11. 2-ethoxynaphthalene; 12. p-naphthoquinone; 13. dibenzylether; 14. 4-hydroxybiphenyl; 15. 1-nitronaphthalene; 16. phenanthrene; 17. benzil; 18. phenanthrenequinone; 19. triphenylmethane; 20. fluoranthene; 21. pyrene; 22. 1-hydroxyanthraquinone; 23. tetraphenylmethane; 24. 1,8-dihydroxyanthraquinone.

Fig. 7 Chromatograms of FAEs on the DPFP column and DB-17 column Conditions: N2 linear velocity, 10 cm/s; 35 ℃ (maintained for 5 min) increased to 160 ℃ at 5 ℃/min, then at 10 ℃/min to 280 ℃; injection split ratio, 30∶1; both injector and detector temperatures, 300 ℃. Peaks: 1. methanol; 2. ethylformate; 3. methylacetate; 4. ethylacetate; 5. 2-methylmethacrylate; 6. n-butylformate; 7. methyl 3,3-dimethacrylate; 8. n-butylacetate; 9. iso-butylacetate; 10. ethyl butyrate; 11. ethyl acetate; 12. iso-amylacetate; 13. n-ethylvalerate; 14. n-butylbutyrate; 15. ethylcaproate; 16. ethylcyanoacetate; 17. carbitolacetate; 18. benzylacetate; 19. methyldecanoate; 20. methyl 10-undecenate; 21. methyldodecanoate; 22. ethylundecylenate.

Fig. 8 Chromatograms of fatty alcohols on the DPFP column and DB-17 column Conditions: N2 linear velocity, 10 cm/s; 35 ℃ (maintained for 8 min) increased to 100 ℃ at 3 ℃/min, then at 5 ℃/min to 300 ℃; injection split ratio, 20∶1; both injector and detector temperatures, 350 ℃. Peaks: 1. methanol; 2. ethylalcohol; 3. iso-propanol; 4. t-butylalcohol; 5. allylalcohol; 6. trichloromethane; 7. n-propanol; 8. 1-methylpropanol; 9. iso-butylalcohol; 10. 2-methyl-2-butanol; 11. 2-methyl-1-propanol; 12. n-butylalcohol; 13. iso-amylalcohol; 14. 3-methyl-1-butanol; 15. n-pentanol; 16. 2-methyl-1-pentanol; 17. n-hexanol; 18. 4-hydroxy-4-methyl-2-pentanone; 19. 3-methyl-2-buten-1-ol; 20. n-heptanol; 21. 2-ethylhexanol; 22. 2-octanol; 23. n-octanol; 24. menthol; 25. n-nonanol; 26. 2-(2-ethoxyethoxy)-ethanol; 27. n-decanol; 28. n-undecanol; 29. laurylalcohol; 30. n-dodecanethiol; 31. myristylalcohol; 32. n-pentadecanol; 33. n-hexadecanol; 34. heptadecanol; 35. stearylalcohol; 36. oleoylalcohol; 37. n-icosanol.
To further evaluate the separation ability of DPFP, FAEs and fatty alcohols were separated on the DPFP and the commercial DB-17 under same conditions. Results of the comparison are shown in Figs. 7 and 8.
Fig. 7 shows the separation results of FAEs. All compounds were well-separated, with sharp and symmetric peak shapes on the DPFP column except peak 14 (n-butyl butyrate, b. p.: 166.5 ℃) and peak 15 (ethyl caproate, b. p.: 167.0 ℃). Furthermore, the results show a much better resolution for the analytes than the commercial DB-17 column that failed to resolve some peak pairs of the analytes, i. e., peak 17 (benzylacetate, b. p.: 206.0 ℃) and peak 18 (carbitolacetate, b. p.: 218.0 ℃). In addition, the elution orders of peak 17 and peak 18 on the DB-17 column were in opposition to those on the DPFP column. Moreover, the DPFP column exhibited different retention behaviors from the commercial column; the weak polar analytes exhibited shorter retentions on DB-17. Overall, the results indicated that this new stationary phase has the advantage over commercial columns because of the separation of the weak polar substances.
Additionally, Fig. 8 shows the chromatogram of fatty alcohols. That baseline separation of most components was achieved indicates the high separation efficiency of the DPFP column. The peaks of alcohols were slightly tailed, which could be attributed to the hydrogen bond basicity of the DPFP stationary phase. Compared with the column DPFP, several peaks could not be separated well on DB-17, i. e., peak 7 (n-propanol, b. p.: 97.2 ℃) and peak 8 (1-methylpropanol, b. p.: 98.0 ℃), peak 16 (2-methyl-1-pentanol, b. p.: 148.0 ℃) and peak 17 (n-hexanol, b. p.: 155.8 ℃), which indicates that the DPFP column has a higher selectivity. In addition, the elution time of the fatty alcohols on DB-17 was shorter than that on DPFP. This result might be related to the unique structure of the new stationary phase, which could increase dipole-induced dipole interaction and H-bonding interactions with the alcohols. Furthermore, the DB-17 column began to show a slight baseline drift at 300 ℃, whereas the DPFP column showed a smooth baseline under the same condition. The above results suggest that the new DPFP stationary phase has a superior separation performance to the commercial one.
2.6 Column repeatability
Table 4 shows the repeatability of retention times of the analytes after the column was conditioned up to the indicated temperatures for 6 h. As shown, the retention times of almost all the analytes remained almost unchanged with the relative standard deviation (RSDs) not more than 2.51%. Moreover, with RSDs of retention time not more than 4.58% for column-to-column, the DPFP columns were well repeatable and reproducible.
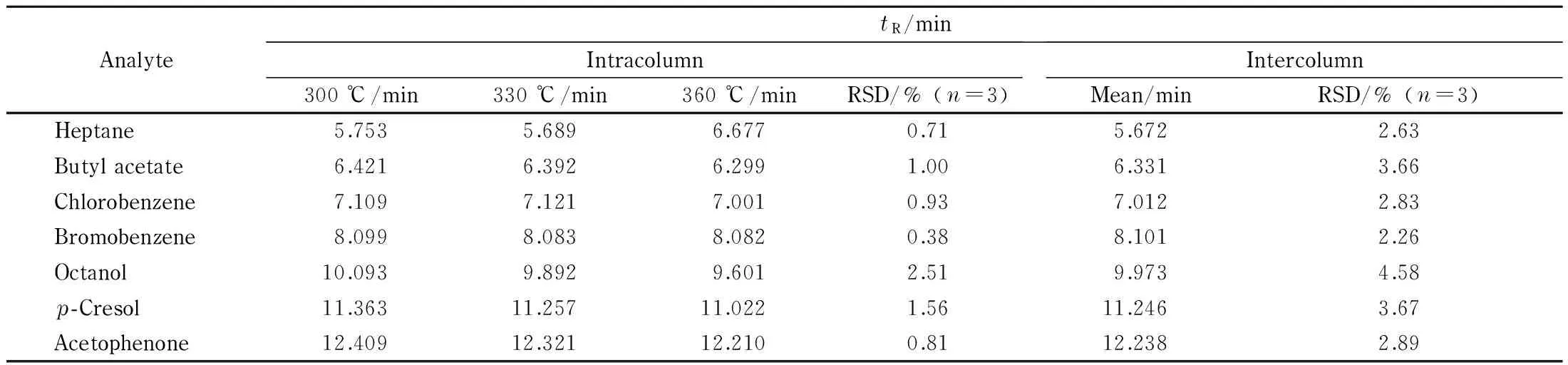
Table 4 Separation repeatability of DPFP column
3 Conclusions
In this study, DPFP was synthesized and coated on the inside wall of the fused-silica capillary columns. It exhibited good coating ability, high thermal stability, and good separation repeatability. McReynolds constants indicated that the polarity of the stationary phase was moderate. Moreover, the solvation parameter evaluation showed that the DPFP stationary phase exhibited strong dipole-induced dipole interactions and H-bond acceptor. However, it had slightly weak dispersive interactions with solutes. The excellent selectivity of the DPFP column can be used to separate substituted benzenes, PAHs, FAEs, and fatty alcohols. This extensive applicability suggests that the DPFP stationary phase has a good application potential in GC.
[1] Liu Y R, Jiang J J, Liu Z L, et al. Chinese Journal of Chromatography, 2016, 34(2): 215
[2] Liu X Q, Tong L, Meng W T, et al. Chinese Journal of Chromatography, 2015, 33(8): 869
[3] Peng S N, Wang Q Q, Fang L L, et al. Chinese Journal of Chromatography, 2014, 32(1): 69
[4] Yan L F, Lü Y, Shao L, et al. Chinese Journal of Chromatography, 2014, 32(12): 1295
[5] Chen G, Zhao X J, Xing J, et al. Chinese Journal of Chromatography, 2014, 32(10): 1117
[6] Curat A, Tisse S, Andrieu A, et al. Chromatographia, 2014, 77 (23): 1671
[7] Peaden P A, Wright B W, Lee M L, et al. Chromatographia, 1982, 15: 335
[8] Takayama Y, Takeichi T, Kawai S, et al. J Chromatogr A, 1990, 514: 259
[9] Mayer B X, Zöllner P, Lorbeer E, et al. J Sep Sci, 2002, 25: 60
[10] Zhao P C, Teng S, Yu M, et al. Anal Methods, 2015, 7: 1333
[11] Zhao P C, Liu L, Yu M, et al. Anal Methods, 2014, 6: 6278
[12] Miller K J, Savchik J A. J Am Chem Soc, 1979, 101: 7206
[13] Grob J K, Grob G, Grob K. J Chromatogr A, 1978, 156: 1
[14] Zhao P C, Niu Y Y, Liu L, et al. RSC Adv, 2015, 5: 22399
[15] Feng S Y. Silicone Polymers and Their Applications. Beijing: Chemical Industry Press, 2004: 79
[16] Mayer B X, Kähilig H, Rauter W, et al. J Chromatogr A, 2003, 993: 59
[17] Anderson J L, Armstrong D W. Anal Chem, 2005, 77: 6453
[18] Mayer B X, Kählig H, Rauter W, et al. Analyst, 2003, 128: 1238
[19] Giddings J C. Anal Chem, 1964, 36: 741
[20] Grob K, Grob G. J Chromatogr A, 1981, 207: 291
[21] McReynolds W O. J Chromatogr Sci, 1970, 8: 685
[22] Han X, He X X, Wang H, et al. J Chromatogr A, 2016, 1449: 118
[23] Abraham M H, Whiting G S, Doherty R M, et al. J Chromatogr A, 1990, 518: 329
[24] Wang Y Z, Qi M L, Fu R N. RSC Adv, 2015, 5: 76007
[25] Poole C F, Poole S K. J Chromatogr A, 2008, 1184: 254
[26] Sun X J, Zhu Y L, Xing J, et al. J Chromatogr A, 2011, 1218: 833
3,4-二(3-苯氧基-4-氟苯基)-2,5-二苯基苯基接枝聚硅氧烷气相色谱固定相的合成与表征
王 欢, 韩 雪, 贺新新, 王 冰, 吴 波*
(山东大学化学与化工学院, 山东 济南 250100)
合成了一种耐高温的3,4-二(3-苯氧基-4-氟苯基)-2,5-二苯基苯基接枝聚硅氧烷(DPFP)固定相,使用静态涂渍法将其涂渍到毛细管柱内壁上,制成气相色谱柱。分离裂解乙烯的色谱图显示DPFP固定相在360 ℃时仍具有良好的分离能力。DPFP固定相的柱效为3 324块/米(保留因子(k)4.24,萘,0.25 mm i. d.)。麦克雷诺常数计算结果显示DPFP固定相属中等极性。溶剂化参数模型结果显示DPFP固定相与溶质之间的主要作用力为偶极-诱导偶极作用力、氢键碱性作用力。Grob试剂分离结果显示DPFP色谱柱具有良好的选择性与惰性。另外,芳香族同分异构体、苯取代物、多环芳烃、脂肪酸酯及脂肪醇都得到了良好的分离,表明DPFP固定相在应用方面有巨大的潜力。
聚硅氧烷;气相色谱;固定相;耐温性;选择性
10.3724/SP.J.1123.2016.07026
Foundation item: National Natural Science Foundation of China (No. 21275090); Technology Development Plan of Shandong Province (No. 2014GGX107010).
O658 Document code: A Article IC:1000-8713(2017)04-0388-10
* Received date: 2016-07-21
* Corresponding author. Tel: (0531)88369277, E-mail: wubo@sdu.edu.cn.
