Densityfunctionalcalculationofphysicalpropertiesofg-C3N4/germaneneheterobilayer-theaffectionsofelectricfields
2016-09-20,*,
, *,
(1. College of Chemistry and Chemical Engineering, Anhui University, Hefei 230601, China;2. Department of Chemistry and Molecular Engineering, East China University
Densityfunctionalcalculationofphysicalpropertiesofg-C3N4/germaneneheterobilayer-theaffectionsofelectricfields
RUANLinwei1,ZHUYujun1*,LUYunxiang2
(1.CollegeofChemistryandChemicalEngineering,AnhuiUniversity,Hefei230601,China;2.DepartmentofChemistryandMolecularEngineering,EastChinaUniversity
ofScienceandTechnology,Shanghai200237,China)
Effectofelectricfieldintensityanddirectiononbindingenergy,densityofstates(DOS),andchargedensityofg-C3N4/germaneneheterobilayerwasinvestigatedbyfirstprinciplecalculations.Thecalculationresultsrevealthelargeimpactofelectricfieldonthephysicalparametersofg-C3N4/germaneneheterobilayer.ApplicationofupwardelectricfieldmovestheDOStowardsleft,whilethedownwardelectricfieldresultsintheright-shiftofDOSing-C3N4/germaneneheterobilayer.Inaddition,nochangesinworkfunctionofheterobilayeroccurunderelectricfields.
g-C3N4;heterobilayer;germanene;electricfields
0 Introduction
Graphiticcarbonnitride(labeledasg-C3N4)hasrecentlyreceivedintensiveattentionsduetoitsgoodphotocatalyticperformanceforwatersplittingandorganicpollutantspurificationundervisiblelightirradiation.Likegraphite,g-C3N4hasatwo-dimensional(2D)planarπconjugationstructure,whichenablestheefficientelectrontransferwithintheπconjugationstructure.However,thephotocatalyticactivityofpristineg-C3N4isstilltoolowtopracticalapplications.Researchershavenowrecognizedthatthesingle-componentg-C3N4cannotachievethehigherphotocatalyticactivityduetotherapidrecombinationofphotogeneratedelectron-holepairs.Tosolvethisissue,formingheterostructuresbycouplingg-C3N4withothermaterialshavebeendemonstratedaseffectivestrategytoimprovethephotocatalyticefficiencyofg-C3N4.Forexample,Daietal.[1]fabricatedg-C3N4/TiO2nanosheethybridsforenhancedphotodegradationoforganiccontaminationsusingvisiblelight.Xingandco-workers[2]usedIn2S3/g-C3N4heterostructurestoimprovethephotocatalyticabilityofg-C3N4.Yangetal.[3]studiedtheinfluenceofpreparationmethodonphotocatalyticperformanceofg-C3N4/WO3compositephotocatalyst,andfoundthatthecompositespreparedthroughhydrothermalmethodexhibitedthehighestphotocatalyticactivity.Zhangetal.[4]designedBi2O3/g-C3N4hybridswithhighvisiblelightactivityformethyleneblueandrhodamineB.Huangetal.[5]studiedeffectofcontactinterfacebetweenTiO2andg-C3N4onthephotoreactivityofg-C3N4/TiO2photocatalyst,anddiscoveredthatthe(101)facethasbetterperformance.
Meanwhile,theoreticinvestigationon2-Dcarbon-basedmaterialhybridshasalsobeenintensivelystudied.Maetal.[6]examinedthebandstructureanddensityofstates(DOS)oftransition-metaldichalcogenideandmxenemonolayer.Medvedevaetal.[7]studiedtheAlN/GaN:Cr(0001)heterostructurebyusingfirstprincipalcalculationandfoundthattheheterobilayerdopedwithCrwidenedthebandgapofg-C3N4.RoomeandCarey[8]calculatedthestructuralstability,electronicandvibrationalpropertiesofdifferentmonolayerconfigurationsofsiliceneandgermaneneheterobilayer.Gaoetal.[9]simulatedthehybridgraphene/anataseTiO2(001)nanocompositesandfoundtheimprovedinterfacialelectrontransferwithgrapheneintroduction.Xuetal.[10]predictedtheimprovedphotocatalyticactivityoverAg3PO4/graphenenanocompositethroughfirstprinciplecalculation.Gengetal.[11]foundthattheelectronicpropertiesofZnOretainedunchangedwhencouplingwithgraphene.Gaoetal.[12]simulatedtheheterobilayersformedbysiliceneandMoS2,anddeducedthatitisacandidatematerialforlogiccircuitsandphotonicdevices.
Itiswellknownthatthephotoelectricpropertiesofsemiconductorareaffectedgreatlybytheappliedexternalelectricfield.Wuetal.[13]foundtheopticalenergygapofg-C3N4bilayercanbeengineeredbytheexternalelectricfield.Zhangetal.[14]calculatedtheeffectsoftransverseelectricfieldonenergygapmodulationofBNribbonsandfoundtheenergygapsnarrowingcausedbythefield-inducedmotionofnearlyfreeelectronstates.Kangetal.[15]investigatedtheaffectionstobandgapsofgraphdiynenanoribbonsfromtransverseelectricfield.Kanetal.[16]verifiedthetransformationofconductivezigzaggraphenenanoribbonintohalfmetalunderelectricfield.Tothebestofourknowledge,thereisnoreportoftheeffectofelectricfieldonthephysicalpropertiesofg-C3N4heterobilayers.
Herein,theauthorsreporttheeffectofappliedelectricfieldontheg-C3N4/Geheterobilayer.Itisrevealedthatthephysicalpropertiesofg-C3N4/Geheterobilayerareaffectedgreatlybytheappliedelectricfield.Theprincipledisclosedbythesimulationcanprovideusefulinformationforthesynthesisofheterobilayers.
1 Methodology and calculation
AllcalculationswereperformedbyDmol3module[17]inMaterialsstudio7.0software.The(001)surfaceofg-C3N4and(111)surfaceofgermaniumwerecleaved,thelatticeparametersofbothsurfacesare19.133 Åand20.155 Å.Wecanassumethatthetwosurfacescangenerateanewheterobilayerbecauseoftheapproximatelatticelength.Theheterobilayerwasbuiltthrough“buildlayer”tabfromthenanosheetsofC3N4andgermaneneobtainedbefore.Inordertogaintheoptimizedstructureofheterobilayer,allstructuresobtainedbeforeusedinthesimulationneedtominimizetheenergy.Generalgradientapproximation(GGA)andPerdew-Burke-Ernzerhof(PBE)[18]functionwereusedinthewholesimulation,andapragmaticmethodtodescribecorrectlyvanderWaalsinteractionsresultingfromdynamicalcorrelationsbetweenfluctuatingchargedistributionshasbeengivenbytheDFT-D2approachofGrimme.DFTsemi-corepseudopotsandDNPbasiswerealsousedinthewholeprocessofsimulation.Thecutoffenergyis900eVandkpointis6×6×3,simultaneously,thenumberofatomsoftotalsystemis96.
TheultimatestructureofheterobilayerwasshowninFig.1,twolayersofstructuremaintainasawholethroughvanderWaalsinteractions.Afterenergyminimization,thegermanenelayercorrugatedcomparedtotheflatC3N4layercankeepthewholesystemstable.Theoriginalandultimatelatticeparametera,b,cofheterobilayeris19.721 7 Å, 19.721 7 Å, 20.000 0 Åand19.722 3 Å, 19.721 6 Å, 20.000 0 Å.Theoriginalandultimateinterlayerdistanceis3.578 Åand3.572 Å.
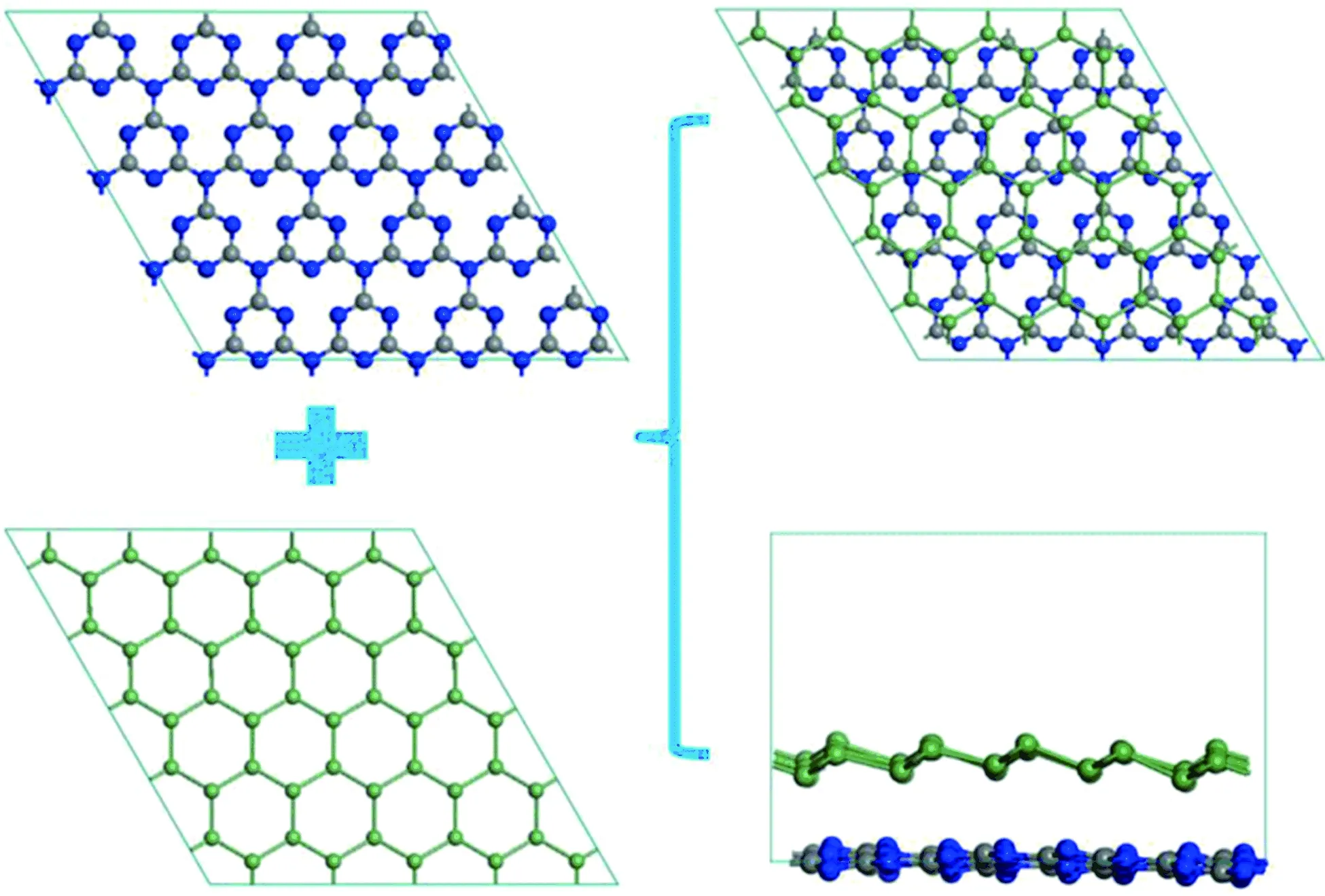
Fig.1 Schematic diagram of heterobilayer formed by g-C3N4 and germanene
2 Results and discussion
Bindingenergycanbeusedtorevealthedifficultiesofheterobilayerformation.Thereforethebindingenergywasfirstcalculatedtostudytheformationofg-C3N4/Geheterobilayer,asshowninFig.2.Bindingenergyinpresentsystemwasdefinedas
Eb= E(heterobilayer)-E(g-C3N4)-E(germanene).
Itwasfoundthatthebindingenergyofg-C3N4/Geheterobilayervarieswiththedirectionofappliedelectricfield.Theupwardelectricfield(+z)causestheincreasedbindingenergy,whilethedownwardelectricfield(-z)resultsinthedecreasedbindingenergy,asshowninFig.2.Thisisbecausethe+zdirectionisthesameasthedirectionofinduceddipole,onthecontrary,the-zdirectioniscontradicttothedirectionoftheinduceddipole.
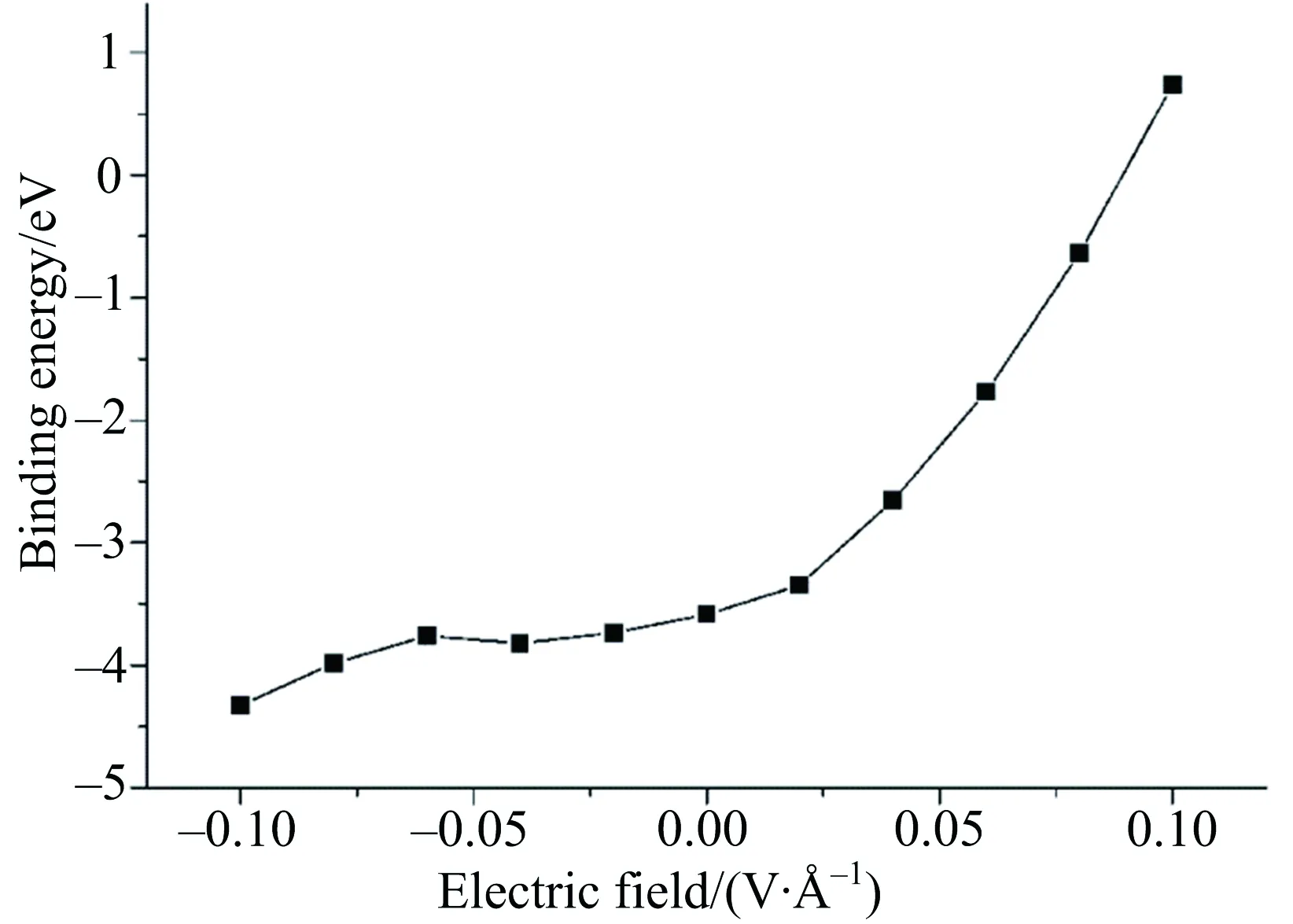
Fig.2 Binding energies of heterobilayer as function of electric field
Thebandgapofg-C3N4/Geheterobilayerisalsoaffectedbytheappliedelectricfielddirection,asillustratedinFig.3.Despitetheelectricfielddirection,thebandgapbecomesnarrowedwhenexternalelectricfieldwasapplied.Thisphenomenonisdifferentfromthechangetendencyinbindingenergy,asdiscussedabove.Thedecreaseinbandgapcanbeattributedtothesmallerelectronenergybarriercausedbytheappliedelectricfield[15].Thedifferenceinbandgapchangewiththeelectricfielddirectioncouldbecausedbythedirectionalflowofelectronsfromg-C3N4togermaneneastheupwardelectricfieldcanpromotethemovementofelectronsfromg-C3N4togermanene.Thisresultisverysimilartheauthors’previousresult[19]thatthebandgapdecreaseswithincreasedexternalpressure,butthedeclinedrangewasnarrowercomparedwiththepreviousresult.
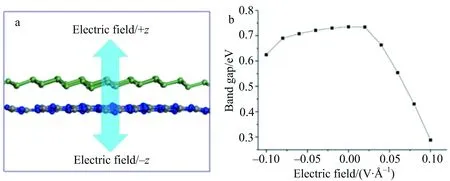
Fig.3 The directions of electric fields (a) and the relation between band gap and electric field (b)
ThecalculatedbandgapEgofg-C3N4/germaneneheterobilayeris0.735eV.Suchsmallbandgapindicatesthatg-C3N4/germaneneheterobilayercanabsorbthefullvisiblelightregion[10].Despitetheelectricfielddirection,theconductionbandedgelinearlydecreaseswiththeappliedelectricfield(Fig.4).Notably,thevalencebandedgeincreaseswiththeincreaseofelectricfieldundertheirradiationofupwardelectricfield(+z).Interestingly,adecreasedvalencebandedgeoccurswhenthedownwardelectricfieldgraduallyincreases.
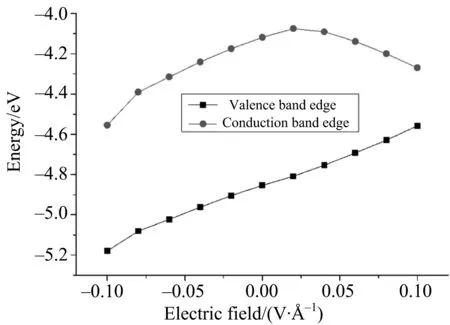
Fig.4 Valence band edge and conduction band edge of heterobilayer
Fig.5shownthedensityofstatesofheterobilayerwithoutexternalelectricfieldapplied.Thedensityofstates(DOS)ofg-C3N4/manganeneheterobilayerwasalsocalculated,asshowninFig.5andFig.6.TheDOSbetween-25and-15eVwasmainlycomposedofC2sorbitals.TheGeporbitalscontributeddominantlytotheDOSfromtheenergyof-12.5eVtotheFermienergy.Inaddition,thetopofthevalencebandwasalsomainlyconstructedbytheGePDOS.

Fig.5 The DOS of heterobilayer without electric field
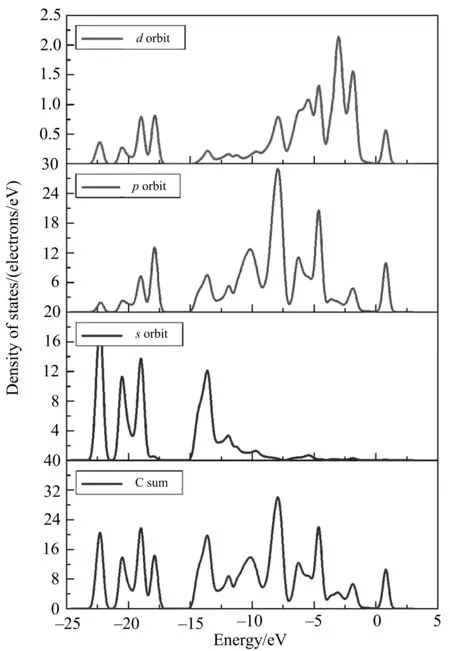
Fig.6 The s, p, d PDOS and sum DOS of carbon atoms
NotethattheelectricfieldalsoaffectstheDOSoftheg-C3N4/germaneneheterobilayer,asshowninFig.7.TheDOSmovestothelowerenergysidewithincreaseofupwardelectricfieldintensity.Thisresultisconsistentwiththephenomenonthattheelectricfieldcannarrowtheenergyofg-C3N4/germaneneheterobilayer[13].Comparatively,thedownwardelectricfieldpushestheDOStothehighenergylevelwiththeincreaseofelectricfieldintensity.

Fig.7 DOS of heterobilayer with different value of electric field (a) and different direction of electric field (b)

Fig.8 Electron density of heterobilayers without electric field (a), with the value 0.1 and -z direction electric field (b), with the value 0.1 and z direction electric field (c)
Fig.8showstheelectrondensityofg-C3N4andGelayer,respectively.Electrondensityatg-C3N4layerishigherthanthatofGelayerwithoutexternalelectricfield.Interestingly,thedirectionofelectricfieldaffectsthegapofg-C3N4layerandGelayer.Theupwardelectricfield(+z)widensthegapbetweeng-C3N4andGelayer,whilethedownwardelectricfield(-z)narrowsthegapbetweeng-C3N4andGelayer.Inaddition,Gelayerownshighelectrondensityincomparisontog-C3N4layerwiththevalue0.1of-zelectricfieldshowninFig.8.Notably,thechemicalbondsbetweeng-C3N4andGelayerformedwhendownwardelectricfieldintensityof0.1wasapplied.Ge-C3N4layerhas-2.515emullikenchargeandGelayerhas2.52emullikenchargeatthissituation.Comparatively,g-C3N4layerpossesses0.95emullikenchargeandGermanenelayerowns-0.95emullikenchargewhenupwardelectricfieldintensityof0.1wasaddedtotheg-C3N4/Geheterobilayer.Althoughthemullikenpopulationanalysisistoocoarsetorevealthecharges’spatialdistribution,themullikenchargeing-C3N4andGelayersverifiestheelectrondensityplotandtheasymmetrybetweenthetwolayers.Thechargeredistributionoflayersoccurredinthiskindofhybridheterobilayerwilldemonstratetheconclusionisrightornot.AfurtherchargeanalysisrevealsthateachGeatomtransferes0.05etog-C3N4,whileeachCatomloses0.338eandNatomobtaines0.292einpresentheterobilayer.ThechargeredistributioninpresentheterobilayerisdifferentfromthatinTiO2/GR[20-23],ZnO/GR[24],TiO2/carbonnanotube[25],C60/TiO2[26]etc,inwhichthechargemerelytransfersfromonecomponenttoanother.Thisresultcouldbeascribedtothevariationofelectrondensitycausedbytheelectricfield,asrevealedbyXu’swork[10],hencechemicalbondformedbetweenatomsbelongtodifferentlayers.Thecorrespondingbondlengthofbetweeng-C3N4andGelayerisillustratedinFig.9.Underelectricfieldirradiation,thelengthofN—GeandC—Gebondvariesfrom2.0to2.2 Å,whichislargerthanthatofC—Cbondlength.Thisresultrevealsthattheinteractionbetweeng-C3N4andGelayersisweakerthanthatofcovalentg-C3N4layer.
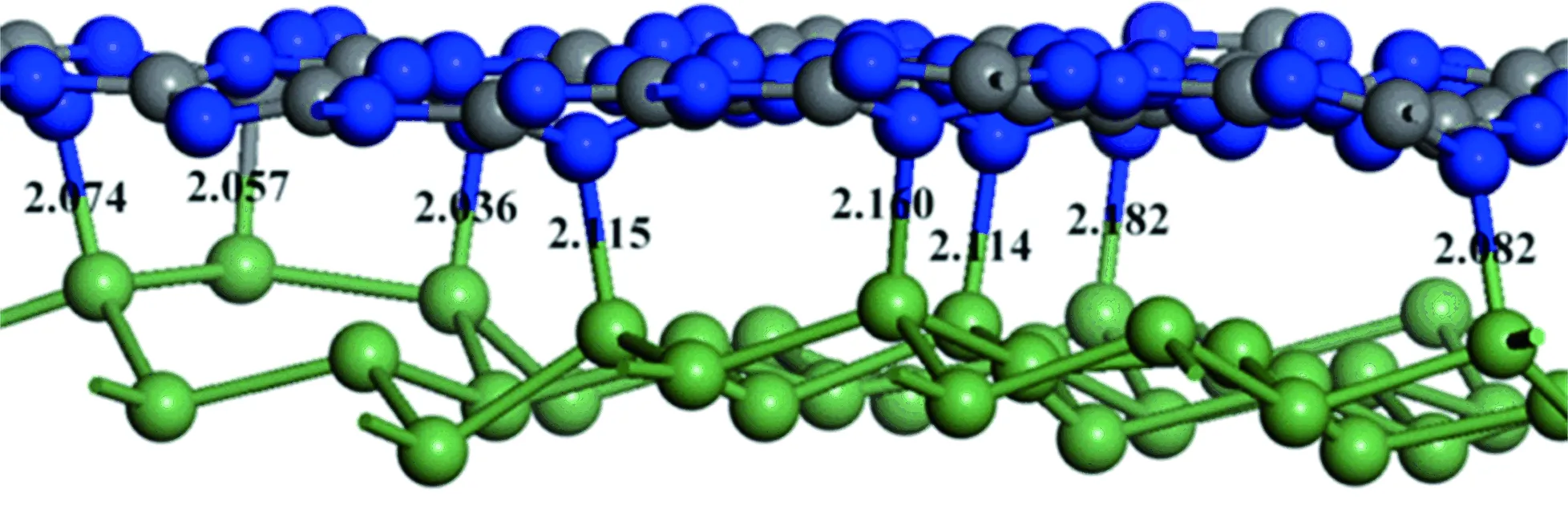
Fig.9 Lengths of chemical bonds formed in 0.1 electric field of -z direction
WorkfunctionistheenergythatcanbeprovidedfortheelectronwithFermienergyescapingfromtheinnermetaltovacuumlevel.Thesimulatedworkfunctionoftwolayersalmostmaintainsunchangedwhentheelectricfieldintensityincreasesfrom0to0.1,thisresultmaybecausedbythesmallexternalelectricfield,whichcannotaffecttheelectronescape.
For(001)surfaceofg-C3N4,theworkfunctionwascalculatedtobe4.5eV[27]inagreementwiththispaper.Electronscanflowfromthelayerwithhighworkfunctiontothelayerwithlowworkfunctionwhentherewasnoelectricfieldapplied.Hence,electronscanmovetog-C3N4layerbecausetheg-C3N4layerownslowerworkfunction.Theelectronflowdirectioncanbechangedunderelectricfieldirradiation.Fig.10illustratestheworkfunctionofg-C3N4/Geheterobilayerwithelectricfielddirection.Aslightlydecreasedworkfunctionof4.425eVofg-C3N4wasobtainedunderelectricfieldirradiationwhencomparedwiththevalueof4.3eVwithoutelectricfieldirradiation[28].

Fig.10 Work function of heterobilayer when electric field from +z and -z direction with value 0.1
3 Conclusion
Thephysicalpropertiesofg-C3N4/Geheterobilayerwerestudiedbyfirstprinciplecalculations.Itwasfoundthatthebindingenergy,bandgapenergy,anddensityofstatesaregreatlydependentontheappliedelectricfielddirection.Despitetheelectricfielddirection,thebandgapwasdecreasedwiththeincreaseofelectricfieldintensity.TheDOSmovestothelowerenergysideunderupwardelectricfieldirradiation,andmovesbacktothehighenergysideunderdownwardelectricfield.Interestingly,workfunctionofheterobilayerchangedalittleincomparisonwiththepristineg-C3N4.Thepresentresultdemonstratesthegreateffectonexternalelectricfieldonthephysicalpropertiesofg-C3N4heterobilayer.
References:
[1]DAIK,LULH,LIANGCH,etal.Heterojunctionoffacetcoupledg-C3N4/surface-fluorinatedTiO2nanosheetsfororganicpollutantsdegradationundervisibleLEDlightirradiation[J].ApplCatalB-Environ, 2014, 156/157: 331-340.
[2]XINGCS,WUZD,JIANGDL,etal.HydrothermalsynthesisofIn2S3/g-C3N4heterojunctionswithenhancedphotocatalyticactivity[J].JColloidInterfaceSci, 2014, 433: 9-15.
[3]YANGM,HUSZ,LIFY,etal.Theinfluenceofpreparationmethodonthephotocatalyticperformanceofg-C3N4/WO3compositephotocatalyst[J].CeramInt, 2014, 40: 11963-11969.
[4]ZHANGJF,HUYF,JIANGXL,etal.DesignofadirectZ-schemephotocatalyst:preparationandcharacterizationofBi2O3/g-C3N4withhighvisiblelightactivity[J].JHazardMater, 2014, 280: 713-722.
[5]HUANGZA,SUNQ,LVKL,etal.EffectofcontactinterfacebetweenTiO2andg-C3N4onthephotoreactivityofg-C3N4/TiO2photocatalyst:(001)vs(101)facetsofTiO2[J].ApplCatalB-Environ, 2015, 164: 420-427.
[6]MAZN,HUZP,ZHAOXD,etal.Tunablebandstructuresofheterostructuredbilayerswithtransition-metaldichalcogenideandMXenemonolayer[J].JPhysChemC, 2014, 118: 5593-5599.
[7]MEDVEDEVAJE,FREEMANAJ,CUIXY,etal.Half-metallicityandefficientspininjectioninAlN/GaN:Cr(0001)heterostructure[J].PhysRevLett, 2005, 94: 146602.
[8]ROOMENJ,CAREYJD.Beyondgraphene:stableelementalmonolayersofsiliceneandgermanene[J].ACSAppMaterInterfaces, 2014, 6: 7743-7750.
[9]GAOHT,LIXH,LVJ,etal.Interfacialchargetransferandenhancedphotocatalyticmechanismsforthehybridgraphene/anataseTiO2(001)nanocomposites[J].JPhysChemC, 2013, 117: 16022-16027.
[10]XUL,HUANGWQ,WANGLL,etal.Mechanismofsuperiorvisible-lightphotocatalyticactivityandstabilityofhybridAg3PO4/graphenenanocomposite[J].JPhysChemC, 2014, 118: 12972-12979.
[11]GENGW,ZHAOXF,LIUHX,etal.InfluenceofinterfacestructureonthepropertiesofZnO/graphenecomposites:atheoreticalstudybydensityfunctionaltheorycalculations[J].JPhysChemC, 2013, 117: 10536-10544.
[12]GAON,LIJC,JIANGQ.Tunablebandgapsinsilicene-MoS2heterobilayers[J].PhysChemChemPhys, 2014, 16: 11673-11678.
[13]WUF,LIUYF,YUGX,etal.Visible-light-absorptioningraphiticC3N4bilayer:enhancedbyinterlayercoupling[J].JPhysChemLett, 2012, 3: 3330-3334.
[14]ZHANGZH,GUOWL.Energy-gapmodulationofBNribbonsbytransverseelectricfields:first-principlescalculations[J].PhysRevB, 2008, 77: 075403.
[15]KANGJ,WUFM,LIJB.Modulatingthebandgapsofgraphdiynenanoribbonsbytransverseelectricfields[J].JPhysCondensMatter, 2012, 24: 165301.
[16]KANEJ,LIZ,YANGJ,etal.Willzigzaggraphenenanoribbonturntohalfmetalunderelectricfield?[J].ApplPhysLett, 2007, 91: 243116.
[17]DELLEYB.Anall-electronnumericalmethodforsolvingthelocaldensityfunctionalforpolyatomicmolecules[J].JChemPhys, 1990, 92 (1): 508-517.
[18]PERDEWJP,BURKEK,ERNZERHOFM.Generalizedgradientapproximationmadesimple[J].PhysRevLett, 1996, 77 (18): 3865-3868.
[19]RUANLW,ZHUYJ,QIULG,etal.Firstprinciplescalculationsofthepressureaffectiontog-C3N4[J].CompMaterSci, 2014, 91: 258-265.
[20]DUA,NGYH,BELLNJ,etal.Hybridgraphene/titaniananocomposite:interfacechargetransfer,holedoping,andsensitizationforvisiblelightresponse[J].JPhysChemLett, 2011, 2: 894-899.
[21]LIXH,GAOHT,LIUGJ.ALDA+Ustudyofthehybridgraphene/anataseTiO2nanocomposites:interfacialpropertiesandvisiblelightresponse[J].ComputationalandTheoreticalChemistry, 2013, 1025: 30-34.
[22]LIULC,LIUZ,LIUAN,etal.EngineeringtheTiO2-grapheneinterfacetoenhancephotocatalyticH2production[J].ChemSusChem, 2014, 7: 618-626.
[23]LIUXY,CONGRD,CAOLF,etal.Thestructure,morphologyandphotocatalyticactivityofgraphene-TiO2multilayerfilmsandchargetransferattheinterface[J].NewJChem, 2014, 38: 2362-2367.
[24]XUPT,TANGQ,ZHOUZ.Structuralandelectronicpropertiesofgraphene-ZnOinterfaces:dispersion-correcteddensityfunctionaltheoryinvestigations[J].Nanotechnology, 2013, 24: 305401.
[25]LONGR.ElectronicstructureofsemiconductingandmetallictubesinTiO2/carbonnanotubeheterojunctions:densityfunctionaltheorycalculations[J].JPhysChemLett, 2013, 4: 1340-1346.
[26]LONGR,DAIY,HUANGBB.FullereneinterfacedwithaTiO2(110)surfacemaynotformanefficientphotovoltaicheterojunction:first-principlesinvestigationofelectronicstructures[J].JPhysChemLett, 2013, 4: 2223-2229.
[27]SUNL,QIY,JIACJ,etal.Enhancedvisible-lightphotocatalyticactivityofg-C3N4/Zn2GeO4heterojunctionswitheffectiveinterfacesbasedonbandmatch[J].Nanoscale, 2014, 6: 2649-2659.
[28]YANGF,KUZNIETSOVV,LUBLOWM,etal.Solarhydrogenevolutionusingmetal-freephotocatalyticpolymericcarbonnitride/CuInS2compositesasphotocathodes[J].JMaterChemA, 2013, 1: 6407-6415.
(责任编辑于敏)
10.3969/j.issn.1000-2162.2016.02.016
g-C3N4/germanene异质结物理性质的密度泛函计算-电场的影响
阮林伟1,朱玉俊1*,卢运祥2
(1.安徽大学 化学化工学院,安徽 合肥230601;2.华东理工大学 化学与分子工程学院,上海200237)
通过第一性原理计算研究电场强度和方向对于g-C3N4/germanene双层的结合能、态密度以及电荷的影响.计算结果显示,电场对于双层的物理性质影响很大,方向朝上的电场使得态密度曲线向左移动,同时方向朝下的电场使得态密度曲线朝右移动.并且在电场的影响下,功函数的变化不大.
g-C3N4; 异质结;germanene; 电场
date:2015-03-26
SupportedbytheNationalNaturalScienceFoundationofChina(51002001);theAnhuiUniversityDoctoralScientificResearchFoundation(02303319)
Author’sbrief:RUANLinwei(1990-),male,borninTaihuofAnhuiprovince,masterdegreecandidateofAnhuiUniversity; *ZHUYujun(correspondingauthor):lecturerofAnhuiUniversity,Ph.D,E-mail:zyj8119@sina.cn.
O641Documentcode:AArticleID:1000-2162(2016)02-0093-08
