汉防己甲素衍生物P-42对人乳腺癌细胞增殖、凋亡的影响及机制探讨
2016-09-05晏文涛潘卫东刘柏岑吴翱兰刘金河刘杰麟
晏文涛,潘卫东,刘柏岑,吴翱兰,刘金河,刘杰麟
(贵州医科大学,贵阳550025)
·论著·
汉防己甲素衍生物P-42对人乳腺癌细胞增殖、凋亡的影响及机制探讨
晏文涛,潘卫东,刘柏岑,吴翱兰,刘金河,刘杰麟
(贵州医科大学,贵阳550025)
目的观察汉防己甲素(TET)衍生物P-42对人乳腺癌细胞株MDA-MB-435增殖、凋亡的影响,并探讨其机制。方法收集对数生长期MDA-MB-435细胞,观察组分别加入不同浓度P-42,空白对照组加入DMEM培养液,培养24 h后,采用MTT法测算细胞增殖抑制率,流式细胞仪检测细胞凋亡率。收集对数生长期MDA-MB-435细胞,观察组分别加入终浓度2、10 μmmol/L的P-42,对照组加入DMEM培养液,另设正常乳腺细胞MCF-10a为空白组,采用Western blot法检测各组细胞布卢姆综合征(BLM)蛋白。结果当P-42 浓度在5、20、80 μmmol/L时,MDA-MB-435细胞增殖抑制率分别为76.00%、84.77%和85.53%,20、80 μmmol/L时细胞增殖抑制率均高于5 μmmol/L时,P均<0.05;80 μmmol/L时细胞增殖抑制率高于20 μmmol/L时,但差异无统计学意义。观察组P-42 浓度在 2、10 μmmol/L时,细胞早期凋亡率分别为39.47%、87.95%,晚期凋亡率分别为2.73%、3.82%,对照组分别为1.40%、0.90%,组间比较,P均<0.05。观察组P-42 浓度在 2、10 μmmol/L时, BLM蛋白的相对表达量为31.57±7.80、33.82±8.61,对照组和空白组BLM蛋白相对表达量分别为12.73±1.14、19.12±1.58,组间比较,P均<0.05。 结论TET衍生物P-42可抑制MDA-MB-435细胞增殖,诱导细胞凋亡,其机制可能与促进BLM蛋白表达有关。
汉防己甲素;乳腺癌;细胞增殖;细胞凋亡;布卢姆综合征基因
乳腺癌是女性常见的恶性肿瘤,病死率较高[1]。细胞增殖与凋亡的失衡在乳腺癌的发生发展中起重要作用[2]。布卢姆综合征(BLM)基因是Recq DNA解螺旋酶家族成员之一,其缺乏或突变可导致细胞发生癌变或凋亡[3]。汉防己甲素(TET)是一种提取自防己科植物粉防己根部的中药成分[4],属于双苄基异喹啉类生物碱,具有止痛、抗高血压、抗风湿、抗炎症和抗肿瘤等效果;TET有多种衍生物,如P-36、P-39、P-40、P-41、P-42、P-44、P-47、P-49、P-51、P-53。本课题组前期研究发现P-42抗肿瘤活性较好,但其具体机制尚不明确。2015年5~12月,我们观察了P-42对人乳腺癌细胞株MDA-MB-435增殖及凋亡的影响,并探讨其可能的作用机制。现报告如下。
1 材料与方法
1.1材料MDA-MB-435细胞来自贵州医科大学组织工程与干细胞实验中心细胞库,培养条件:高糖DMEM基础培养基加入10%胎牛血清(FBS)、1%青-链霉素、0.2 mol/L谷氨酰胺和0.2 U/mL的胰岛素。P-42由贵州省中科院天然药物化学重点实验室提供;高糖DMEM培养基、DMEM/F12(1∶1)培养基、0.25%胰蛋白酶、青-链霉素(Hyclone);FBS(杭州四季青)、Annexin Ⅴ-FITC/PI双染凋亡检测试剂盒(上海贝博);RIPA强裂解液、BCA蛋白浓度测定试剂盒(江苏碧云天),兔抗人BLM多克隆抗体和HRP偶联山羊抗兔IgG(北京中杉金桥);美国BioTek公司ELx800通用酶标仪、Beckman公司FC500MCL/MPL流式细胞分析仪。
1.2MDA-MB-435培养及传代MDA-MB-435培养条件:高糖DMEM基础培养基加入10%FBS、1%青-链霉素、0.2 mol/L谷氨酰胺和0.2 U/mL的胰岛素。置于5%CO2、37 ℃恒温细胞培养箱中单层传代培养。
1.3MDA-MB-435增殖观察采用MTT法。取对数生长期细胞, PBS洗涤1~2遍,0.25%胰酶消化,10% FBS的培养基终止消化,1 500 r/min离心3 min,8×103/孔接种于96孔板,分为4组,每组6个复孔,分别加入终浓度5、20、80 μmmol/L的P-42干预48 h,另设空白对照组(加入DMEM培养液)。取上清液,加入含MTT 50 μL的培养基,37 ℃孵育4 h,每孔加入150 μL的DMSO,采用全自动酶标仪测定490 nm波长处各孔光密度(OD)值,重复3次,取平均值,计算各组细胞增殖抑制率。细胞增殖抑制率=(对照组OD值-观察组OD值)/对照组OD值×100%。
1.4MDA-MB-435凋亡观察采用流式细胞仪。取对数生长期细胞接种于96孔板,CO2培养箱中培养24 h,分为2组,每组6个复孔,分别加入终浓度为2、10 μmmol/L P-42干预16 h,同时设立对照组(加入DMEM培养液),加入 Annexin V-FITC和PI染色,采用流式细胞仪检测各组细胞早期和晚期细胞凋亡情况,计算细胞凋亡率。
1.5BLM蛋白检测 采用Western blot法。取对数生长期细胞,分别用终浓度为2、10 μmmol/L P-42和DMEM培养液(对照组)培养24 h,另设正常乳腺细胞MCF-10a为空白组。PBS洗涤2次,采用BCA试剂盒提取总蛋白, SDS-PAGE电泳,分离,采用半干转膜法将蛋白转印至PVDF膜,将PVDF膜放入含5%脱脂奶粉的TBST缓冲液中,室温封闭2 h;加入一抗,4 ℃孵育过夜,充分洗膜,辣根过氧化物酶标记二抗37 ℃孵育1 h,充分洗膜,ECL显色。结果经成像系统扫描条带,以β-actin为内参,以目的蛋白与内参灰度比值来表示目的蛋白的相对表达量。
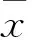
2 结果
2.1P-42对MDA-MB-435增殖的影响 当P-42 浓度分别在5、20、80 μmmol/L时,MDA-MB-435细胞增殖抑制率分别76.00%、84.77%和85.53%。P-42浓度为20、80 μmmol/L时细胞增殖抑制率均高于5 μmmol/L时,P均<0.05;20、80 μmmol/L时细胞增殖抑制率间差异无统计学意义。
2.2P-42对MDA-MB-435细胞凋亡的影响观察组P-42 浓度为2、10 μmmol/L时,细胞早期凋亡率分别为39.47%、87.95%,对照组为1.40%,组间比较,P均<0.05。观察组P-42浓度为2、10 μmmol/L时,细胞晚期凋亡率分别为2.73%、3.82%,对照组为0.90%,组间比较,P均<0.05。
2.3P-42对MDA-MB-435中BLM蛋白表达的影响观察组P-42 浓度为2、10 μmmol/L时, BLM蛋白相对表达量分别为31.57±7.80、33.82±8.61,对照组和空白组BLM蛋白相对表达量分别为12.73±1.14、19.12±1.58,组间比较,P均<0.05。
3 讨论
乳腺癌的病因和发病机制目前尚不明确。BLM作为一种解旋酶在维持基因组稳定方面发挥至关重要的作用[3],主要参与DNA损伤修复、基因重组和染色体错配等。BLM作为一种分子发动机,可以将腺苷三磷酸水解产生的化学能量转化为机械能,进而促使其本身以“蠕动”和“滚动”两种模式沿DNA分子运动,解开双链DNA[5];其具有3′~5′解旋解链活性,能够解旋多种类型的双链DNA,包括3′末端双链DNA、G-四链体DNA、bubble型DNA、复制叉DNA、D型环状DNA及Holiday结构DNA[6]。BLM是细胞内重要的DNA损伤修复酶,无论是基因复制过程中随机发生的DNA错配,或是机械性、物理性、生物性等因素引起的细胞内DNA错配及损伤[5],BLM均可通过代偿性增多发挥修复错配及损伤DNA的效应,以维持细胞内正常代谢及功能。
TET作为中国传统的中药有效成分,可用于治疗关节炎、心律不齐、炎症反应、矽肺病、肿瘤等多种疾病[7],其抗肿瘤机制主要通过介导凋亡[7]、阻断Ca2+通道[8]、引起自噬并调节细胞内活性氧分子[9]等途径抑制和杀伤肿瘤细胞。Bai等[4]研究发现,TET能通过诱导凋亡抑制结肠癌细胞异种移植后的肿瘤生长。Li 等[8]研究表明,TET抗肿瘤效应的一种机制是作为一种非选择性的Ca2+通道阻断剂和Ca2+调节蛋白拮抗剂发挥作用。研究发现,高浓度的TET能诱导肝癌细胞凋亡,低浓度的TET能诱导肝癌细胞自噬,且TET联合其他化疗药物能发挥更明显的抗肿瘤效应[10~12]。本研究结果发现,随P-42浓度升高细胞增殖抑制率逐渐升高。据文献[13,14]报道,顺铂抑制多种肿瘤细胞的半数抑制浓度(IC50)为10~30 μmmol/L。本实验前期研究中发现P-42对MDA-MB-435的 IC50为2 μmmol/L左右,抑制效果明显优于顺铂。当P-42 浓度在 2、10 μmmol/L时,MDA-MB-435细胞早期凋亡率分别为39.47%、87.95%,对照组早期凋亡率为1.40%,组间比较,P均<0.05。P-42 浓度在 2、10 μmmol/L时,MDA-MB-435晚期期凋亡率分别为2.73%、3.82%,对照组晚期凋亡率为0.90%,组间比较,P均<0.05。由于衍生物P-42的强抑制活性,在2、10、20 μmmol的P-42作用下,细胞均无完整的形态,且大部分细胞已被裂解杀死或呈皱缩形态异;药物作用24 h,细胞在不同药物浓度间活性表现出明显差异,随着药物浓度的增加,细胞死亡数呈增多趋势,差异有统计学意义。本研究结果显示,观察组BLM蛋白的相对表达量高于对照组、空白组。推测可能是由于P-42可穿过细胞膜进入细胞内部引起DNA损伤[15,16],同时细胞内部启动DNA修复程序,代偿性合成更多DNA修复酶BLM来修复损伤的DNA,以维持基因组稳定和正常核酸代谢[17~19]。
综上所述,TET衍生物P-42可抑制MDA-MB-435的增殖,诱导其凋亡,其机制可能与促进BLM表达有关,但其具体作用机制尚有待于进一步研究。
[1] Barzegar E, Fouladdel S, Movahhed TK, et al. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines[J]. Iranian J Bas Med Sci, 2015,18(4):334-342.
[2] Tengku DT, Seeni A, Khairi WNM, et al. Effects of rapamycin on cell apoptosis in MCF-7 human breast cancer cells [J]. Asian Paci J Can Pre, 2015,15(24):10659-10663.
[3] Sommers J, Suhasini A, Brosh R. Protein degradation pathways regulate the functions of helicases in the DNA damage response and maintenance of genomic stability [J]. Biomolecules, 2015,5(2):590-616.
[4] Bai BC, Gao JL, Zhang BQ, et al. Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses tumor growth of human colorectal cancer [J]. Mole Pharm, 2011,79(2):211-219.
[5] 张永娟,沈佐君. BLM基因的研究进展[J].分子诊断与治疗杂志, 2012, 4 (4):262-266.
[6] 刘金河, 许厚强, 孟惠惠, 等. 甘草次酸对Bloom解旋酶生物学特性的影响 [J] 中国生物化学与分子生物学报, 2014, 30 (9):919-926.
[7] Qin R, Shen H, Cao Y, et al. Tetrandrine induces mitochondria-mediated apoptosis in human gastric cancer BGC-823 cells [J]. PLoS One, 2013,8(10):1-10.
[8] Li S, Ji Z, Zou M, et al. Preparation, characterization, pharmacokinetics and tissue distribution of solid lipid nanoparticles loaded with tetrandrine[J]. AAPS Pharm Sci Tech, 2011,12(3):1011-1018.
[9] Gong K, Chen C, Zhan Y, et al. Autophagy-related gene 7 (ATG7) and reactive oxygen species/extracellular signal-regulated kinase regulate tetrandrine-induced autophagy in human hepatocellular carcinoma[J]. J Bio Chem, 2012,287(42):35576-35588.
[10] Liu C, Gong K, Mao X, et al. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing akt activity in human hepatocellular carcinoma[J]. Inter J Can Jour Inter, 2011,129(6):1519-1531.
[11] Wan J, Liu T, Mei L, et al. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling[J]. British J Can, 2013,109(2):342-350.
[12] Wu JM, Chen Y, Chen JC, et al. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice[J]. Cancer Letters, 2010,287(2):187-195.
[13] Wu YH, Chiu WT, Young MJ, et al. Solanum incanum extract downregulates aldehyde dehydrogenase 1-mediated stemness and inhibits tumor formation in ovarian cancer cells[J]. Jour Can, 2015,6(10):1011-1019.
[14] Karabacak M, Altintop MD, Ibrahim CH, et al. Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents[J]. Molecules, 2015,20(10):19066-19084.
[15] RazmkhahM, Ghaderi A. HLA Class I Allele Frequencies in Southern Iranian Women with Breast Cancer[J]. Iran J Basic Med Sci, 2013,16(2): 140-143.
[16] Al-Hajj M, Wicha M, Morrison SJ,et al. Prospective identification of tumorigenic breast cancer cells[J]. Proc Natl Acad Sci USA, 2003,100(7):3983-3988.
[17] Ricci-Vitiani L, Lombardi DG,Pilozzi E,et al. Identification and expansion of human colon-cancer-initiating cells[J]. Nature, 2007,445(7123):111-115.
[18] Li X,Xu H,Zhu Z, et al.Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on "oxidation therapy"[J]. Mol Pharm, 2012,9(2):222-229.
[19] Tao L,Zhou X,Shen C,et al.Tetrandrine induces apoptosis and triggers a caspase cascade in U2-OS and MG-63 cells through the intrinsic and extrinsic pathways[J]. Mol Med Rep, 2014, 9(1):345-349.
Effects of tetrandrine derivatrve P-42 on proliferation and apoptosis of human breast cancer cells and its mechanism
YANWentao,PANWeidong,LIUBocen,WUAolan,LIUJinhe,LIUJielin
(GuizhouMedicalUniversity,Guiyang550025,China)
ObjectiveTo observe the effects of tetrandrine (TET) derivative P-42 on the proliferation and apoptosis of human breast cancer cell line MDA-MB-435 and to investigate its mechanism.MethodsMDA-MB-435 cells in the logarithmic phase were collected, and then the observation group was treated with different concentrations of P-42, and the control group was added with DMEM nutrient solution. After 24 hours, MTT assay was used to measure the proliferation inhibition rate of MDA-MB-435 cells and flow cytometry was applied to analyze the apoptosis rate. MDA-MB-435 cells in the logarithmic phase were collected, and then the observation group was treated with 2 and 10 μmmol/L P-42, and the control group was added with DMEM nutrient solution, meanwhile, the normal breast cells MCF-10a were taken as the blank group. Bloom syndrome (BLM) protein in each group was detected Western blotting. ResultsWhen the concentrations of P-42 were 5, 20 and 80 μmmol/L, the proliferation inhibition rates of MDA-MB-435 cells were 76.00%, 84.77% and 85.53%, respectively. The proliferation inhibition rates of MDA-MB-435 cells treated with 20 and 80 μmmol/L P-42 were higher than that treated with 5 μmmol/L P-42, allP<0.05. The proliferation inhibition rate of MDA-MB-435 cells treated with 80 μmmol/L P-42 were higher than that treated with 20 μmmol/L P-42, allP<0.05. When the concentrations of P-42 were 2 and 10 μmmol/L, the early apoptosis rates of MDA-MB-435 cells were 39.47% and 87.95%, and that in the control group was 1.4%, allP<0.05. When the concentrations of P-42 were 2 and 10 μmmol/L, the late-stage apoptosis rates of MDA-MB-435 cells were 2.73% and 3.82%, and that in the control group was 0.90%, allP<0.05.When the concentrations of P-42 were 2 and 10 μmmol/L, the relative expression of BLM protein was 31.57±7.80 and 33.82±8.61, and that in the control group and blank group was 12.73±1.14 and 19.12±1.58, allP<0.05.ConclusionTetrandrine derivative P-42 can inhibit the proliferation and induce apoptosis of MDA-MB-435 cells, adn the underlying mechanism may be associated with the up-regulation of BLM expression.
tetrandrine; breast carcinoma; cell proliferation; apoptosis; Bloom syndrome gene
国家自然科学基金资助项目(81360349)。
晏文涛(1989-),男,硕士,主要研究方向为抗肿瘤药物筛选及抑瘤机制研究。E-mail: 714419389@qq.com
简介:刘杰麟(1963-),男,博士,教授,主要研究方向为小分子天然活性物质抑制RecQ家族解旋酶等肿瘤相关基因。E-mail:779713773@qq.com
10.3969/j.issn.1002-266X.2016.18.001
R392-33
A
1002-266X(2016)18-0001-04
2016-01-01)
