胰腺内副脾表皮样囊肿的影像特征及病理对照
2016-10-28杨雪融周良平
杨雪融 周良平△ 黄 丹
(1复旦大学附属肿瘤医院放射科-复旦大学上海医学院肿瘤学系,2病理科 上海 200032)
胰腺内副脾表皮样囊肿的影像特征及病理对照
杨雪融1周良平1△黄丹2
(1复旦大学附属肿瘤医院放射科-复旦大学上海医学院肿瘤学系,2病理科上海200032)
目的探讨胰腺内副脾表皮样囊肿(epidermoid cyst in intrapancreatic accessory spleen,ECIPAS)的影像学特征,以提高对该少见病变的认识。方法回顾性分析经手术病理证实的5例ECIPAS的临床、影像及病理资料。4例行CT平扫加双期增强检查,其中2例同时行磁共振胰胆管造影(magnetic resonance cholangiopancreatography,MRCP)检查,1例行超声内镜检查,另1例行MR平扫加增强检查。观察病灶的大小、形态、密度信号及强化特点,并与组织病理进行对照。结果所有病灶均位于胰尾部,囊肿最大径1.7~5.0 cm,平均2.7 cm。2例囊内可见分隔。CT平扫3例为边界清楚的类圆形低度灶,1例呈高密度伴边缘点状钙化;增强后3例可见环绕囊肿的实质显著强化,动脉期和静脉期密度均高于胰腺组织。1例MR扫描未见明显实性成分,囊肿呈T1WI低信号,T2WI高信号。MRCP均未见囊肿与胰管相通。结论ECIPAS是少见的胰尾良性病变,其典型影像表现为囊肿伴周边类脾脏强化的实性成分。
胰腺;副脾;表皮样囊肿;体层摄影术;X线计算机;磁共振成像;病理学
胰腺内副脾表皮样囊肿(epidermoid cyst in intrapancreatic accessory spleen,ECIPAS)由Davidson等[1]在1980年首次报道,是一种起源尚未明确的胰腺良性病变,临床甚为少见,缺乏特征性临床症状,误诊率极高[2]。因生物学行为和治疗手段不同,ECIPAS与胰腺囊性肿瘤,如囊腺瘤等的鉴别诊断尤为重要,可靠的术前诊断能避免不必要的手术。目前国内外有关ECIPAS的文献多为个案报道,关注临床病理方面较多而系统全面的影像学研究有限。本研究回顾性分析本院收治的5例ECIPAS患者的临床资料、影像表现和病理结果,重点将术前影像与术后病理进行对照,并结合文献探讨,旨在提高对该少见病的认识及影像诊断准确率。
资 料 和 方 法
一般资料 收集2009年3月至2015年8月复旦大学附属肿瘤医院收治并经手术病理证实的ECIPAS患者5例。其中女性4例,男性1例。年龄27~66岁,平均48岁。除1例因腹胀就诊外,其余为体检时腹部影像发现胰腺占位或肿瘤指标CA19-9升高。所有病例均无急性胰腺炎及腹部外伤史。
影像检查CT检查示:4例行CT平扫及双期增强扫描。患者检查前4 h禁食,扫描前口服温水约800 mL适度充盈胃腔。采用Siemens Somatom Sensation MSCT扫描仪。管电压120 kV,管电流300 mA,层厚5 mm。扫描范围包括膈顶至髂棘水平。经肘静脉团注碘普罗胺(300 mg I/mL),剂量2 mL/kg,速率3.0 mL/s。注射对比剂后分别于25~30 s、60~65 s行动脉期和门脉期扫描。原始数据进行1.5 mm薄层重建及多平面重建。
MRI检查示:1例行MR平扫加增强,2例行磁共振胰胆管造影(magnetic resonance cholangi-opancreatography,MRCP)。采用GE Signa 3.0 T磁共振扫描仪,8通道体部相控阵线圈。平扫序列为横轴面FSPGR T1WI和FSE T2WI,层厚5 mm,间隔1 mm。增强扫描团注对比剂Gd-DTPA,剂量0.1 mmoL/kg,流率2 mL/s,序列LAVA(TR 2.5 ms,TE 1.2 ms),FOV 40 cm×40 cm。MRCP序列包括SSFSE T2WI(TR 1 090 ms,TE 78 ms),屏气BH 2D MRCP(TR 7 000 ms,TE 1 235 ms),呼吸门控RTr 3D MRCP ASSET(TR 3 000 ms,TE 641 ms),FOV 32 cm×32 cm。
图像分析 2名从事影像诊断工作10年以上的医师共同阅片,结论不一致时协商统一。主要观察病变部位、大小、边界,有无钙化,囊内有无分隔和壁结节,有无实性成分,CT、MR的平扫密度信号以及强化特征。另外还包括胰管是否扩张、病变是否侵犯周围组织及有无淋巴结肿大等。
结 果
临床特征 2例患者肿瘤指标CA19-9升高,分别为133.20和87.72 U/mL;CEA值均在正常范围内。术前影像学诊断2例为囊腺瘤,1例为实性假乳头状瘤,2例为胰腺良性囊肿,其中1例伴出血。因临床上不除外肿瘤性病变,所有病例均行手术治疗。1例行保留脾脏的胰尾切除术,其余行胰尾联合脾脏切除。除1例于2015年8月手术外,4例术后随访(平均11个月)实验室检查及影像未见复发(表1)。
影像表现 病灶均位于胰尾部,边缘清楚,呈圆形或椭圆形,囊肿最大径1.7~5.0 cm,平均2.7 cm。1例在超声内镜上无回声区内探及分隔且部分融合,1例在MR T2WI上囊内见细线样低信号分隔。CT平扫1例为高密度,CT值约80 HU,边缘见点状钙化(图2);3例为低密度,CT值28~35 HU。1例囊内壁不平呈结节样改变(图3),余例囊壁光滑。CT增强后3例可见环绕囊肿的实质明显强化,动脉期和静脉期密度均高于周围胰腺组织,强化方式与脾脏类似(图1),实性成分对应在MRCP上呈T2WI高信号,同脾脏信号一致。MR检查1例,未见实性成分,囊肿无强化,呈T1WI低信号,T2WI高信号(图4)。MRCP均未见囊肿与胰管相通。所有患者均未见胰管扩张及淋巴结肿大与侵犯周围征象。
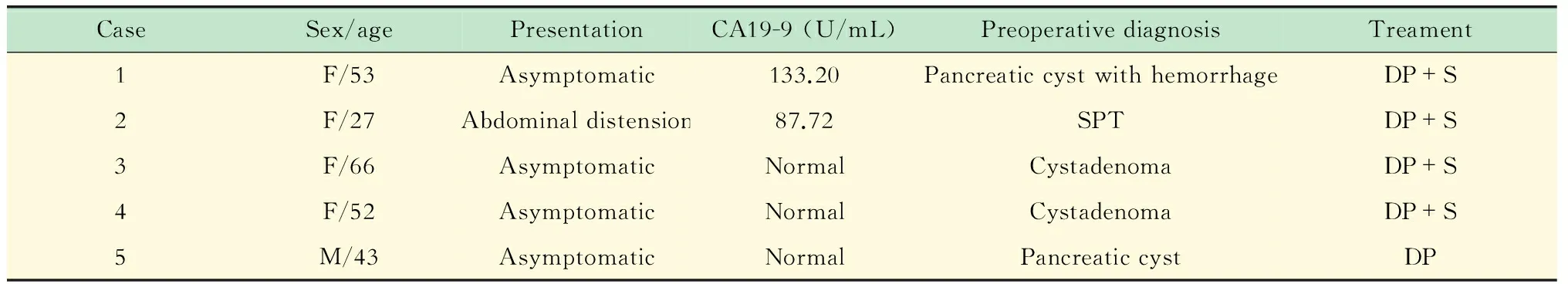
表1 ECIPAS的临床特征
Normal serum level of CA19-9 is 0-27 U/mL.SPT:Solid pseudopapillary tumor;DP: Distal pancreatectomy;S:Splenectomy.
A:Plain CT scan shows a well-defined lesion in the tail of the pancreas;B:Arterial phase scan shows the solid component surrounding the cyst with the same heterogeneous hyperdensity as the spleen;C:On portal phase the solid component shows the same density as the spleen which is higher than the pancreas;D:A microscopic slide reveals a cyst (circle) surrounded by accessory splenic tissue (star) in the pancreas parenchyma (triangle)(HE staining,×20).
图11例66岁2.9 cm女性ECIPAS
Fig 1A 66-years-old women with 2.9 cm ECIPAS
A:Precontrast CT scan shows a high density lesion with stippled calcification;The lesion appears an unenhanced,low-density mass on arterial phase (B)and portal phase (C);D:Microscopic analysis reveal smuch amorphous secretion in the cyst (HE staining,×100).
图21例53岁1.7 cm女性ECIPAS
Fig 2A 53-years-old women with 1.7 cm ECIPAS
病理检查 手术标本表面光滑,切面显示单房或多房囊肿,囊液淡黄至浑浊,周围覆以褐色质软组织。HE染色切片镜下囊壁内衬复层鳞状上皮,部分角化,无皮脂腺等皮肤附属器,周围为副脾组织,可见红髓、白髓及脾小梁。脾与胰腺之间有纤维间隔。1例囊内见大片无定形分泌物,同时伴胆固醇结晶及钙化。1例囊内壁不光整,可见由纤维成分及少量脾组织构成数枚凸起。
Portal phase CT (A) and MR T2WI (B) images show the inner surface of cystic wall unsmooth with a mural nodule;C:The histological section shows thick fibrous tissue with hyaline degeneration and spleen tissue resembling a mural nodule (HE staining,×40).
图31例27岁5.0 cm女性ECIPAS
Fig 3A 27-years-old women with 1.7 cm ECIPAS
The cyst shows low signal intensity on T1WI (A),high signal intensity on T2WI (B),and remains unenhanced after contrast administration (C).No heterotopic splenic tissue is visible.D:Histopathological examination (HE staining,×400) shows that the cyst is lined with stratified squamous epithelium.Subepithelial structure consistents with features of the spleen.
图41例52岁2.0 cm女性ECIPAS
Fig 4A 52-year-old women with 2.0 cm ECIPAS
讨 论
概述 ECIPAS是一种少见的胰腺良性病变,据不完全统计,目前中英文文献报道仅数十例[2,3]。患者年龄12~70岁,平均45岁,女性多于男性(1.45∶1),多无临床症状,部分出现腹痛、腹胀、恶心、呕吐等非特异性表现[3]。值得一提的是发病人群中亚洲人占大多数(28/36,77.7%)[2],有明显种族倾向。本研究中女性4例、男性1例,平均年龄48岁,除1例腹胀外,其余临床表现无殊,与文献基本相符。
本组2例CA19-9升高,同时伴消化道肿瘤指标CA50和CA242轻度升高,或为病灶周围胰腺组织的反应性结果。但Higaki等[4]指出ECIPAS患者血清CA19-9和CEA升高是由囊壁内衬鳞状上皮产生后释放入循环。多个相关研究[5-6]中免疫组化证实内衬上皮细胞CA19-9和CEA表达阳性,并且术后肿瘤指标降低也支持这一理论。本组血清CEA均未升高,有待进一步大样本临床病理研究。
副脾是正常脾组织的先天异位,临床上并不少见,多位于脾门,尸检研究中9.3%的副脾在胰尾部[7]。ECIPAS的组织起源尚有争议,根据原位脾脏表皮样囊肿的发生方式,有的认为由间皮鳞状上皮化生[8]形成,另外还有胚胎鳞状上皮内陷[9]、胰管突入副脾[10]等假说。尽管是良性病变,但某些病例中ECIPAS可迅速增大。本研究中1例体检发现胰尾1.5 cm大小的囊性占位,1年后复查病灶为2.9 cm。Kumamoto等[11]报道1例随访1年的ECIPAS,从2.0 cm增大至3.8 cm。对此类患者建议采用创伤较小、保留脾脏的腹腔镜下胰尾切除术。
影像表现 文献报道ECIPAS均位于胰尾部,大小1.4~15 cm,平均3.9 cm[2]。囊内可有分隔,单房囊肿约为多房囊肿的1.5倍[12]。本组1例在超声内镜、1例在MR上囊内见薄壁分隔,MR具有高软组织分辨率,相比CT能更好地显示内部细微结构。CT平扫一般为低密度灶,本组1例呈高密度,CT值约80 HU,影像诊断囊肿伴出血,病理显示囊内大片无定形分泌物为蛋白样物质。囊肿呈T1WI低信号,T2WI高信号,部分研究中ECIPAS T1WI为高信号[5,13],考虑与囊液角蛋白含量高有关[14]。
囊肿并无突出特征,确认副脾组织存在是诊断ECIPAS的关键,少量副脾影像上不易明确,因此正确的术前诊断很大程度依赖于囊肿周围有足够多的脾组织。Motosugi等[12]回顾性分析33例ECIPAS,其中15例在影像或大体病理上副脾不可见,又将18例可见副脾的形态分为肿块样(三角形或帆状)和环状(厚薄均一),两者比例为13∶5。本组1例MR上副脾不明显,但镜下病理清晰可见脾组织和内衬鳞状上皮结构;3例CT上可见副脾,按Motosugi等[12]的分类都为肿块样环绕囊肿,动脉期明显不均匀强化,静脉期强化程度趋于均匀,二期密度均高于周围胰腺。同时,T2WI上副脾同原位脾一致,呈高信号,与低信号的胰腺区分(本研究MRCP检查未含T1WI序列)。除强化特征外,超顺磁氧化铁对比剂MR成像上,因脾脏网状内皮组织吞噬作用,副脾同原位脾均出现T2WI信号降低[15],也可证实为胰腺内副脾。本组1例见点状钙化,其他报道也多为病灶边缘小钙化[13,16]。
鉴别诊断 胰腺囊性病灶一般分为肿瘤性和非肿瘤性,生物学行为和治疗方式差别很大。非肿瘤性囊性病变最常见的是假性囊肿[17],多有胰腺炎或外伤史,出血,胰液外渗,组织坏死包裹,囊壁为纤维肉芽组织,壁薄伴强化,囊壁可钙化。潴留囊肿是梗阻所致的胰腺导管囊状扩张,识别下游的结石、肿瘤或其他引起导管狭窄的病因后也不难诊断。另外如单纯囊肿、淋巴上皮囊肿等很少见,影像学表现一般考虑为良性。
与ECIPAS鉴别的肿瘤性病变主要为囊腺瘤、实性假乳头状肿瘤(solid pseudopapillary tumor,SPT)和导管内乳头状黏液瘤(intraductal papillary mucinous neoplasm,IPMN),导管腺癌伴囊变通常恶性征象显著,容易除外。本组2例诊断为囊腺瘤,为中老年女性。其中1例CT增强上可见胰尾部环绕囊肿的实质成分强化方式与原位脾一致,为ECIPAS的典型表现,但最初对其缺乏认识而未列入鉴别诊断。另1例未见明显实性成分,T2WI上高信号的囊肿内隐约可见低信号线样分隔。囊腺瘤分浆液性和黏液性,前者为良性而后者具有恶性潜能。浆液性囊腺瘤(serouscystadenoma,SCA)微囊型为多囊的蜂窝状肿块,而寡囊型表现为单个或数个大囊,与副脾不明显的ECIPAS可相似,但寡囊型SCA多位于胰头,呈分叶状,囊壁无强化[18]。黏液性囊性肿瘤(mucinous cystic neoplasm,MCN)多位于胰体尾部,呈多房大囊样(>2cm),可见厚壁强化及壁结节[19]。将ECIPAS的副脾误认为MCN的厚壁,加之两者相似的发病年龄性别及部位,ECIPAS常被误诊为MCN[2]。本组1例囊内壁不平伴结节样改变,Yamanishi等[20]报道1例超声内镜上可见壁结节的ECIPAS,同样都经病理证实结节由纤维组织伴玻璃样变性及脾组织构成。
本组1例考虑到年轻女性(27岁)胰尾囊实性占位,诊断为SPT。SPT是好发于年轻女性的交界性肿瘤,通常较大,胰头和胰尾多见,囊性和实性成分可不同比例混合,实性成分多渐进性强化,但强化程度低于胰腺实质[21]。IPMN起源于主胰管或分支胰管,老年男性居多,常发生在胰头钩突部,MRCP显示胰管扩张与病灶相通等特点[22],易于区分。因ECIPAS的副脾明显强化,有时与富血供的胰腺神经内分泌肿瘤(pancreatic neuroendocrine tumors,PNETs)囊变难以鉴别。无功能性PNETs不伴激素相关临床症状,发现时多较大,可有囊变,Kawamoto等[23]报道13例囊性为主的PNETs有11例表现为周边强化且高于胰腺实质。超声内镜及其引导下针吸活检提供了高分辨率的形态学、对囊液生化分析及细胞学检测,是CT、MR等影像检查的有益补充[24]。
综上所述,ECIPAS为少见的胰尾良性病变,明确诊断能避免不必要的手术或提供适当的手术方式。ECIPAS缺乏特征性临床症状和实验室指标,发病具有女性优势。鉴别胰尾囊性病变时有典型表现如囊肿伴周边类脾脏强化的实性成分应考虑此病。
[1]DAVIDSON ED,CAMPBELL WG,HERSH T.Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen[J].DigDisSci,1980,25(12):964-967.
[2]ZAVRAS N,MACHAIRAS N,FOUKAS P,etal.Epidermoid cyst of an intrapancreatic accessory spleen:a case report and literature review[J].WorldJSurgOncol,2014,12(1):1-7.
[3]王健,胡红杰.胰尾部副脾表皮样囊肿的CT和MR影像学诊断分析[J].中华胰腺病杂志,2015,15(3):204-206.
[4]HIGAKI K,JIMI A,WATANABE J,etal.Epidermoid cyst of the spleen with CA19-9 or carcinoembryonic antigen productions:report of three cases[J].TheAmJSurgPathol,1998,22(6):704-708.
[5]HORIBE Y,MURAKAMI M,YAMAO K,etal.Epithelial inclusion cyst (epidermoid cyst) formation with epithelioid cell granuloma in an intrapancreatic accessory spleen[J].PatholInt,2001,51(1):50-54.
[6]RU K,KALRA A,UCCI A.Epidermoid cyst of intrapancreatic accessory spleen[J].DigDisSci,2007,52(5):1229-1232.
[7]UNVER DOGAN N,UYSAL II,DEMIRCI S,etal.Accessory spleens at autopsy[J].ClinAnat,2011,24(6):757-762.
[8]BÜRRIG KF.Epithelial (true) splenic cysts:pathogenesis of the mesothelial and so-called epidermoid cyst of the spleen[J].AmJSurgPathol,1988,12(4):275-281.
[9]LIFSCHITZ-MERCER B,OPEN M,KUSHNIR I,etal.Epidermoid cyst of the spleen:a cytokeratin profile with comparison to other squamous epithelia[J].VirchowsArch,1994,424(2):213-216.
[10]SASOU S,NAKAMURA S,INOMATA M.Epithelial splenic cysts in an intrapancreatic accessory spleen and spleen[J].PatholInt,1999,49(12):1078-1083.
[11]KUMAMOTO Y,KAIZU T,TAJIMA H,etal.A rapidly growing epidermoid cyst in an intrapancreatic accessory spleen treated by laparoscopic spleen-preserving distal pancreatectomy:Report of a case[J].IntSurg,2015,[Epub ahead of print].
[12]MOTOSUGI U,YAMAGUCHI H,ICHIKAWA T,etal.Epidermoid cyst in intrapancreatic accessory spleen:radiological findings including superparamagnetic iron oxide-enhanced magnetic resonance imaging[J].JComputAssistTomogr,2010,34(2):217-222.
[13]ITANO O,SHIRAGA N,KOUTA E,etal.Epidermoid cyst originating from an intrapancreatic accessory spleen[J].JHepatobiliaryPancreaSurg,2008,15(4):436-439.
[14]LIM J,CHO K.Epidermoid cyst with unusual magnetic resonance characteristics and spinal extension[J].WorldJSurgOncol,2015,13(1):240.
[15]HERÉDIA V,ALTUN E,BILAJ F,etal.Gadolinium-and superparamagnetic-iron-oxide-enhanced MR findings of intrapancreatic accessory spleen in five patients[J].MagnResonImaging,2008,26(9):1273-1278.
[16]HU S,ZHU L,SONG Q,etal.Epidermoid cyst in intrapancreatic accessory spleen:computed tomography findings and clinical manifestation[J].AbdomImaging,2012,37(5):828-833.
[17]KLÖPPEL G,KOSMAHL M.Cystic lesions and neoplasms of the pancreas:the features are becoming clearer[J].Pancreatology,2001,1(6):648-655.
[18]COHEN-SCALI F,VILGRAIN V,BRANCATELLI G,etal.Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT:initial observations[J].Radiology,2003,228(3):727-733.
[19]MANFREDI R,VENTRIGLIA A,MANTOVANI W,etal.Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas:MR imaging characterization[J].EurRadiol,2015,25(4):940-949.
[20]YAMANISHI H,KUMAGI T,YOKOTA T,etal.Epithelial cyst arising in an intrapancreatic accessory spleen:a diagnostic dilemma[J].InternMed,2011,50(18):1947-1952.
[21]KAWAMOTO S,SCUDIERE J,HRUBAN RH,etal.Solid-pseudopapillary neoplasm of the pancreas:spectrum of findings on multidetector CT[J].ClinImaging,2011,35(1):21-28.
[22]KIM JH,HONG SS,KIM Y J,etal.Intraductal papillary mucinous neoplasm of the pancreas:differentiate from chronic pancreatits by MR imaging[J].EurJRadiol,2012,81(4):671-676.
[23]KAWAMOTO S,JOHNSON PT,SHI C,etal.Pancreatic neuroendocrine tumor with cystlike changes:evaluation with MDCT[J].AJRAmJRoentgenol,2013,200(3):283-290.
E-mail:zhoulp-2003@163.com
The imaging features of epidermoid cyst in intrapancreatic accessory spleen:correlated with pathological findings
YANG Xue-rong1, ZHOU Liang-ping1△, HUANG Dan2
(1DepartmentofRadiology-DepartmentofOncology,ShanghaiMedicalCollege,2DepartmentofPathology,ShanghaiCancerCenter,FudanUniversity,Shanghai200032,China)
ObjectiveTo investigate the imaging features of epidermoid cyst in intrapancreatic accessory spleen (ECIPAS) for better understanding of the rare disease.MethodsThe clinical,radiological and pathological data of 5 patients with pathologically confirmed ECIPAS were retrospectively reviewed.Four cases underwent plain and dual-phase enhancement CT scan,of which 2 cases received magnetic resonance cholangiopancreatography (MRCP),and 1 case received endoscopic ultrasonography,and the other 1 case underwent plain and contrast-enhanced MRI scan.The size,shape,density/signal and enhancement mode of the lesions were analyzed and correlated with pathologic findings.ResultsAll lesions were situated in the pancreatic tail,wherein the mean size of the cyst was 2.7 cm,ranging from 1.7 cm to 5.0 cm.The cyst appeared multilocular in 2 cases.On plain CT,3 cases showed oval well-defined low density foci,while 1 case showed high density with stippled calcification in the periphery.In 3 cases,the solid component surrounding the cyst displayed marked enhancement on postcontrast CT,which density were higher than the pancreatic parenchymaduring both arterial and portal venous phase.Solid component was not seen on MR in 1 case,the cyst was hypo-intense on T1WI and hyper-intense on T2WI.No communication with pancreatic duct was shown on MRCP.ConclusionsECIPAS is a rare benign lesion situated in the tail of the pancreas,the typical imaging manifestation is a cyst with surrounding solid component that enhancing similar to the spleen.
pancreas;accessory spleen;epidermoid cyst;body section radiography;X-ray computed tomography; magnetic resonance imaging;pathology
R816.5
Bdoi: 10.3969/j.issn.1672-8467.2016.04.010
2015-10-15;编辑:沈玲)
