冷诱导RNA结合蛋白通过激活NF-κB信号通路影响H1N1甲型流感病毒的复制
2016-11-08聂培婷
聂培婷,汤 承,岳 华
(西南民族大学生命科学与技术学院,成都 610041)
冷诱导RNA结合蛋白通过激活NF-κB信号通路影响H1N1甲型流感病毒的复制
聂培婷,汤承,岳华*
(西南民族大学生命科学与技术学院,成都 610041)
冷诱导RNA结合蛋白(CIRP)是NF-κB和ERK信号通路的上游调控因子,而这两条信号通路是甲型流感病毒复制和机体起始免疫所必需的,为探讨CIRP对H1N1甲型流感病毒复制的影响及可能的分子机制,构建了CIRP过表达BHK-21细胞系(Cirp+BHK-21),用Western blot检测NF-кB和ERK1/2的磷酸化水平,研究CIRP对NF-кB和ERK1/2的调节作用;用Real-time RT-PCR检测H1N1甲型流感病毒感染后Cirp+BHK-21和对照细胞中病毒拷贝数的动态变化,以及在特异性阻断剂PDTC阻断NF-кB通路的Cirp+BHK-21细胞中病毒拷贝数的动态变化。Western blot检测结果显示:过表达CIRP显著促进了BHK-21细胞中NF-κB的磷酸化水平(P<0.05),而对ERK1/2的磷酸化水平无显著影响;病毒定量检测结果显示:过表达CIRP能显著促进H1N1甲型流感病毒的增殖,感染后3、9、15、21 h 病毒在Cirp+BHK-21细胞中的拷贝数分别为对照组的111%、103%、167%和235% (P<0.05);阻断NF-κB信号通路后病毒的拷贝数显著下降,在感染后3、9、15、21 h分别为未阻断组的98%、42%、19%(P<0.05)和7%(P<0.05)。从本研究结果可见,CIRP可通过活化NF-κB信号通路促进H1N1甲型流感病毒的复制。
冷诱导RNA结合蛋白;H1N1甲型流感病毒;病毒复制;NF-κB;BHK-21细胞
甲型流感病毒(influenza A virus)为正黏病毒科(orthomyxoviridae)成员,基因组为分节段的单股负链RNA[1]。甲型流感病毒感染后触发了宿主细胞一系列的分子事件,一方面帮助病毒完成复制周期,同时也激活了机体的免疫应答以抵抗病毒感染[2-7]。最近,有三个研究团队利用全基因组RNA干涉技术鉴定出一大批与流感病毒复制有关的宿主因子[4-5]。涉及病毒复制和转录、机体的免疫应答、机体与病毒vRNP相互作用和病毒RNA合成等相关的多种宿主因子[8-16],其中包括一些RNA结合蛋白(RNA-binding proteins,RanBP)家族成员,如RNA结合蛋白GFSF-1与流感病毒蛋白的合成有关[17-18],RNA结合蛋白PKR的胞内抑制剂P58IPK能促进流感病毒蛋白的合成[19]。RNA结合蛋白3(RanBP3)与甲型流感病毒的出核转运有关[20],在流感病毒感染过程中还存在干扰素IFN-β合成的RNA结合蛋白调控网络,激酶PKR、多重剪接RNA结合蛋白(RNA-binding protein with multiple splicing,RBPMS)、白介素增强子结合因子3(interleukin enhancer-binding factor 3,ILF3)、FMR1、DHX9、ZNF346和HNRPC参与其中[11]。
冷诱导RNA结合蛋白(CIRP)是一种在脊椎动物间高度保守的多功能蛋白,参与对温和低温[21]、H2O2[22]、缺氧[23]、渗透压[24]等多种应激的转录应答,发挥细胞保护作用[25-26],同时参与胚胎发育、神经调节[27]和生物钟调节等生理过程,此外,CIRP还参与机体对新城疫病毒(NDV)[28]、细菌内毒素(LPS)[29]的转录应答,但未见CIRP对流感病毒感染应答的研究。业已证明,ERK通路和核因子κB通路(NF-κB)是流感病毒复制所必需的[30-34],而CIRP作为这两条信号通路的上游调控因子,可能通过这两条信号通路影响病毒复制。本研究旨在探讨CIRP对流感病毒复制的影响及其分子机制,为深入解析流感病毒与宿主之间相互作用的分子机制提供参考。
1 材料与方法
1.1细胞株和毒株
过表达CIRP的BHK-21细胞系(Cirp+BHK-21)和对照细胞系(BHK-21 GFP)由作者实验室构建[35];H1N1甲型流感病毒PR/8毒株(HA效价为28)由四川省疾病预防控制中心惠赠。
1.2主要试剂和仪器
高糖DMEM培养基(HyClone公司);胎牛血清(GIBCO公司);RNA提取试剂盒(Invitrogen公司);反转录试剂盒(TaKaRa公司);NF-κB特异性抑制剂PDTC、TPCK胰酶(Sigma 公司);BCA试剂盒、RIPA蛋白裂解液(碧云天公司);CIRP兔抗人多克隆抗体(Proteintech公司);一抗为鼠抗NF-κB P65、ERK1/2磷酸化抗体(p-ERK1/2)、ERK1/2抗体(碧云天公司);二抗为HRP标记的羊抗鼠IgG抗体(北京中杉金桥公司);ECL发光试剂盒(TransGen公司);电泳仪、转膜仪(Bio-Rad公司);ABI 7300荧光定量PCR仪(美国ABI公司);Casy TT细胞计数仪(Roche公司)。
1.3NF-κB和ERK1/2的磷酸化水平的测定
NF-кB和ERK1/2的磷酸化水平(p-ERK1/2)是判断这两条信号通路是否被激活的重要指标,检测ERK磷酸化水平时需要同时检测ERK1/2的表达水平以确保试验结果的准确性。根据RIPA裂解液说明书提取Cirp+BHK-21和BHK-21 GFP细胞总蛋白质,BCA法蛋白质定量,并制备蛋白质含量10 μg·μL-1的电泳样品,取8 μL(80 μg总蛋白质)蛋白质经SDS-PAGE后转印到PVDF膜上,5%的脱脂奶粉4 ℃封闭过夜。分别加入3 mL 1∶300稀释一抗(NF-κB P65、p-ERK1/2和ERK1/2),4 ℃封闭过夜,随后加入3 mL 1∶5 000稀释的二抗,37 ℃孵育1 h,按照ECL试剂盒说明书显色,最后用X-胶片曝光。以β-actin作为内参,quantity one分析软件检测蛋白质灰度值,应用SPSS软件进行数据处理,分析过表达CIRP对BHK-21细胞中NF-κB和ERK1/2磷酸化水平的影响。
1.4CIRP对H1N1甲型流感病毒复制的影响
1.4.1PR-8感染Cirp+BHK-21和BHK-21 GFP细胞将Cirp+BHK-21和BHK-21 GFP分别培养于6孔细胞培养板,待细胞形成致密单层时,接种1MOI (1拷贝·cell-1)PR/8,置于37 ℃、5% CO2培养箱中孵育1 h后弃去接种物,加入2.5 mL·孔-1维持液(4%胎牛血清、5 μg·mL-1TPCK胰酶)置于37 ℃、5% CO2的培养箱中培养。
1.4.2病毒拷贝数的测定在感染后3、9、15和21 h分别收集Cirp+BHK-21和BHK-21 GFP上清各3孔,用RNA试剂盒提取总RNA,并用反转录试剂盒合成cDNA,用Real-time RT-PCR方法[36]检测病毒在Cirp+BHK-21和BHK-21 GFP中的拷贝数,用Ppia作为内参基因[37],用甲型H1N1流感病毒M基因Real-time RT-PCR方法的标准曲线公式(y=30.62-3.53x)计算病毒拷贝数。
1.5阻断NF-κB信号通路对流感病毒复制的影响1.5.1PDTC对NF-κB信号通路阻断效果测定将Cirp+BHK-21细胞培养在75 cm2培养瓶中,待形成致密单层后,加入DMSO溶解的25 μmol·L-1PDTC按照文献[38]进行处理,另取3瓶加入等体积的DMSO作为对照组,37 ℃、5% CO2培养3 h,按“1.3”方法提取总蛋白质并检测NF-κB的磷酸化水平,以β-actin作为内参基因,评价PDTC的阻断效果。1.5.2阻断NF-κB信号通路对流感病毒复制的影响待Cirp+BHK-21细胞在6孔培养板中长成致密单层后,按“1.5.1”方法加入PDTC,15 min后弃去上清,以阻断剂稀释液代替PDTC处理Cirp+BHK-21作为对照组;两组细胞按“1.4.1”方法感染PR/8,分别于感染后3、9、15和21 h收集两组病毒各3孔,按“1.4.2”的方法检测病毒拷贝数。
1.6数据分析
所有待检样本均进行3次平行重复试验,并用SPSS18.0软件进行分析。
2 结 果
2.1过表达CIRP 显著提高了NF-κB的磷酸化水平
对Cirp+BHK-21中和BHK-21 GFP细胞中NF-κB P65、ERK1/2和p-ERK1/2的表达磷酸化水平的检测结果显示,Cirp+BHK-21中ERK1/2(图1A、1B)和p-ERK1/2(图1C)的磷酸化水平略升高,但与对照组无显著差异(P>0.05);而NF-κB P65(图1D)的表达水平显著提高(P<0.05),说明过表达CIRP能显著提高BHK-21细胞中NF-κB的磷酸化水平,激活NF-κB信号通路,但其对ERK信号通路无明显影响。
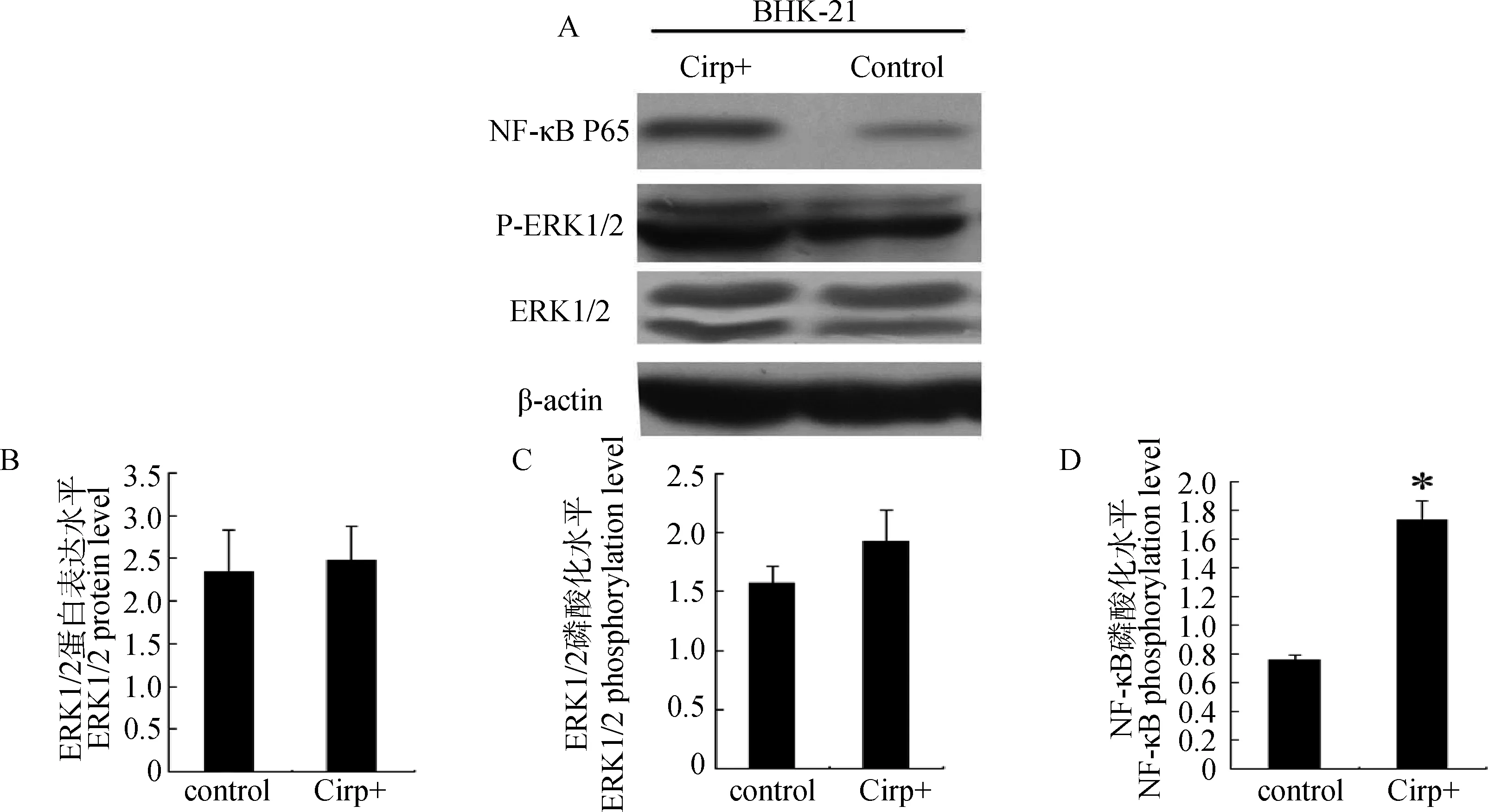
A.Western blot 检测结果;B.ERK1/2半定量测定结果;C.p-ERK1/2半定量测定结果;D.NF-κB p65半定量测定结果;*.差异显著(P<0.05) A.The result of Western blot;B.The semi-quantitative result of ERK1/2;C.The semi-quantitative result of p-ERK1/2;D.The semi-quantitative result of NF-κB p65;*.Significant difference (P<0.05)图1 Crip+BHK-21和BHK-21 GFP细胞中ERK1/2、P-ERK1/2和 NF-κB P65的表达水平Fig.1 Expression of NF-κB P65,P-ERK1/2,ERK1/2 in Crip+BHK-21 whole-cells and control whole-cells
2.2过表达CIRP促进流感病毒的增殖
病毒定量检测结果显示,在感染后3、9、15和21 h,Crip+BHK-21中病毒的拷贝数分别为对照组的111%、103%、167%和235%(P<0.05)(表1),说明过表达CIRP促进了H1N1甲型流感病毒在BHK-21细胞中的增殖。
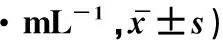
细胞系Cellline时间/hTimespostinfection(h)391521Cirp+BHK-211.29±0.103.46±1.589.76±2.2421.8±3.30*BHK-21GFP1.16±0.433.35±2.145.97±2.949.64±1.76
*.P<0.05
2.3PDTC对NF-κB信号通路的阻断效果
阻断试验结果表明,PDTC显著抑制了NF-κB的磷酸化作用,阻断了NF-κB信号通路(图2)。
2.4阻断NF-κB信号通路抑制了流感病毒的复制
阻断NF-κB信号通路,导致H1N1甲型流感病毒在Cirp+BHK-21细胞中的拷贝数明显降低,在感染后3、9、15和21 h分别为对照组的98%、42%、19%(P<0.05)和7%(P<0.05)(图3)。可见,CIRP通过激活NF-κB信号通路促进H1N1甲型流感病毒在BHK-21细胞中的复制。
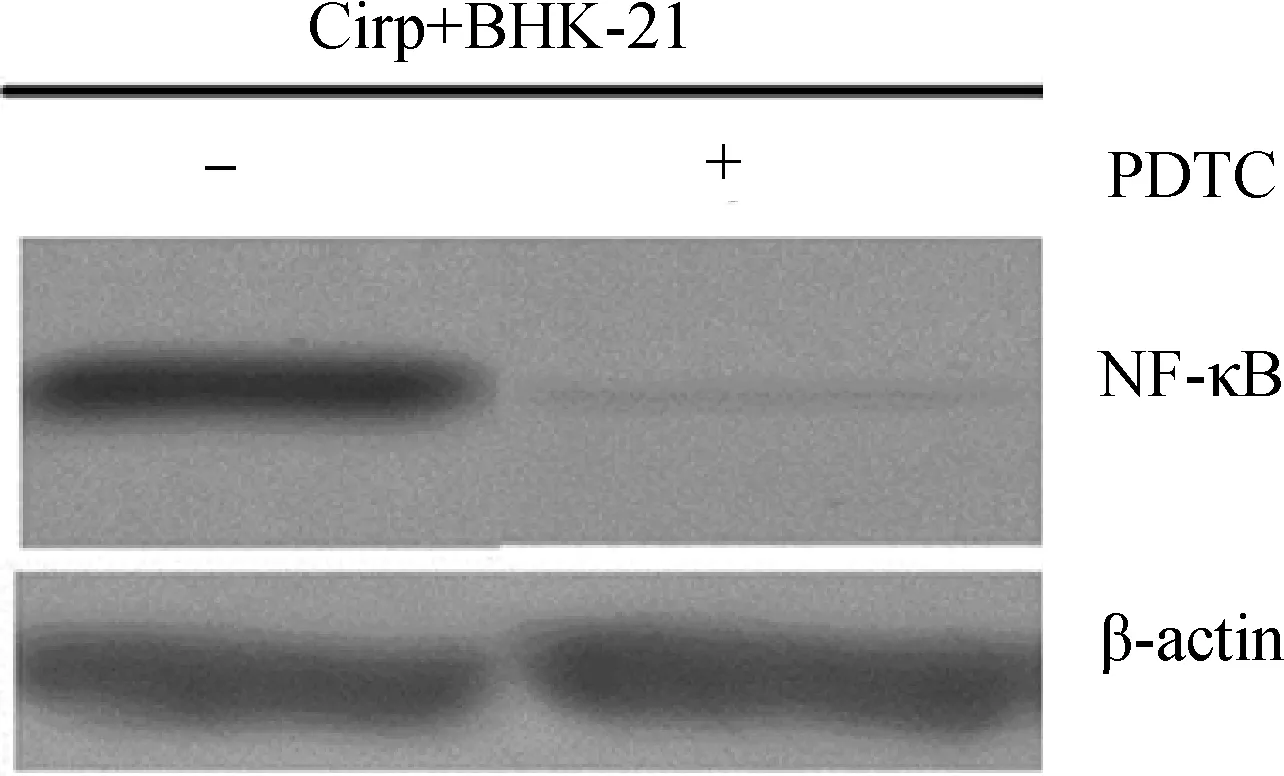
图2 PDTC对NF-κB信号通路的阻断效果Fig.2 The blocking effect of PDTC
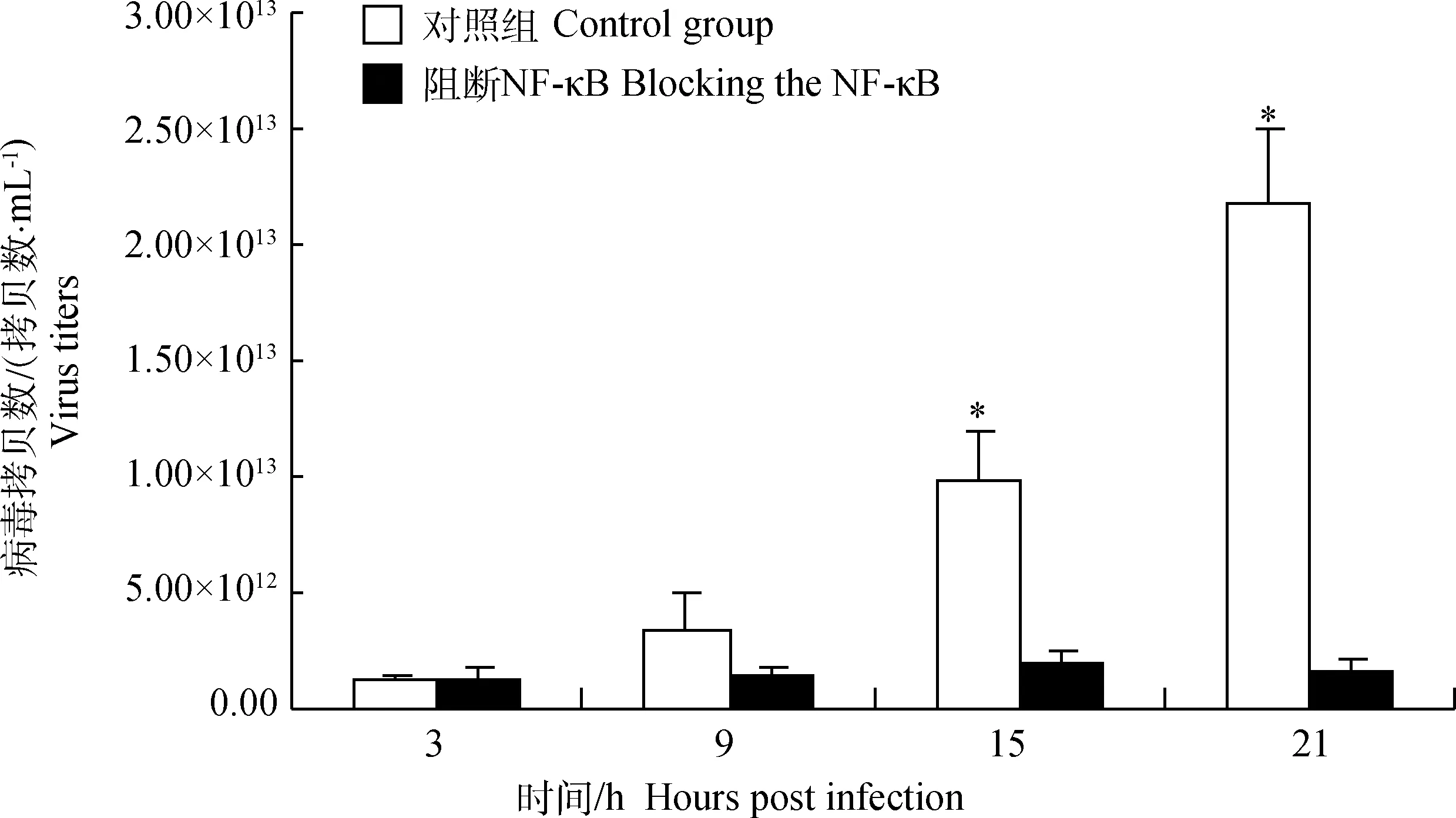
图3 阻断NF-κB抑制了CIRP过表达引起的H1N1甲型流感病毒滴度增加Fig.3 Inhibition of NF-κB p65 expression blocked the increase of virus titers caused by CIRP overexpression
3 讨 论
3.1过表达的CIRP能显著促进流感病毒在BHK-21细胞中的增殖
本研究中,在感染后21 h病毒拷贝数在过表达的BHK-21细胞中极显著升高,病毒滴度提高1倍以上,说明CIRP可能是通过激活细胞中一系列分子事件最终促进流感病毒增殖。BHK-21对多种动物病毒敏感,是包括流感病毒在内的多种动物病毒疫苗生产常用的培养系统,筛选和培育病毒高产细胞系一直是疫苗生产最迫切追求的目标,本研究证实稳定过表达CIRP能大大提高流感病毒在BHK-21中的滴度,为培育病毒高产细胞系提供了新思路。
3.2CIRP通过激活NF-κB信号通路促进流感病毒复制
研究证明,过表达的CIRP能引起NF-κB磷酸化水平的上调[30],CIRP是NF-κB信号通路的上游调控因子,而这条信号通路是流感病毒复制所必需的[31,34]。NF-κB是一个具有抗凋亡活性的转录因子,参与免疫应答和转录调控,广泛地影响参与细胞存活、分化和增殖的基因的表达[5],并在流感病毒复制中发挥重要作用,阻断人肺上皮细胞A549和U1752中NF-κB信号通路的激活可以抑制流感病毒的感染和增殖[30,39]。在本研究中,阻断NF-κB信号通路也同时阻断了由CIRP过表达引起的病毒滴度的增加,说明CIRP通过激活NF-κB信号通路促进流感病毒复制。业已证明NF-κB信号通路促进流感病毒复制的分子机制主要有以下三种:第一,NF-κB通过调控凋亡因子如肿瘤坏死因子相关凋亡诱导配体(TRAIL)或FasL[40],随后激活caspases途径,从而促进病毒核糖核蛋白复合体(RNPs)的输出[41-42];第二,涉及NF-κB的依赖性反作用类型的干扰素诱导基因(ISG)的表达,可能会通过上调细胞因子信号转导抑制因子3(SOCS-3)和/或通过直接抑制ISG启动子区域[43-44];第三,在流感病毒感染的细胞中,NF-κB抑制剂能特异性地降低病毒RNA(vRNA)水平及RNA的转录水平,P65亚基似乎与vRNA合成调节有关,提示NF-κB可能调节流感病毒RNA的合成[45]。但CIRP激活NF-κB通路的分子机制还不十分清楚,有待进一步研究。
[1]殷震,刘景华.动物病毒学[M].2版.北京:科学出版社,1997:704-712.
YIN Z,LIU J H.Animal virology[M].Beijing:Science Press,1997:704-712.(in Chinese)
[2]EMARA M M,BRINTON M A.Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly[J].ProcNatlAcadSciUSA,2007,104(21):9041-9046.
[3]COOMBS K M,BERARD A,XU W,et al.Quantitative proteomic analyses of influenza virus-infected cultured human lung cells[J].JVirol,2010,84(20):10888-10906.
[4]KÖNIG R,STERTZ S,ZHOU Y,et al.Human host factors required for influenza virus replication[J].Nature,2010,463(7282):813-817.
[5]YANG X X,DU N,ZHOU J F,et al.Gene expression profiles comparison between 2009 pandemic and seasonal H1N1 influenza viruses in A549 cells[J].BiomedEnvironSci,2010,23(4):259-266.
[6]CHAKRABARTI A K,VIPAT V C,MUKHERJEE S,et al.Host gene expression profiling in influenza A virus-infected lung epithelial (A594)cells:a comparative analysis between highly pathogenic and modified H5N1 viruses[J].VirolJ,2010,7:219.
[7]LEE S M,GARDY J L,CHEUNG C Y,et al.Systems-level comparison of host-responses elicited by avian H5N1 and seasonal H1N1 influenza viruses in primary human macrophages[J].PLoSOne,2009,4(12):e8072.
[8]BRASS A L,DYKXHOORN D M,BENITA Y,et al.Identification of host proteins required for HIV infection through a functional genomic screen[J].Science,2008,319(5865):921-926.
[9]KARLAS A,MACHUY N,SHIN Y,et al.Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication[J].Nature,2010,463(7282):818-822.
[10]SHAPIRA S D,GAT-VIKS I,SHUM BO,et al.A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection[J].Cell,2009,139(7):1255-1267.
[11]DENG T,ENGELHARDT O G,THOMAS B,et al.Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex[J].JVirol, 2006,80(24):11911-11919.
[12]ENGELHARDT O G,SMITH M,FODOR E.Association of the influenza A virus RNA-dependent RNA polymerase with cellular RNA polymerase II[J].JVirol,2005,79(9):5812-5818.
[13]HUARTE M,SANZ-EZQUERRO J J,RONCAL F,et al.PA subunit from influenza virus polymerase complex interacts with a cellular protein with homology to a family of transcriptional activators[J].JVirol,2001,75(18):8597-8604.
[14]JORBA N,JUAREZ S,TORREIRA E,et al.Analysis of the interaction of influenza virus polymerase complex with human cell factors[J].Proteomics,2008,8(10):2077-2088.
[15]KAWAGUCHI A,NAGATA K.De novo replication of the influenza virus RNA genome is regulated by DNA replicative helicase,MCM[J].EMBOJ,2007,26(21):4566-4575.
[16]WATANABE T,WATANABE S,KAWAOKA Y.Cellular networks involved in the influenza virus life cycle[J].CellHostMicrobe,2010,7(6):427-439.
[17]PARK YW,WILUSZ J,KATZE MG.Regulation of eukaryotic protein synthesis:selective influenza viral mRNA translation is mediated by the cellular RNA-binding protein GRSF-1[J].ProcNatlAcadSciUSA,1999,96(12):6694-6699.
[18]KASH J C,CUNNINGHAM D M,SMIT M W,et al.Selective translation of eukaryotic mRNAs:functional molecular analysis of GRSF-1,a positive regulator of influenza virus protein synthesis[J].JVirol,2002,76(20):10417-10426.
[19]GOODMAN A G,SMITH J A,BALACHANDRAN S,et al.The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism[J].JVirol,2007,81(5):2221-2230.
[20]PREDICALA R,ZHOU Y.The role of ran-binding protein 3 during influenza A virus replication[J].JGenVirol,2013,94(Pt 5):977-984.
[21]NISHIYAMA H,ITOH K,KANEKO Y,et al.A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth[J].JCellBiol,1997,137(4):899-908.
[22]XUE J H,NONOGUCHI K,FUKUMOTO M,et al.Effects of ischemia and H2O2on the cold stress protein CIRP expression in rat neuronal cells[J].FreeRadieBiolMed,1999,27(11-12):1238-1244.
[23]WELLMANN S,BÜHRER C,MODEREGGER E,et al.Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism[J].JCellSci,2004,117(Pt 9):1785-1794.
[24]PAN F,ZARATE J,CHOUDHURY A,et al.Osmotic stress of salmon stimulates upregulation of a cold inducible RNA binding protein (CIRP) similar to that mammals and amphibians[J].Biochimine,2004,86(7):451-461.
[25]YANG C,CARRIER F.The UV-inducible RNA-binding protein A18(A18 hnRNP)plays a protective role in the genotoxic stress response[J].JBiolChem,2001,276(50):47277-47284.
[26]DE LEEUW F,ZHANG T,WAUQUIER C,et al.The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor[J].ExpCellRes,2007,313(20):4130-4144.
[27]SAITO K,FUKUDA N,MATSUMOTO T,et a1.Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein[J].BrainRes,2010,1358:20-29.
[28]LAN D,TANG C,LI M,et al.Screening and identification of differentially expressed genes from chickens infected with Newcastle disease virus by suppression subtractive hybridization[J].AvianPathol,2010,39(3):151-159.
[29]周鸿淼,汤承,岳华,等.冷诱导RNA结合蛋白参与小鼠对LPS的应答[J].畜牧兽医学报,2014,45(8):1348-1354.
ZHOU H M,TANG C,YUE H,et al.Cold-inducible RNA binding protein involves in response to LPS in mice[J].ActaVeterinariaetZootechnicaSinica,2014,45(8):1348-1354.(in Chinese)
[30]ARTERO-CASTRO A,CALLEJAS F B,CASTELLVI J,et al.Cold-inducible RNA-binding protein bypasses replicative senescence in primary cells through extracellular signal-regulated kinase 1 and 2 activation[J].MolCellBiol,2009,29(7):1855-1868.
[31]PLESCHKA S,WOLFF T,EHRHARDT C,et al.Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade[J].NatCellBiol,2001,3(3):301-305
[32]LUDWIG S,PLANZ O.Influenza viruses and the NF-κB signaling pathway-towards a novel concept of antiviral therapy[J].BiolChem,2008,389(10):1307-1312.
[33]SCHMOLKE M,VIEMANN D,ROTH J,et al.Essential impact of NF-kappaB signaling on the H5N1 influenza A virus-induced transcriptome[J].JImmunol,2009,183(8):5180-5189.
[34]NIMMERJAHN F,DUDZIAK D,DIRMEIER U, et al.Active NF-кB signalling is a prerequisite for influenza virus infection[J].JGenVirol,2004,85(Pt 8):2347-2356.
[35]TANG C,WANG Y,LAN D,et al.Analysis of gene expression profiles reveals the regulatory network of cold-inducible RNA-binding protein mediating the growth of BHK-21 cells[J].CellBiolInt,2015,39(6):678-689.
[36]聂培婷,汤承,岳华.H1N1甲型流感病毒在BHK21中的增殖规律[J].西南民族大学学报,2012,38(5):764-769.
NIE P T,TANG C,YUE H.Study on the proliferation profile of influenza A virus in BHK21 cells[J].JournalofSouthwestUniversityforNationalities,2012,38(5):764-769.(in Chinese)
[37]吴巧,张斌,汤承,等.H5N1禽流感病毒感染小鼠后内参基因的筛选[J].中国畜牧兽医,2013,40(9):55-60.
WU Q,ZHANG B,TANG C,et al.Selection of reference gene in mice infected with H5N1 avian influenza virus[J].ChinaAnimalHusbandry&VeterinaryMedicine,2013,40(9):55-60.(in Chinese)
[38]MOGENSEN T H,MELCHJORSEN J,HÖLLSBERG P,et al.Activation of NF-κB in virus-infected macrophages is dependent on mitochondrial oxidative stress and intracellular calcium:downstream involvement of the kinases TGF-beta-activated kinase 1,mitogen-activated kinase/extracellular signal-regulated kinase kinase 1,and l kappa B kinase[J].JImmunol,2003,170(12):6224-6233.
[39]WURZER W J,EHRHARDT C,PLESCHKA S,et al.NF-кB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation[J].JBiolChem,2004,279(30):30931- 30937.
[40]WURZER W J,PLANZ O,EHRHARDT C,et al.Caspase 3 activation is essential for efficient influenza virus propagation[J].EMBOJ,2003,22(11):2717-2728
[41]FALEIRO L,LAZEBNIK Y.Caspases disrupt the nuclear-cytoplasmic barrier[J].JCellBiol,2000,151(5):951-959.
[42]KRAMER A,LIASHKOVICH I,OBERLEITHNER H,et al.Apoptosis leads to a degradation of vital components of active nuclear transport and a dissociation of the nuclear lamina[J].ProcNatlAcadSciUSA,2008,105(32):11236-11241.
[43]PAULI E K,SCHMOLKE M,WOLFF T,et al.Influenza A virus inhibits type I IFN signaling via NF-κB-dependent induction of SOCS-3 expression[J].PLoSPathog,2008,4(11):e1000196.
[44]WEI L,SANDBULTE M R,THOMAS P G,et al.NF-кB negatively regulates interferon-induced gene expression and anti-influenza activity[J].JBiolChem,2006,281(17):11678- 11684.
[45]KUMAR N,XIN Z T,LIANG Y,et al.NF-κB signaling differentially regulates influenza virus RNA synthesis[J].JVirol,2008,82(20):9880- 9889.
(编辑白永平)
Activation of Cold-inducible RNA-binding Protein by H1N1 Influenza Virus Contributes to Viral Replication Via Activating NF-κB Pathway
NIE Pei-ting,TANG Cheng,YUE Hua*
(CollegeofLifeScienceandTechnology,SouthwestUniversityforNationalities,Chengdu610041,China)
The cold-inducible RNA-binding protein (CIRP) is an upstream regulator of the NF-κB and ERK pathways,which are essential for the replication of the influenza virus and the initial immune response.In order to investigate the effect of CIRP on the replication of H1N1 influenza A virus and its possible molecular mechanism,in this study,CIRP overexpression BHK-21 (Cirp+BHK-21) cells were constructed,and the phosphorylation levels of NF-κB and ERK in Cirp+BHK-21 cells were detected by Western blot to confirm the effect of CIRP on regulation of NF-κB and ERK1/2;Real-time RT-PCR was used to detect the dynamic changes of virus load in Cirp+BHK-21 and control cells after infected with influenza A virus,the method was also used to detect the dynamic changes of virus load in Cirp+BHK-21 cells which were blocked by the NF-κB inhibitor PDTC.The results of Western blot exhibited that overexpressed CIRP could significantly increase the expression of phosphorylation level of NF-κB (P<0.05),but had no significant effect on the phosphorylation level of ERK.The results of quantitative detection of virus showed that overexpressed CIRP could significantly enhance the proliferation of influenza A virus,the virus load in the Cirp+BHK-21 cells were 111%,103%,167% and 235% (P<0.05) at 3,9,15 and 21 h PI,respectively,compared to the control group;Blocking the NF-κB was significantly decreased the virus load in the Cirp+BHK-21,and the virus load in treatment group were 98%,42%,19% (P<0.05),7% (P<0.05) at 3,9,15 and 21 h PI,respectively,compared to the unblock group.Therefore,this study confirmed that overexpressed CIRP could enhance the proliferation of influenza A virus via activation of NF-κB pathway.
cold-inducible RNA binding protein;influenza A virus;replication;NF-κB;BHK-21 cells
10.11843/j.issn.0366-6964.2016.10.020
2016-05-06
国家自然科学基金项目(31172307);四川省教育厅创新团队项目(13TD0057)
聂培婷(1989-),女,新疆霍城人,硕士,主要从事感染与免疫相关研究,E-mail:540016852@qq.com
岳华,Tel:028-85528276,E-mail:yhua900@163.com
S852.23
A
0366-6964(2016)10-2108-07
