热应激对性成熟猪睾丸TGF-β3和Claudin-11蛋白表达的影响
2016-11-08范小瑞席华明梁亚俊贺俊平
张 禛,范小瑞,席华明,梁亚俊,贺俊平
(山西农业大学动物科技学院,太谷 030801)
热应激对性成熟猪睾丸TGF-β3和Claudin-11蛋白表达的影响
张禛,范小瑞,席华明,梁亚俊,贺俊平*
(山西农业大学动物科技学院,太谷 030801)
旨在探讨猪舍温度37~40 ℃热应激条件下,转化生长因子β3(TGF-β3)和Claudin-11在猪睾丸的表达与定位。6头性成熟长白公猪分成2组,3头为热应激组,置于温度控制在37~40 ℃的猪舍环境,每天3 h,连续7 d,每天于热处理结束后将猪赶回20~27 ℃正常猪舍环境中;另3头为对照组,饲养于20~27 ℃正常猪舍环境中。7 d后取睾丸组织,采用qRT-PCR、Western blotting及免疫组织化学对猪睾丸TGF-β3和Claudin-11的表达进行研究。qRT-PCR结果显示,与对照组相比,TGF-β3在热应激组的mRNA相对表达量显著升高(P<0.01),Claudin-11的mRNA相对表达量较对照组降低(P<0.05)。Western blotting结果显示,热应激处理组TGF-β3的蛋白表达较对照组升高(P<0.05),Claudin-11的蛋白表达较对照组下降(P<0.05)。免疫组织化学结果显示,热应激组TGF-β3免疫反应强阳性物定位于各级生精细胞和支持细胞,免疫阳性着色深度和范围高于对照组,提示热应激导致TGF-β3的表达增高;热应激组Claudin-11表达与对照组相比明显下降,对照组Claudin-11在血睾屏障位置呈明显的带状表达,而热应激组Claudin-11的表达局限在支持细胞周围,失去明显的血睾屏障带状表达。热应激影响猪睾丸TGF-β3和Claudin-11的表达与定位,提示热应激可能通过调节TGF-β3和Claudin-11的表达来影响精子发生。
热应激;猪睾丸;精子发生;TGF-β3;Claudin-11
多数哺乳动物睾丸在温度较体温低2~8 ℃的阴囊中进行精子发生[1]。阴囊和睾丸温度升高会干扰精子发生,导致精子畸形率升高、密度减少和活力降低。流行病学研究发现,焊工、面包师、铸造工等,由于阴囊和睾丸持续受热,是临床男性不育的高发人群[2-3]。高温影响其他哺乳动物精液品质也有大量研究报道。高温季节公牛的精液品质明显下降[4]。43 ℃热处理猴睾丸30 min后,生精细胞凋亡增加[5-6]。在高温条件下猪的精液品质下降,精子的成活率降低[7]。热应激影响精子发生和精液品质的分子机制尚不清楚。
支持细胞是睾丸曲精小管内唯一与生精细胞直接接触的体细胞,并为生精细胞的发育提供营养供给[8]。相邻支持细胞之间由紧密连接形成血睾屏障(Blood-testis barrier,BTB),血睾屏障的存在及其完整性的保持是功能性精子发生所必需[9]。Claudin-11是支持细胞间紧密连接的基本组成蛋白之一[10],属于Claudin家族的成员。Claudin-11表达于人[11]、小鼠[12]、兔[13]等睾丸曲精小管。来自恒河猴的研究表明,热应激能改变恒河猴睾丸中支持细胞的形态和功能,进而诱导生精细胞凋亡,导致精子减少[14]。
一种转化生长因子TGF-β3可能会下调C1audin-11的表达[15]。TGF-β3属于TGF-βs[16],是TGF-β超家族的一员,具有广泛的生物学效应,TGF-β3对生殖系统的调控机制是目前研究的前沿与热点。研究发现,支持细胞紧密连接的组装过程中,TGF-β3可以在短时间内抑制C1audin-11的表达,进而对支持细胞紧密连接屏障造成干扰[17]。热应激处理小鼠睾丸后,TGF-β3表达量可逆性增高,推断TGF-β3可能参与了对紧密连接相关蛋白表达的下调[18]。
睾丸热应激严重影响猪精子发生和精液品质,但其分子机制尚不清楚。高温是否影响TGF-β3和Claudin-11的表达,从而影响精子发生和精液品质,尚无相关报道。本研究以性成熟的公猪为对象,研究TGF-β3和C1audin-11在37~40 ℃热应激情况下的基因表达变化,旨在探索热应激影响猪精子发生的分子机制。
1 材料与方法
1.1试验动物及样品采集
6头18月龄性成熟长白公猪来自山西省太谷县的某养殖场,其中3头为热应激组(Heat stress group),置于温度控制在37~40 ℃的猪舍环境,每天3 h,连续7 d,每天于热处理后驱赶回20~27 ℃的猪舍环境;另外3头为对照组(Control group),于20~27 ℃正常猪舍环境中饲养。7 d后手术摘除两侧睾丸,将睾丸组织切成小块,部分组织放入液氮中,用于Western blotting检测,部分睾丸组织置于Bouin’s固定液中,经浸蜡包埋后进行免疫组织化学检测。
1.2主要试剂
RIPA强裂解液(碧云天公司产品);RNA提取试剂盒(Trizol Readent,Invitrogen公司产品);反转录试剂盒(QIAGEN公司产品);QuantiFast SYBR Green PCR Kit(QIAGEN公司产品);兔抗TGF-β3及Claudin-11多克隆抗体(北京博奥森生物技术有限公司产品);羊抗兔GAPDH单克隆抗体(Abcam公司产品);HRP-羊抗兔IgG及高灵敏度发光试剂盒(康为世纪公司产品);蛋白Marker(Thermo公司产品);硝酸纤维素膜(NC)(武汉博士德生物公司产品);DAB显色剂(福州迈新试剂产品)。
1.3方法
1.3.1RNA提取和qRT-PCR的扩增Trizol法提取总RNA,凝胶电泳检测其完整性,用ND-1000(NanDrop Technologies)测定其浓度。按照QIAGEN公司反转录试剂盒进行cDNA合成,反应体系为gDNA Wipeout Buffer(7×)2 μL;总 RNA 1 μg;加去RNA酶水至14 μL。体系混匀后,42 ℃反应2 min。将Quantiscript RT Buffer(5×)4 μL;RT Primer Mix 1 μL;Reverse-transcription master mix 1 μL,充分混匀后,置于PCR仪中,反应程序:42 ℃30 min;95 ℃3 min进行反应,-20 ℃保存cDNA。利用Primer premier 5.0软件,并通过NCBI设计TGF-β3和Claudin-11的引物,引物由华大基因公司合成。引物序列见表1,退火温度为60 ℃。
表1荧光定量PCR引物序列及扩增条件
Table 1Fluorescence quantitative PCR primer sequences and amplification conditions
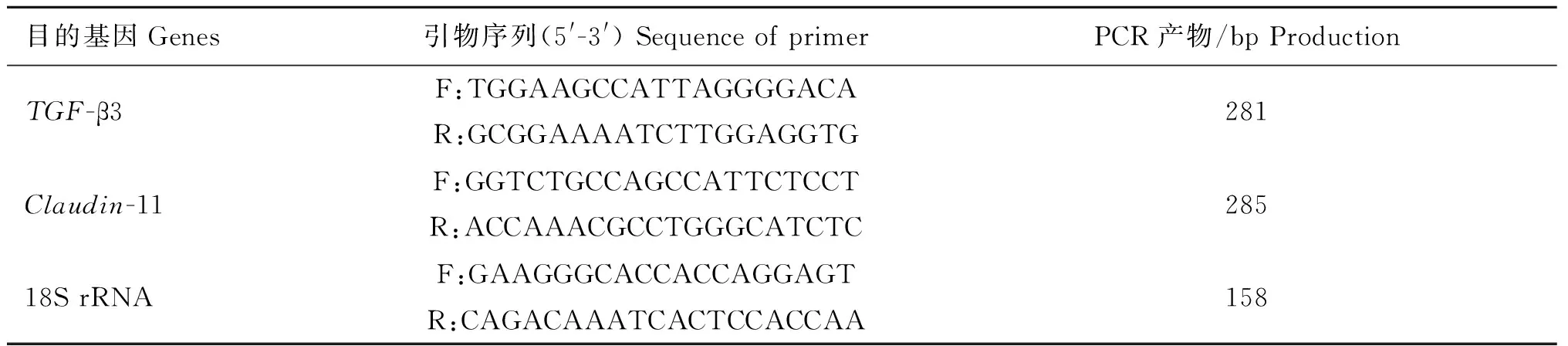
目的基因Genes引物序列(5'-3')SequenceofprimerPCR产物/bpProductionTGF-β3F:TGGAAGCCATTAGGGGACAR:GCGGAAAATCTTGGAGGTG281Claudin-11F:GGTCTGCCAGCCATTCTCCTR:ACCAAACGCCTGGGCATCTC28518SrRNAF:GAAGGGCACCACCAGGAGTR:CAGACAAATCACTCCACCAA158
按照QIAGEN试剂盒进行荧光定量PCR,反应结束后,由熔解曲线判定PCR反应的特异性,并根据扩增曲线CT值,利用2-△△CT法计算TGF-β3和Claudin-11在热应激组和对照组猪睾丸中相对表达水平。
1.3.2Western blotting使用RIPA强裂解液提取睾丸组织总蛋白,蛋白质的浓度用ND-1000微量核酸蛋白测定仪测定。上样后进行SDS-PAGE电泳,电泳完毕转移至NC膜,摇床上5%脱脂奶粉摇动封闭1 h。孵育一抗(1∶300 TGF-β3多克隆抗体,1∶200 Claudin-11多克隆抗体,1∶1 000 GAPDH单克隆抗体),4 ℃孵育过夜。TBST洗膜10 min×3次,HRP-羊抗兔IgG覆盖NC膜,37 ℃孵育1 h。TBST洗膜5 min×6次,加入高灵敏度发光试剂进行显色,暗室曝光获取图像,用Image-ProPlus6.0软件对TGF-β3和C1audin-11结果分析,数据均用“Means±SE”表示,用SPSS19.0软件进行单因素方差分析,P<0.05有统计学意义。
1.3.3免疫组织化学石蜡切片经二甲苯脱蜡、梯度酒精水化,加3%H2O2,置37 ℃孵育10 min,PBS缓冲液(pH=7.4)冲洗2 min×3次;用5%牛血清白蛋白(BSA)稀释一抗,滴加1∶50稀释的兔抗TGF-β3多克隆抗体和1∶50稀释的兔抗Claudin-11多克隆抗体,4 ℃过夜;置37 ℃反应30 min,PBS缓冲液冲洗2 min×3次;滴加HRP标记的羊抗兔IgG,置37 ℃孵育40 min,PBS缓冲液冲洗2 min×3次;DAB显色3 min。苏木精复染15 min,经梯度酒精脱水、二甲苯透明、中性树胶封片,显微镜下观察。部分切片以非免疫兔血清代替一抗作为阴性对照切片。
2 结 果
2.1qRT-PCR扩增
qRT-PCR结果表明,TGF-β3在对照组中mRNA的相对表达量为(1.254±0.197),而在热应激组中mRNA相对表达量升高,为(2.873±0.055),是对照组的2.291倍(P<0.01),二者表达差异极显著(图1A);对照组Claudin-11 mRNA的相对表达量为(0.917±0.050),在热应激组Claudin-11 mRNA的相对表达量降低,为(0.710±0.101),对照组是热应激组的1.292倍(P<0.05),二者表达差异显著(图1B)。
2.2Western blotting检测
兔抗TGF-β3多克隆抗体可以与猪睾丸蛋白提取物中分子量约为47 ku的蛋白条带发生免疫阳性反应(图2A)。通过SPSS19.0软件分析数据得到TGF-β3蛋白在对照组相对表达量为(1.171±0.178),热应激组相对表达量升高,为(1.350±0.200),热应激组TGF-β3表达量是对照组的1.153倍(P<0.05),两者差异显著(图2B)。
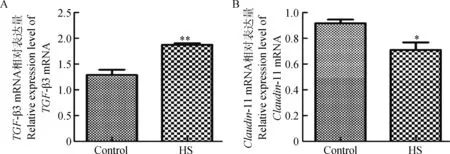
A.TGF-β3;B.Claudin-11。Control.对照组;HS.热应激组;*.P<0.05,**.P<0.01。下同A.TGF-β3;B.Claudin-11.Control.Control group;HS.Heat stress group;*.P<0.05;**.P<0.01.The same as below图1 TGF-β3和Claudin-11 mRNA在对照组和热应激组猪睾丸的相对表达量Fig.1 Relative expression level results of TGF-β3 and Claudin-11 mRNA in boar testis collected from control group and heat stress group
兔抗C1audin-11多克隆抗体可以与猪睾丸蛋白提取物中分子量约为22 ku的蛋白条带发生免疫阳性反应(图2A)。通过SPSS19.0软件分析数据得到C1audin-11蛋白在对照组相对表达量为(0.698±0.062),热应激组相对表达量下降,为(0.443±0.034),对照组C1audin-11的表达量是热应激组的1.576倍(P<0.05),两者差异显著(图2C)。
2.3免疫组织化学染色
2.3.1TGF-β3在猪睾丸中的免疫组织化学染色对照组TGF-β3免疫反应阳性物着色于精原细胞、精母细胞及圆形精子细胞的胞质中,呈阳性表达,在支持细胞胞质染色较浅,表达较弱(图3A);热应激组TGF-β3于各级生精细胞的胞质着色较对照组着色深,呈强阳性表达,在支持细胞胞质的着色也变深,表达增强(图3B)。阴性对照切片以正常兔血清代替一抗,无特异性着色(图3C)。
2.3.2Claudin-11在猪睾丸中的免疫组织化学染色对照组中Claudin-11定位于支持细胞质膜相应位置,在血睾屏障位置处呈明显的带状表达,形成一条强阳性表达的连续带(图4A);热应激组Claudin-11表达与对照组相比差异明显,Claudin-11的表达局限在支持细胞周围,失去明显的血睾屏障带状表达(图4B)。阴性对照切片以正常兔血清代替一抗,无特异性着色(图4C)。
3 讨 论
TGF-β3在哺乳动物睾丸中的表达已见有各种报道。V.Caussanel等[19]研究发现,TGF-β3表达于成熟期猪睾丸的支持细胞和分裂前期的生殖细胞。TGF-β3表达于雄性大鼠睾丸生精上皮的减数分裂前的精母细胞、支持细胞和圆形精子细胞[20-21]。本研究对照组免疫组织化学结果显示,TGF-β3蛋白主要表达在猪睾丸各级精母细胞和支持细胞胞质中,与W.Xia等[20]、W.Y.Lui等[21]对大鼠睾丸,A.Wagener等[22]对鹿睾丸中的表达结果基本一致。
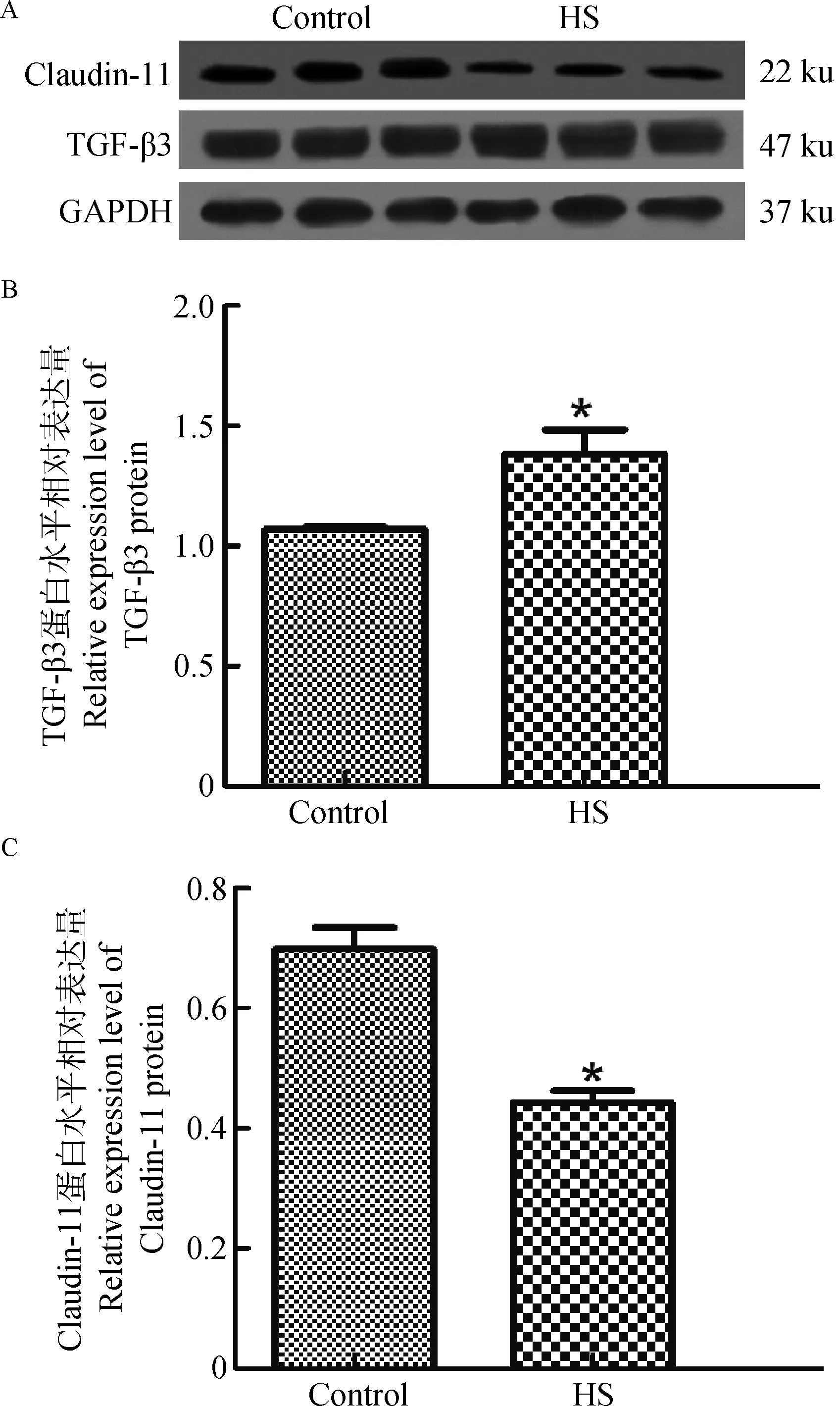
A.47和22 ku分别为TGF-β3多克隆抗体和Claudin-11多克隆抗体印记;B.TGF-β3在对照组和热应激组中的相对表达量;C.Claudin-11在对照组和热应激组中的相对表达量A.47 and 22 ku,respectively TGF-β3 polyclonal antibody and Claudin-11 polyclonal antibody imprint;B.Relative expression levels of TGF-β3 protein in control group and heat stress group;C.Relative expression levels of Claudin-11 protein in control group and heat stress group图2 TGF-β3和Claudin-11表达免疫印迹分析结果Fig.2 Western blotting analysis results of the expression of TGF-β3 and Claudin-11
A.对照组;B.热应激组;C.阴性对照组.Sg.精原细胞;Sp.精母细胞;Sc.支持细胞;RS.圆形精子细胞;标尺=25 μm。图4同A.Control group;B.HS group;C.Negative control group.Sg.Spermatogonia;Sp.spermatocyte;Sc.Sertoli cell;R.S.Round spermatid;bar=25 μm.The same as Figure 4图3 TGF-β3在猪睾丸的免疫组织化学表达和定位Fig.3 Immunohistochemical expression and localization result of TGF-β3 in boar testis
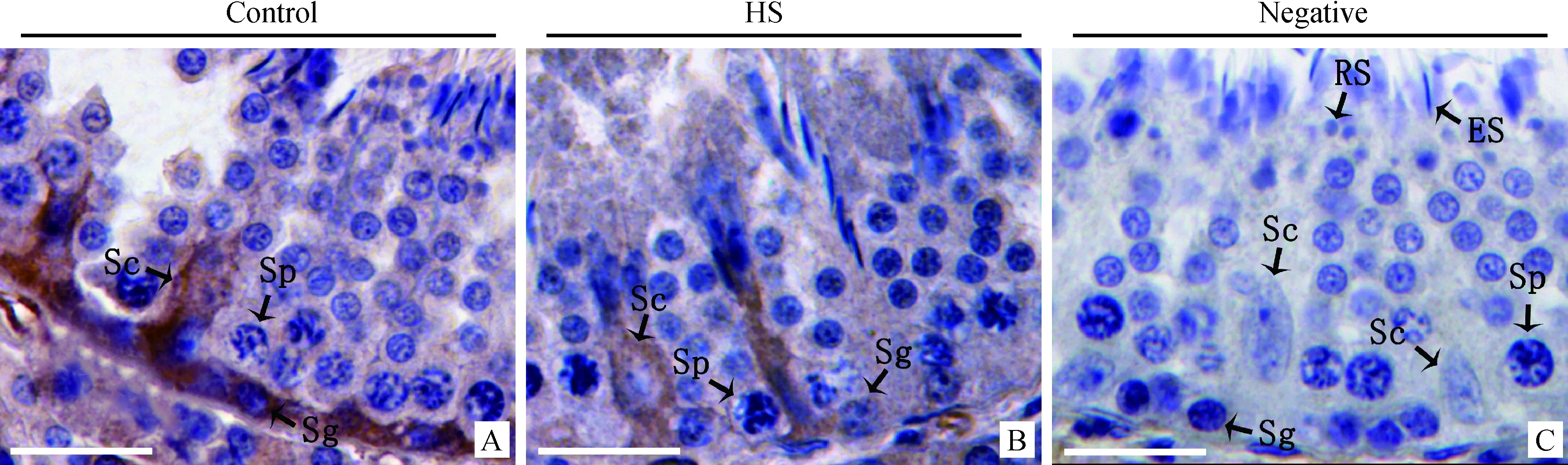
ES.长形精子细胞ES.Elongated spermatid图4 Claudin-11在猪睾丸的免疫组织化学表达和定位Fig.4 Immunohistochemical expression and localization result of Claudin-11 in boar testis
Claudin-11是构成血睾屏障中支持细胞紧密连接重要的蛋白分子,C.J.Park等[23]研究表明,Claudin-11在野鸡睾丸平行表达于生精上皮底部的基膜层。Claudin-11表达于成年羊驼睾丸支持细胞的基底部,在生精上皮形成连续带[24]。C.J.Park等[25]发现Claudin-11表达在软壳龟睾丸支持细胞胞质的下方。本研究对照组免疫组织化学结果显示,Claudin-11主要平行表达于支持细胞的基底部,在血睾屏障对应处形成一条强阳性表达的连续带,与C.J.Park等[23]对野鸡睾丸,Q.Y.Guo等[24]对羊驼睾丸,C.J.Park等[25]对软壳龟睾丸中的表达定位结果相似。
本研究热应激组免疫组织化学结果显示,TGF-β3于各级生精细胞和支持细胞胞质的着色较对照组深;Claudin-11的表达局限在支持细胞周围,失去明显的血睾屏障带状表达。根据qRT-PCR和Wertern blotting结果显示,TGF-β3在热应激组的mRNA和蛋白相对表达量较对照组升高;而热应激处理却导致C1audin-11的mRNA和蛋白相对表达量较对照组降低。推断37~40 ℃热处理猪睾丸后,TGF-β3表达升高,升高的TGF-β3可能下调Claudin-11的表达,这与H.Cai等[18]对小鼠睾丸,W.Y.Lui等[17]对大鼠睾丸的研究结果相似,但其机制有待进一步研究。
用睾丸支持细胞离体培养系统研究发现,TGF-β3通过激活p38 MAPK信号通路,调节紧密连接蛋白Claudin-11的表达,进而干扰支持细胞紧密连接屏障[21],这提示TGF-β3是调控支持细胞紧密连接屏障的一个重要因子。目前在所有的Claudin蛋白家族中,人们对Claudin-11的研究最多。据报道,敲除小鼠睾丸支持细胞中的Claudin-11,曲精小管内精子畸形率增高,精子活力降低,导致小鼠不育[26]。这提示Claudin-11对睾丸精子的发生有着重要意义。
综上表明,推测热应激导致猪睾丸TGF-β3表达升高,下调Claudin-11的表达,导致正常的精子发生受阻,进而影响精子发生和精液品质。
4 结 论
本研究结果表明,TGF-β3及血睾屏障紧密连接蛋白Claudin-11特异性定位和表达于性成熟猪睾丸中。37~40 ℃热应激处理猪睾丸,TGF-β3表达升高,Claudin-11表达下降,提示热应激可能经由调节这两个基因的表达来影响精子发生。
[1]DANNO S,ITOH K,MATSUDA T,et al.Decreased expression of mouse Rbm3,a cold-shock protein,in Sertoli cells of cryptorchid testis[J].AmJPathol,2000,156(5):1685-1692.
[2]MIEUSSET R,BUJAN L.Testicular heating and its possible contributions to male infertility:a review[J].IntJAndrol,1995,18(4):169-184.
[3]THONNEAU P,BUJAN L,MULTIQNER L.Occupational heat exposure and male fertility:a review[J].HumReprod,1998,13(8):2122-2125.
[4]AX R L,GILBERT G R,SHOOK G E.Sperm in poor quality semen from bulls during heat stress have a lower affinity for binding hydrogen-3 heparin[J].JDairySci,1987,70(1):195-200.
[5]ZHANG X S,LUE Y H,GUO S H,et al.Expression of HSP105 and HSP60 during germ cell apoptosis in the heat-treated testes of adult cynomolgus monkeys (Macacafascicularis)[J].FrontBiosci,2005,10:3110-3121.
[6]LUE Y,WANG C,LIU Y X,et al.Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macacafascicularis)[J].JClinEndocrinol,2006,91(2):539-545.
[7]TRUDEAU V,SANFORD L M.Effect of season and social environment on testis size and semen quality of the adult Landrace boar[J].JAnimSci,1986,63(4):1211-1219.
[8]GRISWOLD M D.Interactions between germ cells and Sertoli cells in the testis[J].BiolReprod,1995,52(2):211-216.
[9]SMITH B E,BRAUN R E.Germ cell migration across Sertoli cell tight junctions[J].Science,2012,338(6108):798-802.
[10]FURUSE M,HIRASE T,ITOH M,et al.Occludin:a novel integral membrane protein localizing at tight junctions[J].JCellBiol,1993,123(6 Pt 2):1777-1788.
[11]FINK C,WEIGEL R,FINK L,et al.Claudin-11 is over-expressed and dislocated from the blood-testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men[J].HistochemCellBiol,2009,131(6):755-764.
[12]HELLANI A,JI J,MAUDUIT C,et al.Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/Claudin 11 in mouse testis[J].Endocrinology,2000,141(8):3012-3019.
[13]PARK C J,LEE J E,OH Y S,et al.Postnatal changes in the expression of Claudin-11 in the testes and excurrent ducts of the domestic rabbit (Oryctolaguscuniculusdomesticus)[J].JAndrol,2011,32(3):295-306.
[14]ZHANG Z H,HU Z Y,SONG X X,et al.Disrupted expression of intermediate filaments in the testis of rhesus monkey after experimental cryptorchidism[J].IntJAndrol,2004,27(4):234-239.
[15]FELDMAN G J,MULLIN J M,RYAN M P.Occludin:structure,function and regulation[J].AdvDrugDelivRev,2005,57(6):883-917.
[16]OLASO R,PAIRAULT C,SAEZ J M,et al.Transforming growth factor β3 in the fetal and neonatal rat testis:immunolocalization and effect on fetal Leydig cell function[J].HistochemCellBiol,1999,112(3):247-254.
[17]LUI W Y,LEE W M,CHENG C Y.Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrierinvitropossibly mediated via its effects on Occludin,zonula Occludens-1,and Claudin-11[J].Endocrinology,2001,142(5):1865-1877.
[18]CAI H,REN Y,LI X X,et al.Scrotal heat stress causes a transient alteration in tight junctions and induction of TGF-β expression[J].IntJAndrol,2011,34(4):352-362.
[19]CAUSSANEL V,TABONE E,HENDRICK J C,et al.Cellular distribution of transforming growth factor betas 1,2,and 3 and their types I and II receptors during postnatal development and spermatogenesis in the boar testis[J].BiolReprod,1997,56(2):357-367.
[20]XIA W,MRUK D D,LEE W M,et al.Differential interactions between transforming growth factor-beta3/T betaR1,TAB1,and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion[J].JBiolChem,2006,281(24):16799-16813.
[21]LUI W Y,LEE W M,CHENG C Y.Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway[J].BiolReprod,2003,68(5):1597-1612.
[22]WAGENER A,FICKEL J,SCHON J,et al.Seasonal variation in expression and localization of testicular transforming growth factors TGF-beta1 and TGF-beta3 corresponds with spermatogenic activity in roe deer[J].JEndocrinol,2005,187(2):205-215.
[23]PARK C J,LEE J E,OH Y S,et al.Expression of Claudin-1 and-11 in immature and mature pheasant (Phasianuscolchicus) testes[J].Theriogenology,2011,75(3):445-458.
[24]GUO Q Y,GAO Z Z,ZHAO L,et al.Expression of growth differentiation factor 9 (GDF9),ALK5,and Claudin-11 in adult alpaca testis[J].ActaHistochem,2013,115(1):16-21.
[25]PARK C J,HA C M,LEE J E,et al.Claudin 11 inter-Sertoli tight junctions in the testis of the Korean soft-shelled turtle (Pelodiscusmaackii)[J].BiolReprod,2015,92(4):96.
[26]MAZAUD-GUITTOT S,MEUGNIER E,PESENTI S,et al.Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis[J].BiolReprod,2010,82(1):202-213.
(编辑程金华)
Effect of Heat Stress on the Expression of TGF-β3 and Claudin-11 Protein in Mature Boar Testis
ZHANG Zhen,FAN Xiao-rui,XI Hua-ming,LIANG Ya-jun,HE Jun-ping*
(CollegeofAnimalScienceandVeterinaryMedicine,ShanxiAgriculturalUniversity,Taigu030801,China)
The purposes of this study was to explore the expression and location of TGF-β3 and Claudin-11 in the boar testis under heat stress (37-40 ℃) and normal temperature (20-27 ℃).Six boars (Landrace,18 months of age) were used and divided into 2 groups.3 boars were homed in a thermo-controlled temperature (37-40 ℃,3 h daily,consecutive 7 d) house as a heat stress group.After heat treatment,the boars were driven back to normal temperature (20-27 ℃).The other 3 boars were homed in 20-27 ℃ house as a control group.7 days later,all boars were castrated and the testis tissues were harvested.qRT-PCR,Western blotting and immunohistochemistry were used to explore the changes of mRNA and protein in response to heat treatment.qRT-PCR showed that relative expression levels of TGF-β3 mRNA significantly increased (P<0.01),while relative expression levels of Claudin-11 mRNA decreased(P<0.05) in heat treatment group compared with the control.Western blotting found that the expression levels of TGF-β3 protein significantly increased(P<0.05)in heat treatment group,while the expression levels of Claudin-11 protein decreased(P<0.05)compared with the control.Immunohistochemistry results showed that:TGF-β3 immunoreactivity staining was observed in all stages of germ cells and Sertoli cells in both heat stress group and the control,and the depth and area of the positive staining in heat stress group were higher than that of the control;Claudin-11 immunoreactivity staining decreased in heat stress boars compared with the control in that Claudin-11 immunoreactivity staining localized in a consecutive strand area corresponding to the blood-testis barrier in the testis of control boars,while Claudin-11 immunoreactivity staining was limited to Sertoli cells and no obvious immunoreactivity strand that could be found in heat stress group.Then we could get a conclusion that heat stress damaged the sperm quality and spermatogenesis maybe partly via heat stress increased the expression of TGF-β3 and decreased the expression of Claudin-11 in boar testis.
heat stress;boar testis;spermatogenesis;TGF-β3;Claudin-11
10.11843/j.issn.0366-6964.2016.10.023
2016-04-25
国家自然科学基金项目(31470124)
张禛(1991-),男,山西阳泉人,硕士生,主要从事基础兽医学研究,E-mail:18935445461@163.com
贺俊平,E-mail:dnhjp@163.com
S852.1
A
0366-6964(2016)10-2136-07
