内源IAA、GA3调控对百合试管苗生长的影响研究
2016-11-10谢忠奎郭志鸿张玉宝
杨 柳 王 乐 谢忠奎 郭志鸿 张玉宝
(1.中国科学院寒区旱区环境与工程研究所,兰州 730000; 2.中国科学院大学,北京 100049)
内源IAA、GA3调控对百合试管苗生长的影响研究
杨 柳1,2王 乐1,2谢忠奎1*郭志鸿1张玉宝1
(1.中国科学院寒区旱区环境与工程研究所,兰州 730000;2.中国科学院大学,北京 100049)
以东方百合‘Sorbonne’试管苗为对象,对不同浓度IAA及其抑制剂(TIBA)添加后试管苗茎叶和根的生长情况进行了研究,测定了试管苗中内源激素(IAA,GA3,Zeatin)的变化。数据显示10 mg·L-1IAA促进试管苗茎叶、根生长的效果最佳而40 mg·L-1IAA抑制试管苗的生长,TIBA抑制试管苗茎叶、根生长的效果显著。内源激素测定结果表明外源IAA及TIBA处理影响了内源激素的变化,IAA对生长的影响可能是通过调节内源激素的变化而实现的。结合生长情况及内源激素变化的分析结果表明:过高浓度的IAA、过低浓度的GA3都不利于试管苗生长,内源IAA和GA3在适宜浓度下才能发挥促进生长的作用;内源Zeatin在试管苗的生长调控中可能不发挥作用。本实验为百合种植中外源激素的合理使用提供了依据,有助于提升百合种植者的经济效益。
百合;IAA处理;生长;内源激素
植物激素是植物体内合成的一类有机物质,由产生部位运输到作用部位,以极低的浓度发挥生理作用,参与包括植物休眠、萌发、生长、分化、生殖、成熟和衰老在内的每一个发育过程和所有的生理活动[1~5]。自1928年生长素发现以来植物激素一直是植物科学及相关领域的研究热点。
IAA在植物生长调节中发挥重要的作用,广泛应用于各种植物的体外培养,但多与其他生长调节物质共同使用。IAA+GA处理可以促进马铃薯(Solanumtuberosum)试管苗的生长,明显提高试管苗的高度,且随着IAA浓度升高芽的长度增加;IAA+GA+BAP处理增加试管薯鲜重[6~7]。IAA配合6-BA、NAA、KT、IBA处理可以诱导红掌(Anthuriumandraeanum)产生不定芽[8]、促进蜀葵(Althaearosea)芽的伸长生长和甜菜试管苗(Betavulgaris)生根[9~10]、有利于玫瑰(Rosarugosa)不定根的生长[11]。培养基中单一添加IAA更易于研究其对植物生长所发挥的作用。IAA可以诱导红辣椒(Capsicum)、薰衣草(Lavandulavera)和大叶相思(Acaciaauriculiformis)试管苗的生根[12~14]、促进红辣椒外植体芽的形成[12],是试管苗生长调节的重要激素。
切花百合是具有重要经济价值的高档花卉,在中国的种植范围逐年扩大[15]。组培是百合新品种快繁和种性恢复的重要手段。IAA在百合组培中已有应用,与6-BA、NAA等共同调控试管苗的增殖分化和生长[16~17],对试管苗丛生芽生长、生根及根伸长产生影响[18~20]。但外源激素对内源IAA的调控以及内源激素变化对百合试管苗生长的影响还不是很清楚。
本研究以东方百合‘Sorbonne’(Liliumoriental‘Sorbonne’)试管苗为材料,通过外源添加不同浓度生长素(IAA)及其输出抑制剂(2,3,5-Triiodobenzoic acid,TIBA)的方法,定期测定试管苗生长发育进程中内源激素的变化,研究不同内源激素对试管苗生长发育的影响,探讨外源IAA调控植物生长的内在机制。
1 实验材料与方法
1.1 实验材料
选取健康无病害的百合‘Sorbonne’,将外层鳞片(第1~2层)剥离后使用75%酒精、0.1%(w/v)HgCl2先后进行表面消毒30 s、6 min。消毒后的外层鳞片切取1 cm×1 cm的小块,近轴面朝上放置于MS培养基中。经过8个月的生长之后,选取长势一致的小苗,切掉根叶后转移至添加了1.0,10,20,30,40 mg·L-1IAA和1.0,10,20,30,40 mg·L-1TIBA的培养基中进行培养。在激素培养的第40、60、80 d测量试管苗的茎叶鲜重、根鲜重和根长。每个处理测量3瓶试管苗,每瓶5株,取平均值。每个处理的小鳞茎分为3组,测定内源激素含量。
1.2 激素的提取与纯化
准确称取小鳞茎样品3 g,加入10 mL 80%(v/v)甲醇、50 μL 30 mg·mL-1铜试剂(Sodium diethyldithiocarbamate,抗氧化剂)、0.1 g polyvinylpolypyrrolidone(PVPP,吸附色素类和多酚类杂质),研磨匀浆[21~22]。所有器皿和试剂均在4℃预冷,全部操作在冰上完成,提取过程全程避光。匀浆提取液于冰水中避光超声提取15 min后,20 000 r·min-1下4℃离心15 min,取上清液。残渣中加入5 mL 80%(v/v)甲醇后再次冰水中超声提取15 min,上述相同条件离心15 min。合并两次上清液,混合提取液于相同条件下离心15 min后,氮气吹上清液至甲醇完全挥发,剩余的水溶液冷冻干燥法干燥。用10 mL超纯水溶解干燥后的残留物。用10 mL甲醇和80 mL超纯水活化、平衡C18 Sep-Pak柱(美国Waters公司)。然后用C18 Sep-Pak柱收集残留物溶解后水溶液中的激素,5 mL 60%(v/v)甲醇洗脱C18 Sep-Pak柱,收集洗脱液。洗脱液过0.45 μm滤膜后(美国Millipore公司)即为待测样品。每个样品测定3次。
1.3 仪器与色谱条件
Agilent 1200液相色谱仪(美国Agilent公司)。植物激素标准样品为Sigma公司色谱级试剂,甲醇、乙腈、醋酸为色谱级试剂(山东禹王实业有限公司),实验用水为超纯水(美国Millipore公司)。分析过程参照文献并做改动[23]。色谱条件:Agilent XDB-C18(150 mm×4.6 mm,5 μm)。流动相A为甲醇,流动相B为乙腈,流动相C为0.05%醋酸;梯度洗脱程序为:0~4.5 min:A/B/C=25∶4∶71;4.5~19 min:A/B/C=41∶9∶50;19~30 min:A/B/C=80∶20∶0;30 min结束:A/B/C=25∶4∶71。分析过程采用各激素的最佳吸收波长进行检测,程序如下:0~7 min:270 nm(Zeatin);7~13.5 min:200 nm(GA3);13.5~16.5 min:210 nm(IAA);16.5 min结束:270 nm(ABA)。温度:20℃;流速:0.6 mL·min-1;进样量:10 μL。
1.4 数据分析
试管苗茎叶重量、根重量和根长数据的单因素方差分析采用SPSS 19.0统计软件中的one-way ANOVA,并用Duncan进行显著性检验。图表在Origin 8.5中绘制。
2 实验结果
2.1IAA及其抑制剂对百合试管苗茎叶鲜重的影响
从外源IAA添加后40、60和80 d试管苗茎叶生长的情况看,10和20 mg·L-1IAA相比其他浓度具有更为显著的促进作用。10 mg·L-1IAA在60和80 d时发挥作用,而20 mg·L-1IAA在40和60 d时发挥作用,所以10 mg·L-1IAA的作用更为持久。TIBA对试管苗茎叶生长的抑制作用显著,随着培养时间延长更为明显。TIBA的作用具有浓度效应,高浓度TIBA处理的茎叶重量最低,随着浓度降低抑制作用减弱(表1)。
表1外源IAA及其抑制剂处理对百合试管苗茎叶鲜重的影响
Table1EffectofexogenousIAAanditsinhibitor(2,3,5-Triiodobenzoicacid,TIBA)onthefreshweightofstemsandleavesofinvitroseedlingofL.oriental‘Sorbonne’
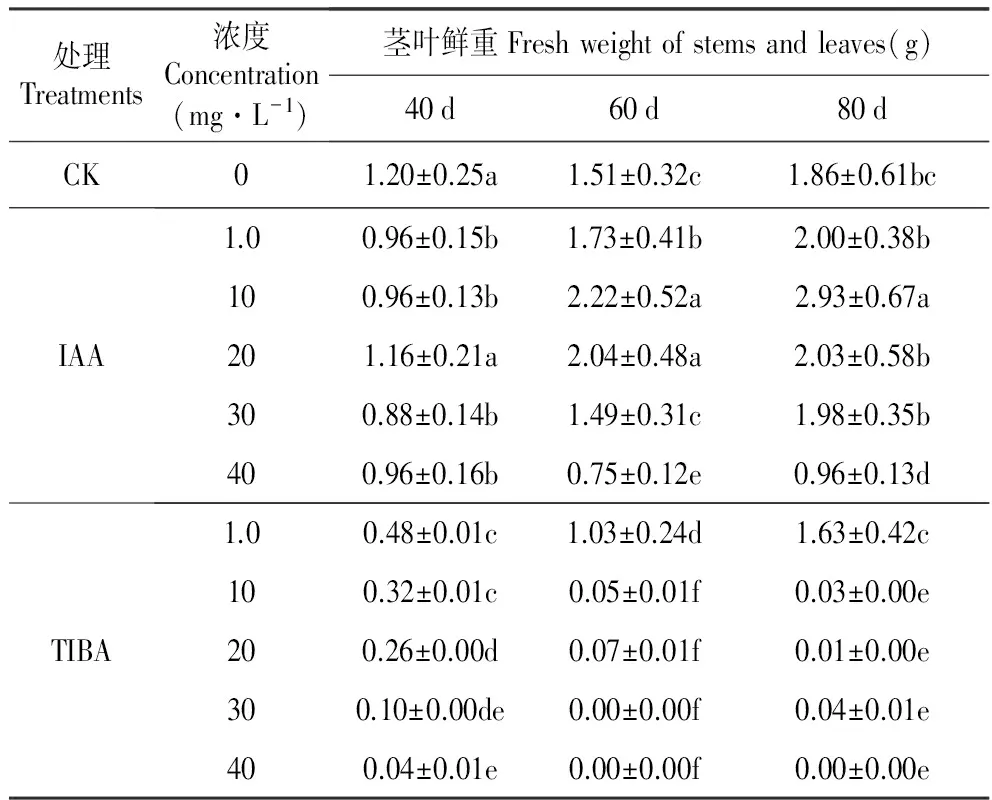
处理Treatments浓度Concentration(mg·L-1)茎叶鲜重Freshweightofstemsandleaves(g)40d60d80dCK01.20±0.25a1.51±0.32c1.86±0.61bcIAA1.00.96±0.15b1.73±0.41b2.00±0.38b100.96±0.13b2.22±0.52a2.93±0.67a201.16±0.21a2.04±0.48a2.03±0.58b300.88±0.14b1.49±0.31c1.98±0.35b400.96±0.16b0.75±0.12e0.96±0.13dTIBA1.00.48±0.01c1.03±0.24d1.63±0.42c100.32±0.01c0.05±0.01f0.03±0.00e200.26±0.00d0.07±0.01f0.01±0.00e300.10±0.00de0.00±0.00f0.04±0.01e400.04±0.01e0.00±0.00f0.00±0.00e
注:同列不同小写字母表示差异显著(P≤0.05),下同。
Note:Different lowercase letters in the same row indicate significant difference(P≤0.05),the same as below.
2.2 IAA及其抑制剂对百合试管苗根鲜重的影响
结果显示,培养基中添加IAA后40 d时,与对照相比,最高浓度IAA(40 mg·L-1)对百合试管苗的根重量没有影响,其他浓度全部显著促进试管苗根的生长,其中30 mg·L-1IAA的作用最为显著。培养至60 d时,30 mg·L-1IAA对试管苗根生长的促进作用不再显著,10和20 mg·L-1IAA处理后的根重量显著高于对照和其他处理。80 d时,10 mg·L-1IAA处理的根重量最高。综合来看,10 mg·L-1IAA对根重量的促进作用最为持久。TIBA对百合试管苗根重量的影响在处理后40 d时已经显示出来。高浓度TIBA(30和40 mg·L-1)完全抑制了根的生长,40 mg·L-1IAA的抑制作用在培养80 d时仍然存在,30 mg·L-1IAA的抑制作用在处理后的80 d时稍有减弱。TIBA对百合试管苗根生长的抑制作用也有浓度效应,随着处理浓度降低,抑制效果减弱(表2)。
表2外源IAA及其抑制剂处理对百合试管苗根鲜重的影响
Table2EffectofexogenousIAAanditsinhibitor(2,3,5-Triiodobenzoicacid,TIBA)onthefreshrootweightofinvitroseedlingofL.oriental‘Sorbonne’
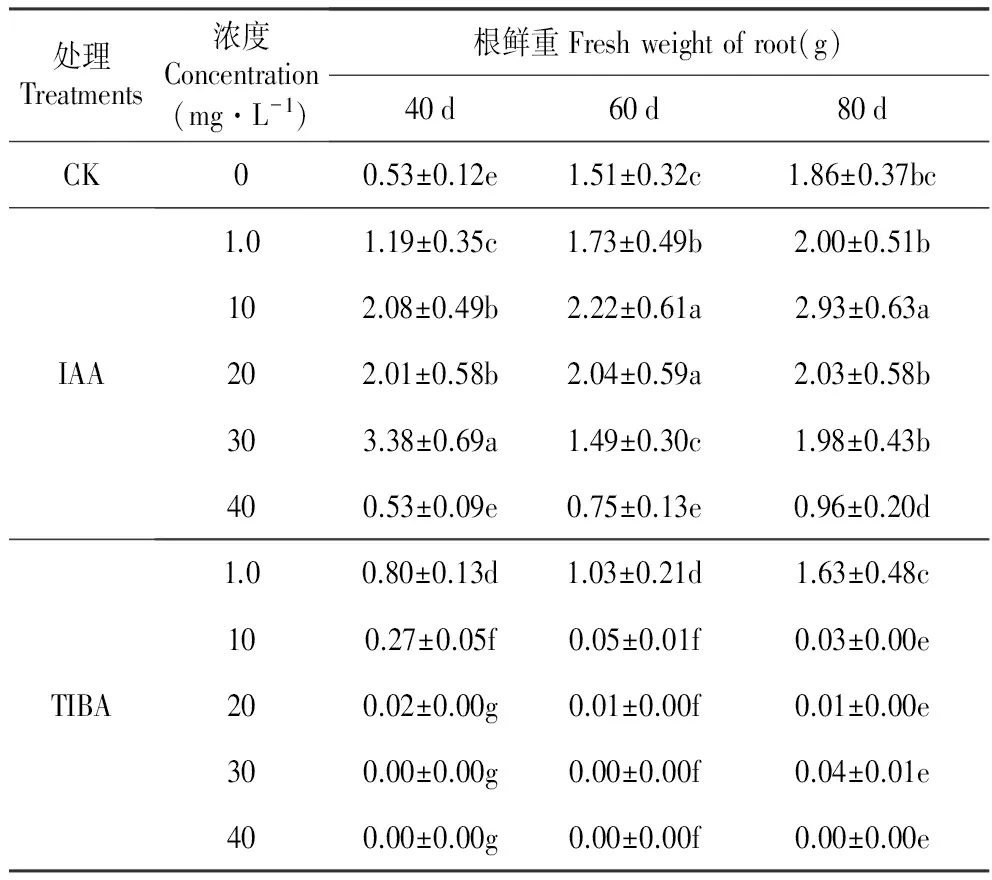
处理Treatments浓度Concentration(mg·L-1)根鲜重Freshweightofroot(g)40d60d80dCK00.53±0.12e1.51±0.32c1.86±0.37bcIAA1.01.19±0.35c1.73±0.49b2.00±0.51b102.08±0.49b2.22±0.61a2.93±0.63a202.01±0.58b2.04±0.59a2.03±0.58b303.38±0.69a1.49±0.30c1.98±0.43b400.53±0.09e0.75±0.13e0.96±0.20dTIBA1.00.80±0.13d1.03±0.21d1.63±0.48c100.27±0.05f0.05±0.01f0.03±0.00e200.02±0.00g0.01±0.00f0.01±0.00e300.00±0.00g0.00±0.00f0.04±0.01e400.00±0.00g0.00±0.00f0.00±0.00e
表3外源IAA及其抑制剂处理对百合试管苗根长的影响
Table3EffectoftheexogenousIAAanditsinhibitor(2,3,5-Triiodobenzoicacid,TIBA)ontherootlengthofinvitroseedlingofL.oriental‘Sorbonne’
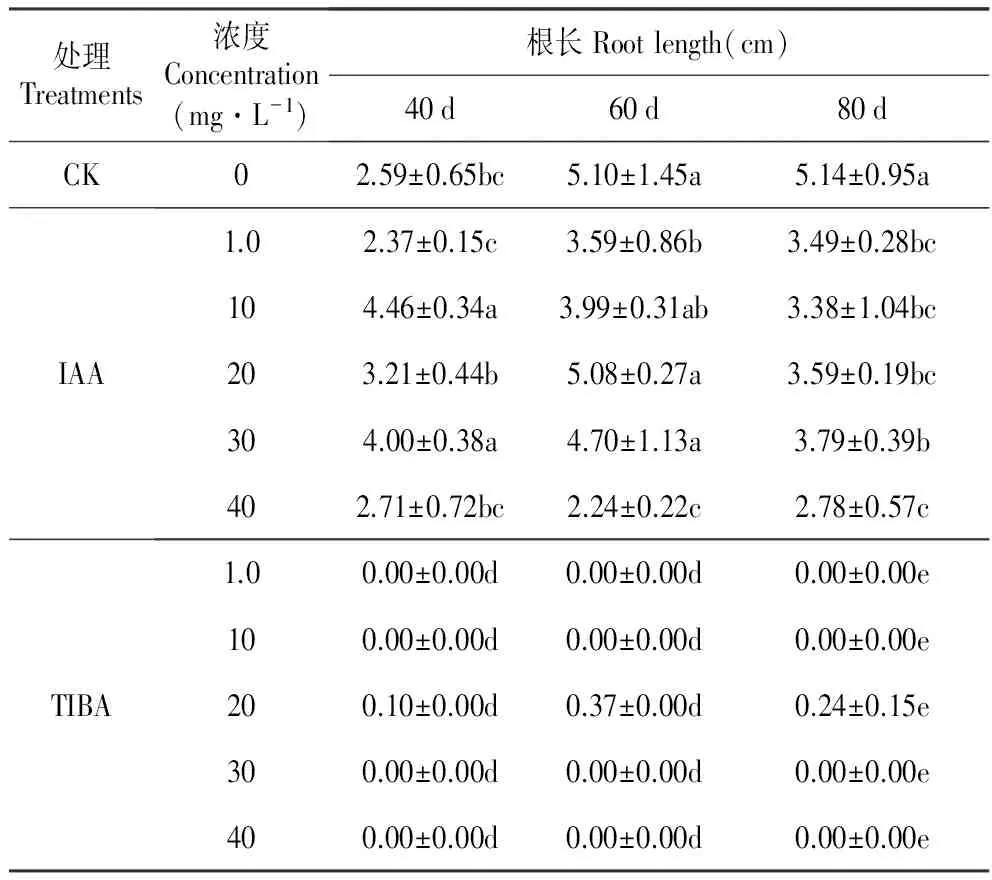
处理Treatments浓度Concentration(mg·L-1)根长Rootlength(cm)40d60d80dCK02.59±0.65bc5.10±1.45a5.14±0.95aIAA1.02.37±0.15c3.59±0.86b3.49±0.28bc104.46±0.34a3.99±0.31ab3.38±1.04bc203.21±0.44b5.08±0.27a3.59±0.19bc304.00±0.38a4.70±1.13a3.79±0.39b402.71±0.72bc2.24±0.22c2.78±0.57cTIBA1.00.00±0.00d0.00±0.00d0.00±0.00e100.00±0.00d0.00±0.00d0.00±0.00e200.10±0.00d0.37±0.00d0.24±0.15e300.00±0.00d0.00±0.00d0.00±0.00e400.00±0.00d0.00±0.00d0.00±0.00e
2.3 IAA及其抑制剂对百合试管苗根长的影响
对照百合试管苗根的长度在处理后60 d时达到最高值,培养至80 d时根长不再增加。IAA处理改变了试管苗根长的生长规律。IAA处理(10和30 mg·L-1)在40 d时显著增加了根的长度,60 d时除20 mg·L-1IAA外其他浓度均抑制了根长的增加;80 d时,所有浓度的IAA处理都抑制了根的伸长生长。高浓度TIBA(30和40 mg·L-1)完全抑制了百合试管苗根的伸长生长,随着TIBA浓度降低和培养时间延长抑制作用逐渐解除,培养至80 d时1.0 mg·L-1TIBA处理对根长已经没有抑制作用(表3)。
2.4IAA及其抑制剂对百合试管苗小籽球中内源激素的影响
处理后40 d时,所有浓度IAA均促进了百合试管苗小鳞茎中内源IAA的合成,且具有浓度效应,1.0 mg·L-1IAA处理后的增量为对照的1.4倍,40 mg·L-1IAA处理后内源IAA含量最高(为对照的131.6倍)。随着培养时间延长,所有IAA处理后的内源IAA含量都降低,80 d时所有处理与对照的内源IAA处于相同水平(图1A)。高浓度TIBA(40,30,20 mg·L-1)几乎完全抑制了百合试管苗小鳞茎内源IAA的合成。随着浓度降低、时间延长,TIBA的抑制作用减弱,1.0 mg·L-1TIBA处理的试管苗内源IAA在60和80 d时已经超过对照水平(图1B)。培养期间不同浓度外源IAA处理后内源GA3的变化没有规律,但最高浓度IAA(40 mg·L-1)的内源GA3始终处于最低水平(图2A)。除80 d时最低浓度(1.0 mg·L-1)外,TIBA处理不同程度的降低了内源GA3含量(图2B)。培养期间,所有IAA处理后的内源Zeatin高于对照或与对照处于相同水平(图3A)。最低浓度(1.0 mg·L-1)TIBA降低了Zeatin的含量,其他的高浓度TIBA均促进了内源Zeatin的合成(图3B)。本研究未能检测到‘Sorbonne’试管苗小鳞茎中的ABA。
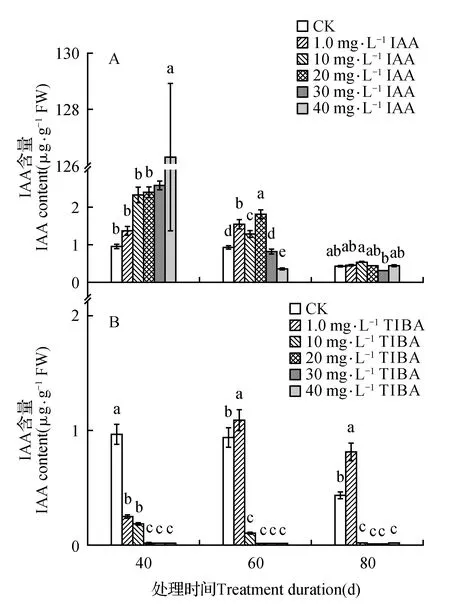
图1 东方百合‘Sorbonne’试管苗小鳞茎中内源IAA对不同浓度外源IAA(A)及其输出抑制剂TIBA(B)处理的响应Fig.1 Response of endogenous IAA in bulblet of in vitro seedling of L.oriental ‘Sorbonne’ to exogenous IAA(A) and its transport inhibitor TIBA(B) applied in different concentrations
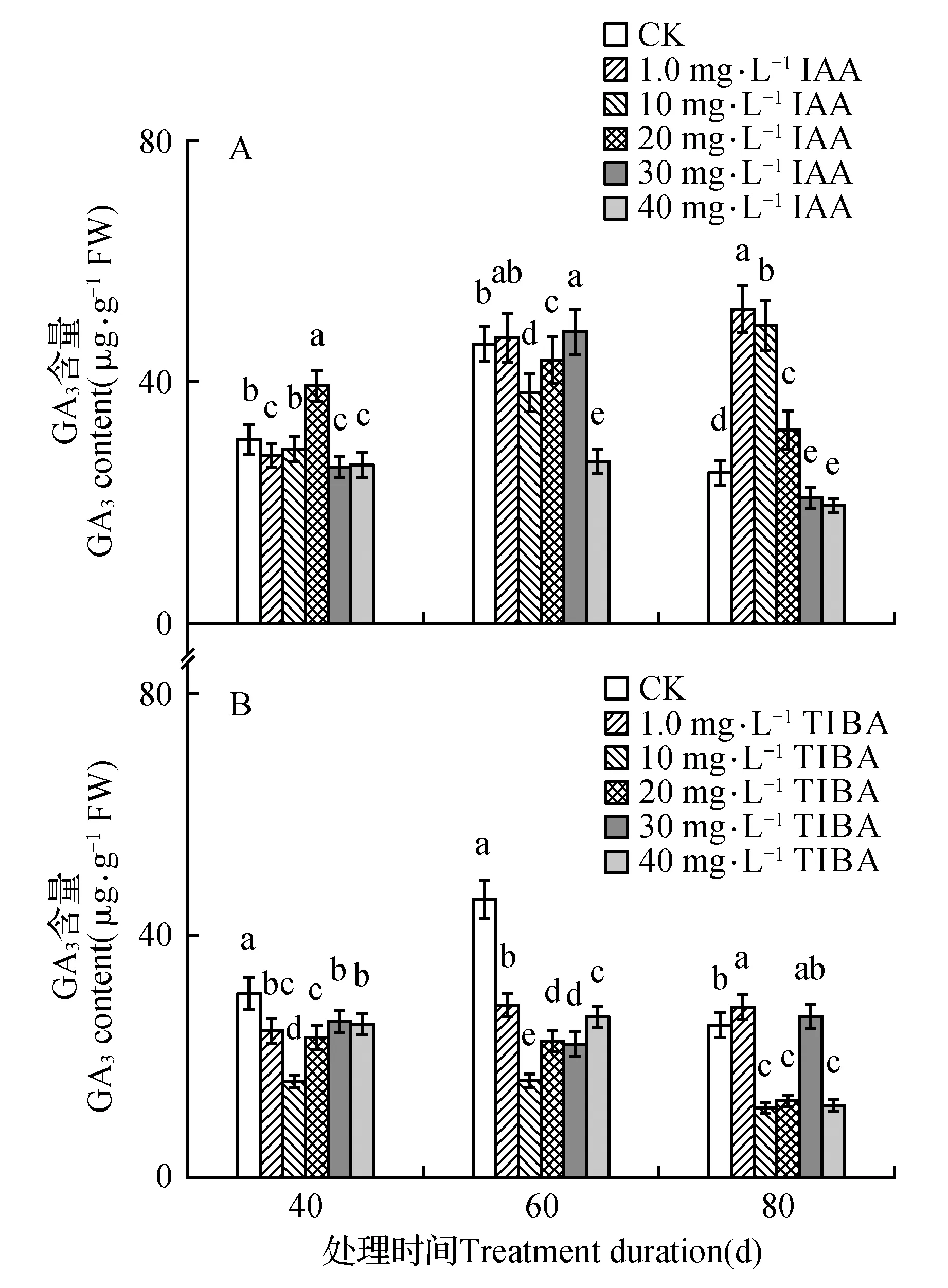
图2 东方百合‘Sorbonne’试管苗小鳞茎中内源GA3对不同浓度外源IAA(A)及其输出抑制剂TIBA(B)处理的响应Fig.2 Response of endogenous GA3 in bulblet of in vitro seedling of L.oriental ‘Sorbonne’ to exogenous IAA (A) and its transport inhibitor TIBA (B) applied in different concentrations
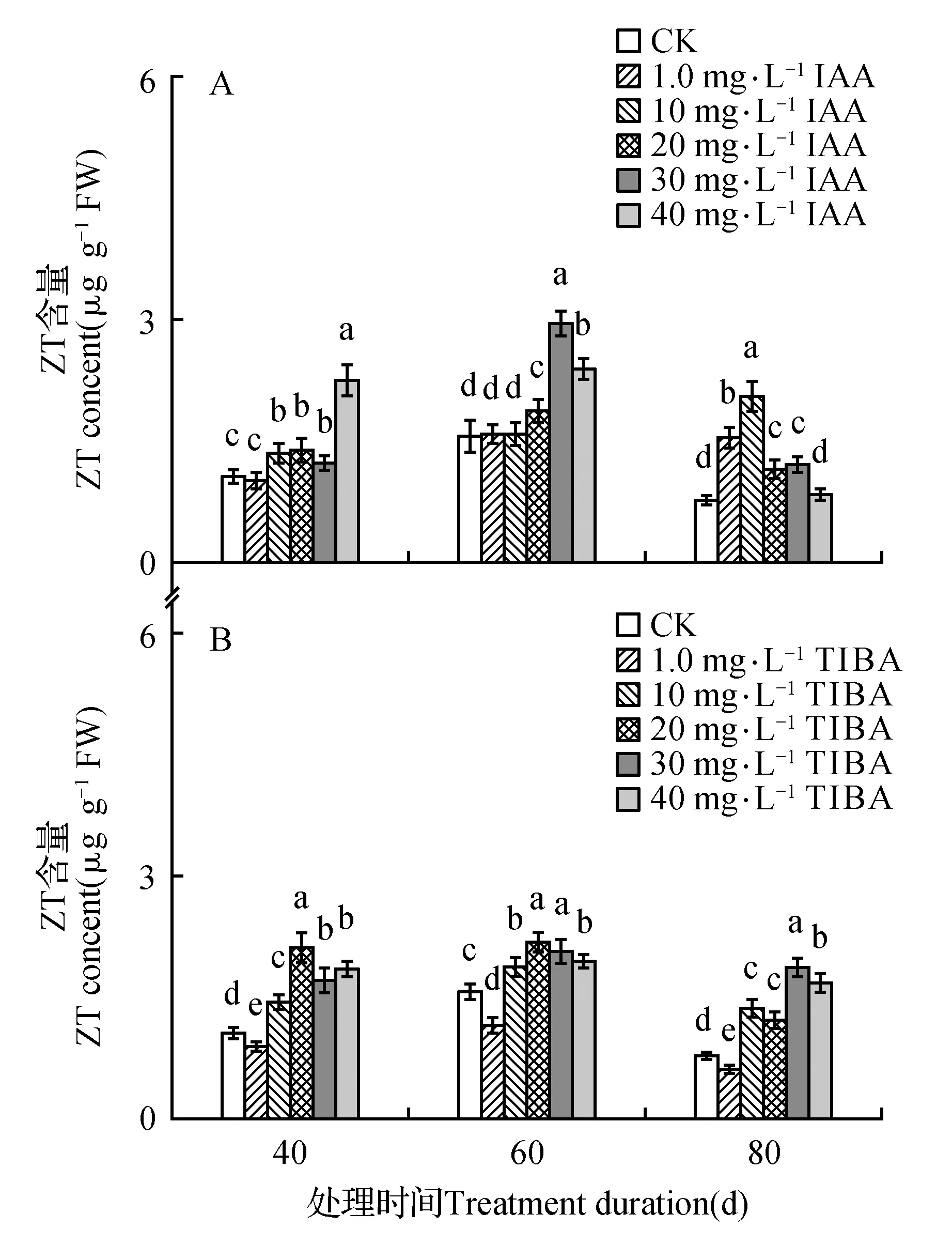
图3 东方百合‘Sorbonne’试管苗小鳞茎中内源Zeatin(ZT)对不同浓度外源IAA(A)及其输出抑制剂TIBA(B)处理的响应Fig.3 Response of endogenous Zeatin(ZT) in bulblet of in vitro seedling of L.oriental ‘Sorbonne’ to exogenous IAA(A) and its transport inhibitor TIBA(B) applied in different concentrations
3 讨论
3.1 IAA对百合试管苗生长的影响
IAA可以促进植物生长,巴西固氮螺菌(Azospirillumbrasilense)对狼尾草(Pennisetumamericanum)生长的促进作用已证明主要是由于其合成的IAA起作用[24]。基于豌豆(Pisumsativum)黄化苗茎切段的实验结果也表明IAA能够促进茎切段的伸长[25]。适宜浓度(10 mg·L-1)的IAA也促进了‘Sorbonne’试管苗茎叶的生长,而IAA输出抑制剂TIBA处理后试管苗的茎叶生长被显著抑制。在大豆(Glycinemax)[26]、香石竹(Dianthuscaryophyllus)[27]、棉花(GossypiumMCU 12)[28]中TIBA也发挥了同样的抑制生长的作用。
生长素是促进植物不定根形成的主要激素,且生长素的作用与处理时间、施用浓度有关[29]。IAA对‘Sorbonne’试管苗根生长的调控,体现在根的重量和根的伸长生长两个方面,综合来看10 mg·L-1IAA的促进作用最为显著。IAA促进了体外培养番茄(Lycopersiconesculentum)叶片[30]、马铃薯(S.tuberosum‘Bintje’)切块[31]、黄瓜(Cucumissativus)离体子叶[32]和豌豆(P.sativum)幼嫩切段[33]不定根的形成和发育,对大豆(G.max)和大蒜(Alliumsativum)根具有低浓度促进生长高浓度抑制生长的作用[34~35]。TIBA对黄瓜下胚轴切段、离体子叶和绿豆(Vignaradiata)下胚轴切段不定根的发生具有抑制作用[32,36]。TIBA处理后,玉米(Zeamays)试管苗根的伸长生长速率以浓度依赖的方式降低[37]。在‘Sorbonne’试管苗中,高浓度TIBA完全抑制了根的生长,低浓度TIBA的抑制作用减弱。
3.2 内源激素的变化与生长的关系
内源IAA含量变化参与调节不定根的形成过程[29],IAA对葡萄(Vitisvinifera)和向日葵(Helianthusannuus)幼苗下胚轴切段的生根是必需的,越高浓度的IAA会诱导产生更多数量的不定根[38~39]。桃(Amygdaluspersica)和白桦(Betulaplatyphylla)的枝条经生根促进剂处理后都检测到了内源IAA含量升高[40~41]。赵仲仁认为钾离子对黄瓜(C.sativus)生根的促进作用是通过影响内源IAA的水平而实现的[32]。另外,内源IAA也会抑制植物的生长发育,对玉米(Z.mays)和茶(Camelliasinensis)的根长、新梢生长起着负调控的作用[42~43]。不同浓度的外源IAA处理‘Sorbonne’试管苗后,小鳞茎中内源IAA的含量全部升高。诱导了最高内源IAA的40 mg·L-1IAA抑制试管苗茎叶和根的生长,促进试管苗茎叶和根生长的10 mg·L-1IAA处理后的内源IAA含量居中。TIBA处理,尤其高浓度,抑制了百合试管苗茎叶和根的生长,显著降低了内源IAA的含量,高浓度时几乎完全抑制了内源IAA的合成。Audus证明TIBA作为IAA的直接竞争性抑制剂可将被处理植物根中的IAA降低至接近零[44]。以上结果证明,过高或过低的内源IAA含量都不利于试管苗茎叶和根的生长。也就是说对百合栽培种‘Sorbonne’试管苗的生长来说,内源IAA是重要的影响因子,但是需要适宜的浓度才能发挥最佳的促进作用。
有研究结果显示内源GA在植物的生长过程中发挥着调节作用。高水平的活性GA对车前子(Plantagomajor)植株和芒果(Mangiferaindica)枝条的生长及马铃薯(S.tuberosum)匍匐茎和块茎的形成非常重要[45~47],GA3是浮水稻(Oryzasativa)和莴苣(Lactucasativa)节间生根和根伸长生长的重要调节物质[48~49]。烯效唑(GA3合成抑制剂)对浮萍(Lemnaminor)根切段伸长的显著抑制作用可被外源GA3完全消除,作者认为内源GA3通过调整细胞伸长从而控制着根的伸长生长[50]。另一方面内源GA3含量与体外培养苹果(Maluspumila‘Jonathan’)的生根能力和马铃薯(S.tuberosum‘Spunta’)芽切段的块茎形成之间存在着负相关关联[51~52]。最高浓度IAA(40 mg·L-1)抑制了‘Sorbonne’试管苗的生长,此浓度IAA处理后的内源GA3浓度降至最低;除80 d时1.0和30 mg·L-1外,TIBA处理后的GA3全部低于对照。说明低浓度的内源GA3限制了‘Sorbonne’试管苗茎叶和根的生长,百合试管苗的生长需要相对高浓度的内源GA3。
体外培养的苹果(M.pumila‘Jonathan’)随着继代培养时间延长而不定根的数量增多,CK含量随着继代培养时间延长而升高[51]。内源细胞分类素参与豌豆(P.sativum‘Weibulls Marma’)枝插条不定根形成的调控[53]。白桦(B.platyphylla)嫩枝经生根促进剂处理后在根原基发育期间能显著增加内源ZR(Zeatin-riboside)的含量以达到有利于生根的水平[41]。但细胞分裂素抑制了拟南芥(Arabidopsisthaliana)根尖和茎尖分生组织的发育[54]。除最低浓度(1.0 mg·L-1)TIBA外,其余的TIBA和IAA处理后内源Zeatin变化趋势相似,而1.0 mg·L-1TIBA对试管苗的生长没有促进作用,所以推测内源Zeatin可能不参与‘Sorbonne’试管苗生长的调控。
适宜浓度的外源IAA能够促进百合试管苗的生长。试管苗的内源激素受到外源IAA的影响从而发生改变,外源IAA的作用可能通过内源激素的变化得以实现。适中浓度的内源IAA和相对高浓度的内源GA3有利于试管苗的生长,这两种内源激素以其适宜的浓度相互协调,共同调控试管苗的生长。
1.Soeno K,Goda H,Ishii T,et al.Auxin biosynthesis inhibitors,identified by a genomics-based approach,provide insights into auxin biosynthesis[J].Plant and Cell Physiology,2010,51(4):524-536.
2.许智宏,李家洋.中国植物激素研究:过去、现在和未来[J].植物学通报,2006,23(5):433-442.
Xu Z H,Li J Y.Plant hormones research in China:past,present and future[J].Chinese Bulletin of Botany,2006,23(5):433-442.
3.黄晓荣,张平治,吴新杰,等.植物内源激素测定方法研究进展[J].中国农学通报,2009,25(11):84-87.
Huang X R,Zhang P Z,Wu X J,et al.Review on plant endogenous hormones determination methods[J].Chinese Agricultural Science Bulletin,2009,25(11):84-87.
4.熊国胜,李家洋,王永红.植物激素调控研究进展[J].科学通报,2009,54(18):2718-2733.
Xiong G S,Li J Y,Wang Y H.Advances in the regulation and crosstalks of phytohomones[J].Chinese Science Bulletin,2009,54(18):2718-2733.
5.白玉,杜甫佑,白玉,等.植物激素检测技术研究进展[J].生命科学,2010,22(1):36-44.
Bai Y,Du F Y,Bai Y,et al.Recent development in determination of plant hormones[J].Chinese Bulletin of Life Sciences,2010,22(1):36-44.
6.Zhang Z J,Zhou W J,Li H Z.The role of GA,IAA and BAP in the regulation ofinvitroshoot growth and microtuberization in potato[J].Acta Physiologiae Plantarum,2005,27(3):363-369.
7.吴秋云,汤浩,蔡南通,等.外源激素对马铃薯试管薯形成和发育的影响[J].福建农业学报,2006,21(2):95-97.
Wu Q Y,Tang H,Cai N T,et al.Effect of exogenous hormone on formation and development of potato microtuberinvitro[J].Fujian Journal of Agricultural Sciences,2006,21(2):95-97.
8.黄丽芳.红掌组培苗工厂化生产的技术优化研究[D].长沙:湖南农业大学,2008.
Huang L F.Improvement on industrial production techniqueinvitroculture ofAnthuriumandraeanum[D].Changsha:Hunan Agricultural University,2008.
9.张文,王楠,王振东,等.变异蜀葵的组织培养研究[J].现代农业科技,2008(24):9-11.
Zhang W,Wang N,Wang Z D,et al.Research ininvitroAlthaearoseamutant[J].Modern Agricultural Science and Technology,2008,(24):9-11.
10.Welander T.Effects of nitrogen,sucrose,IAA and kinetin on explants ofBetavulgarisgrowninvitro[J].Physiologia Plantarum,1976,36(1):7-10.
11.Roy P K,Mamun A N K,Ahmad G.Invitroplantlets regeneration of rose[J].Plant Tissue Culture,2004,14(2):149-154.
12.Gunay A L,Rao P S.Invitroplant regeneration from hypocotyl and cotyledon explants of red pepper(Capsicum)[J].Plant Science Letters,1978,11(3-4):365-372.
13.谢国强.外源激素对薰衣草试管苗生长影响[D].泰安:山东农业大学,2008.
Xie G Q.Effect of exogenous hormones on growth ofLavandulaveraplantlets[D].Taian:Shandong Agricultural University,2008.
14.Mittal A,Agarwal R,Gupta S C.Invitrodevelopment of plantlets from axillary buds ofAcaciaauriculiformis-a leguminous tree[J].Plant Cell,Tissue and Organ Culture,1989,19(1):65-70.
15.岳铭鉴.辽宁省切花百合生产现状与发展建议[J].辽宁农业科学,2010,(3):69-70.
Yue M J.Current situation and development suggestions for cut-flower lily production in Liaoning province[J].Liaoning Agricultural Sciences,2010,(3):69-70.
16.陈丽静,张晓光,马爽,等.东方百合和麝香百合的快速繁殖技术研究[J].北方园艺,2010,(14):92-96.
Chen L J,Zhang X G,Ma S,et al.Study on the rapid propagation ofLiliumoritentialandLiliumlongifolorum[J].Northern Horticulture,2010,(14):92-96.
17.李文英.百合‘普瑞头’组织培养与开放组培初探[D].哈尔滨:东北林业大学,2012.
Li W Y.Study on Tissue Culture and The Preliminary Study Open-Tissue-Culture ofLiliumasiaticHybrids‘Prato’ [D].Harbin:Northeast Forestry University,2012.
18.陈学林,李卫锋,黄海涛.荷兰百合索尔邦的离体组织培养[J].安徽农业科学,2007,35(20):6045-6046.
Chen X L,Li W F,Huang H T.Tissue cultureinvitroof Holland Lily Sorbonne[J].Journal of Anhui Agricultural Sciences,2007,35(20):6045-6046.
19.李卫锋,王丹,黄海涛.荷兰百合新品种索尔邦的快繁技术研究[J].北方园艺,2007,(9):191-192.
Li W F,Wang D,Huang H T.Study on tissue culture and propagation of Holland Lilyinvitro[J].Northern Horticulture,2007,(9):191-192.
20.付文奇.东方百合‘帝伯’(Tiber)组织培养技术研究[D].杨凌:西北农林科技大学,2008.
Fu W Q.Research on technology about tissue culture of oriental hybrids ‘Tiber’ [D].Yangling:Northwest Agricultural & Forestry University,2008.
21.符继红,孙晓红,王吉德,等.植物激素定量分析方法研究进展[J].科学通报,2010,55(33):3163-3176.
Fu J H,Sun X H,Wang J D,et al.Progress in quantitative analysis of plant hormones[J].Chinese Science Bulletin,2010,55(33):3163-3176.
22.曾庆钱,陈厚彬,鲁才浩,等.HPLC测定荔枝不同器官中内源激素流程的优化[J].果树学报,2006,23(1):145-148.
Zeng Q Q,Chen H B,Lu C H,et al.An optimized HPLC procedure for analyzing endogenous hormones in different organs of litchi[J].Journal of Fruit Science,2006,23(1):145-148.
23.安丽萍,谢忠奎,李翊华,等.东方百合鳞片生小鳞茎生长过程中的激素变化[J].中国沙漠,2012,32(3):705-708.
An L P,Xie Z K,Li Y H,et al.Variation of endogenous phytohormones ofLiliumoriental‘Sorbonne’during scale propagation[J].Journal of Desert Research,2012,32(3):705-708.
24.Tien T M,Gaskins M H,Hubbell D H.Plant growth substances produced byAzospirillumbrasilenseand their effect on the growth of pearl millet(PennisetumamericanumL.)[J].Applied and Environmental Microbiology,1979,37(5):1016-1024.
25.蒋宇霞,甘立军,夏凯.IAA和GA3在调控豌豆黄化苗茎切段伸长生长中的相互作用[J].植物生理学通讯,2006,42(2):207-212.
Jiang Y X,Gan L J,Xia K.Interactions between IAA and GA3in regulation of etiolated stem segment elongation inPisumsativumL.[J].Plant Physiology Communications,2006,42(2):207-212.
26.Tanner J W,Ahmed S.Growth analysis of soybeans treated with TIBA[J].Crop Science,1974,14(3):371-374.
27.马智宏,李艾君,刘振林,等.PP333、TIBA和Pix对盆栽香石竹矮化效应的研究[J].河北农业技术师范学院学报,1999,13(1):33-36.
Ma Z H,Li A J,Liu Z L,et al.Dwarfing effects of PP333,TIBA and Pix on potcultivated carnation plants[J].Journal of Hebei Agrotechnical Teachers College,1999,13(1):33-36.
28.Djanaguiraman M,Sheeba J A,Devi D D,et al.Response of cotton to Atonik and TIBA for growth,enzymes and yield[J].Journal of Biological Sciences,2005,5(2):158-162.
29.王金祥,严小龙,潘瑞炽.不定根形成与植物激素的关系[J].植物生理学通讯,2005,41(2):133-142.
Wang J X,Yan X L,Pan R Z.Relationship between adventitious root formation and plant hormones[J].Plant Physiology Communications,2005,41(2):133-142.
30.Coleman W K,Greyson R I.Promotion of root initiation by gibberellic acid in leaf discs of tomato(Lycopersiconesculentum) culturedinvitro[J].New Phytologist,1977,78(1):47-54.
31.Hartmans K J,van Es A.The influence of growth regulators GA3,ABA,kinetin and IAA on sprout and root growth and plant development using excised potato buds[J].Potato Research,1979,22(4):319-332.
32.赵仲仁,李广仁,黄桂琴,等.几种抑制剂对钾和IAA诱导的离体黄瓜子叶不定根形成的影响[J].植物学报,1997,39(1):64-67.
Zhao Z R,Li G R,Huang G Q,et al. Effects of some inhibitors on Pitassiu- and IAA-induced adventitious of excised Cucumber cotyledon[J].Acta Botanica Sinica,1997,39(1):64-67.
33.Rasmussen A,Hosseini S A,Hajirezaei M R,et al.Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis[J].Journal of Experimental Botany,2015,66(5):1437-1452.
34.李欣欣,赵静,廖红.吲哚乙酸、吲哚丁酸和萘乙酸对大豆幼根生长的影响[J].植物生理学报,2013,49(6):573-578.
Li X X,Zhao J,Liao H.Effects of indoleacetic acid,indolebutyric acid and naphthylacetic acid on soybean(Glycinemax(L.) Merr) root growth[J].Plant Physiology Journal,2013,49(6):573-578.
35.赵东利,冯冠军,蒋龙,等.吲哚乙酸(IAA)对大蒜根生长发育的影响[J].大连大学学报,2008,29(6):89-91.
Zhao D L,Feng G J,Jiang L,et al.Effect of IAA toAlliumsativumroots growth and development[J].Journal of Dalian University,2008,29(6):89-91.
36.王利琳,庞基良,胡江琴,等.黄瓜和绿豆下胚轴不定根发生的研究[J].云南植物研究,2002,24(4):508-514.
Wang L L,Pang J L,Hu J Q,et al.Studies on adventitious root formation in hypocotyls of Cucumber and Phaseolus Culturedinvitro[J].Acta Botanica Yunnanica,2002,24(4):508-514.
37.Bronsema F B F,van Oostveen W J F,van Lammeren A A M.Influence of 2,4-D,TIBA and 3,5-D on the growth response of cultured maize embryos[J].Plant cell,Tissue and Organ Culture,2001,65(1):45-56.
38.Kracke H,Cristoferi G,Marangoni B.Hormonal changes during the rooting of hardwood cuttings of grapevine rootstocks[J].American Journal of Enology and Viticulture,1981,32(2):135-137.
39.Liu J H,Reid D M.Adventitious rooting in hypocotyls of sunflower(Helianthusannuus) seedlings.Ⅳ.The role of changes in endogenous free and conjugated indole-3-acetic acid[J].Physiologia Plantarum,1992,86(2):285-292.
40.徐继忠,陈四维.桃硬枝插条内源激素(ABA、IAA)含量变化对生根的影响[J].园艺学报,1989,16(4):275-278.
Xu J Z,Chen S W.The effect of the changes of the endogenous hormone’s contents(ABA and IAA) in hard wood cuttings of peach to rooting[J].Acta Horticulturae Sinica,1989,16(4):275-278.
41.詹亚光,杨传平,金贞福,等.白桦插穗生根的内源激素和营养物质[J].东北林业大学学报,2001,29(4):1-4.
Zhan Y G,Yang C P,Jin Z F,et al.Endogenous hormones and nutritive material in softwood cuttings ofBetulaplatyphylladuring rooting[J].Journal of Northeast Forestry University,2001,29(4):1-4.
42.Pilet P E,Saugy M.Effect on root growth of endogenous and applied IAA and ABA A critical reexamination[J].Plant Physiology,1987,83(1):33-38.
43.潘根生,钱利生,吴伯千,等.茶树新梢生育的内源激素水平及其调控机理(第四报)外源激素对茶树内源激素的影响及其与新梢生长的关系[J].茶叶,2001,27(2):25-29.
Pan G S,Qian L S,Wu B Q,et al.Endogenous plant hormone level and its regulation mechanism during growth of tea plant shoot(Ⅳ) effects of exogenous growth regulators on endogenous hormones and their relation to shoot growth in tea plant[J].Journal of Tea,2001,27(2):25-29.
44.Audus L J,Thresh R.The effects of synthetic growth-regulator treatments on the levels of free endogenous growth-substances in plants[J].Annals of Botany,1956,20(3):439-459.
45.Dijkstra P,Reegen H T,Kuiper P J C.Relation between relative growth rate,endogenous gibberellins,and the response to applied gibberellic acid forPlantagomajor[J].Physiologia Plantarum,1990,79(4):629-634.
46.Davenport T L,Pearce D W,Rood S B.Correlation of endogenous gibberellic acid with initiation of mango shoot growth[J].Journal of Plant Growth Regulation,2001,20(3):308-315.
47.Abdala G,Castro G,Miersch O,et al.Changes in jasmonate and gibberellin levels during development of potato plants(Solanumtuberosum)[J].Plant Growth Regulation,2002,36(2):121-126.
48.Suge H.Ethylene and gibberellin:regulation of internodal elongation and nodal root development in floating rice[J].Plant & Cell Physiology,1985,26(4):607-614.
49.Tanimoto E.Gibberellin-dependent root elongation inLactucasativa:recovery from growth retardant-suppressed elongation with thickening by low concentration of GA3[J].Plant & Cell Physiology,1987,28(6):963-973.
50.Inada S,Shimmen T.Regulation of elongation growth by gibberellin in root segments ofLemnaminor[J].Plant & Cell Physiology,2000,41(8):932-939.
51.Takeno K,Taylor J S,Sriskandarajah S,et al.Endogenous gibberellin- and cytokinin-like substances in cultured shoot tissues of apple,Maluspumilacv.Jonathan,in relation to adventitious root formation[J].Plant Growth Regulation,1982,1(4):261-268.
52.Abdala G,Guiazú M,Tizio R,et al.Effect of 2-chloroethyltrimethyl ammonium chloride on tuberization and endogenous GA3in roots of potato cuttings[J].Plant Growth Regulation,1995,17(2):95-100.
53.Bollmark M,Kubt B,Eliasson L.Variation in endogenous cytokinin content during adventitious root formation in pea cuttings[J].Journal of Plant Physiology,1988,132(3):262-265.
54.Werner T,Motyka V,Laucou V,et al.Cytokinin-deficient transgenicArabidopsisplants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity[J].The Plant Cell,2003,15(11):2532-2550.
The National Natural Science Foundation of China(31201651);The Ningxia Agricultural Comprehensive Development Office(NTKJ2015-05-01)
introduction:YANG Liu(1982—),female,Doctor,major in plant physiology and ecology.
date:2016-05-08
InfluenceofEndogenousIAAandGA3oninvitroLilySeedlingGrowth
YANG Liu1,2WANG Le1,2XIE Zhong-Kui1*GUO Zhi-Hong1ZHANG Yu-Bao1
(1.Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou 730000;2.University of Chinese Academy of Sciences,Beijing 100049)
Theinvitroseedlings ofLiliumoriental‘Sorbonne’ were used to study the response of plants to exogenous IAA and its inhibitor (2,3,5-Triiodobenzoic acid, TIBA), including the growth of stems, leaves and roots, and the dynamic change of endogenous hormones was analyzed by HPLC. The data showed that 10 mg·L-1IAA have the best significant role on growth of stems, leaves and roots simultaneously while 40 mg·L-1IAA and TIBA treatments inhibited growth significantly. By the hormone analysis, the endogenous hormones was influenced by exogenous IAA and TIBA application, the effect of IAA on growth was achieved probably by endogenous hormones regulation. Analysis based on the growth and the change of endogenous hormones revealed that higher level of endogenous IAA and lower level of GA3were disadvantageous for growth, and endogenous IAA and GA3played optimal role in their optimum concentrations. Endogenous Zeatin perhaps did not take part in the regulation of seedling growth. These results would be helpful in the rational use of exogenous hormones in lily planting and useful to improve economic benefit of growers.
Lilies;IAA;growth;endogenous hormones
国家自然科学基金(31201651);宁夏农业综合开发科技推广项目(NTKJ2015-05-01)
杨柳(1982—),女,博士研究生,主要从事植物生理生态研究。
* 通信作者:E-mail:wxhcas@lzb.ac.cn
2016-05-08
* Corresponding author:E-mail:wxhcas@lzb.ac.cn
Q949.71+8.23
A
10.7525/j.issn.1673-5102.2016.05.018
