长链非编码RNA与胃癌相关性的研究进展
2016-08-11黄艳霞朱金水
黄艳霞 张 靖 王 歌 朱金水
长链非编码RNA与胃癌相关性的研究进展
黄艳霞张靖王歌朱金水
200233上海交通大学附属第六人民医院消化内科
摘要:长链非编码RNA(lncRNA)因其长度大于200个核苷酸,缺乏编码蛋白能力而得名。在表观遗传学控制、转录和转录后调控等多个层面,lncRNA参与了机体多种生理及病理过程的调节。研究已经证明,lncRNA在胃癌生物学的调节过程中起关键的作用,lncRNA与胃癌的发生、发展、侵袭、转移及预后密切相关。该文对lncRNA在胃癌中的研究进展作一综述。
关键词:lncRNA;胃癌;转移
长链非编码RNA(lncRNA)因其长度大于200个核苷酸,缺乏编码蛋白能力而得名。lncRNA Xist 为哺乳动物中首先被发现的lncRNA,可导致染色质结构与组成的重塑。尽管lncRNA发挥了重要的生物学功能,但多数情况下仍被认为是转录中的“噪音”。其后发现lncRNA HOTAIR可与多梳蛋白复合体相互作用,参与机体生长、发育过程的调节。随着高通量测序等生物信息学技术的快速发展,已发现lncRNA在表观遗传学控制、转录和转录后调控等多个层面参与了机体多种生理及病理过程的调节。
1lncRNA的结构、分类及功能
lncRNA可位于细胞核和细胞质中,多由RNA聚合酶Ⅱ转录。其长度变异性较大,部分可延长至100 kb。人类基因组约有15 000种lncRNA,其转录水平远低于蛋白编码基因,且具有组织特异性。目前分子结构明确的lncRNA所占比例甚少,Niazi等[1]对204种功能性lncRNA进行分析后发现,内含子数量少、鸟嘌呤(G)和胞嘧啶(C)含量低、起始密码子和开放阅读框架的缺乏可能是lncRNA的一些结构特征。根据lncRNA基因与编码基因间的位置关系,可以将其分为正义lncRNA、反义lncRNA、双向lncRNA、内含子lncRNA和基因间lncRNA五类[2]。lncRNA的异常表达与人类疾病密切相关,如老化相关的阿尔茨海默病及认知障碍相关疾病都被认为与lncRNA的异常表达有关;其在心血管疾病、内分泌疾病(如糖尿病)中亦发挥了作用[3]。值得关注的是,lncRNA与肿瘤的发生发展密切相关,lncRNA在前列腺癌、结直肠癌、乳腺癌、膀胱癌、肝细胞癌及胃癌等多种恶性肿瘤中均有异常表达,可作为致癌或抑癌因子参与肿瘤细胞转录、染色质重塑、微血管侵袭等过程的调控。
2lncRNA与胃癌
lncRNA AC096655首先被发现与胃癌相关,并被命名为GACAT1[4]。2012年Yang等[5]发现lncRNA H19表达上调可促进胃癌细胞增殖。其后lncRNA在胃癌中的重要性得到越来越多的关注,它们几乎参与胃癌发生发展的全过程,包括肿瘤细胞增殖、凋亡、侵袭、转移、预后及耐药性等多个方面。此外,lncRNA对胃癌的诊断也有帮助,可能是胃癌诊断的潜在分子标志物。
2.1lncRNA在胃癌中的生物学功能
lncRNA在胃癌的发生发展中具有促癌或抑癌双重作用。如lncRNA H19、HULC、Linc00152、HOTAIR、PVT1、HIF1A-AS2、LINC00982、MALAT1及SPRY4-IT1等具有促进肿瘤细胞增殖、入侵及转移的作用[6-14],而lncRNA MEG3、GAS5、FENDRR、LEIGC、FER1L4及WT1-AS等具有抑制肿瘤细胞增殖、促进肿瘤细胞凋亡的作用[15-20]。此外,lncRNA在胃癌组织中的表达水平也不一致,lncRNA的表达水平与胃癌临床病理特征的关系见表1。
2.2lncRNA在胃癌耐药中的作用
肿瘤细胞耐药是化学治疗失败的重要原因之一,越来越多的研究发现lncRNA参与肿瘤细胞耐药的调节,如Zhang等[29]的研究发现lncRNA PVT1在对顺铂耐药患者的胃癌组织及胃癌细胞中高表达,过表达lncRNA PVT1促进了胃癌细胞耐药。Ding等[30]亦证实lncRNA PVT1表达升高能促进胃癌细胞对紫杉醇的耐药。此外,癌细胞中高表达的lncRNA MRUL促进了胃癌耐药细胞中ABCB1的表达,从而促进了胃癌细胞对药物的耐药性[31]。然而,一些lncRNA却具有提高肿瘤细胞对化学治疗药物敏感性的作用,如Han等[32]发现lncRNA LEIGC可增加胃癌细胞对5-氟尿嘧啶的敏感性。
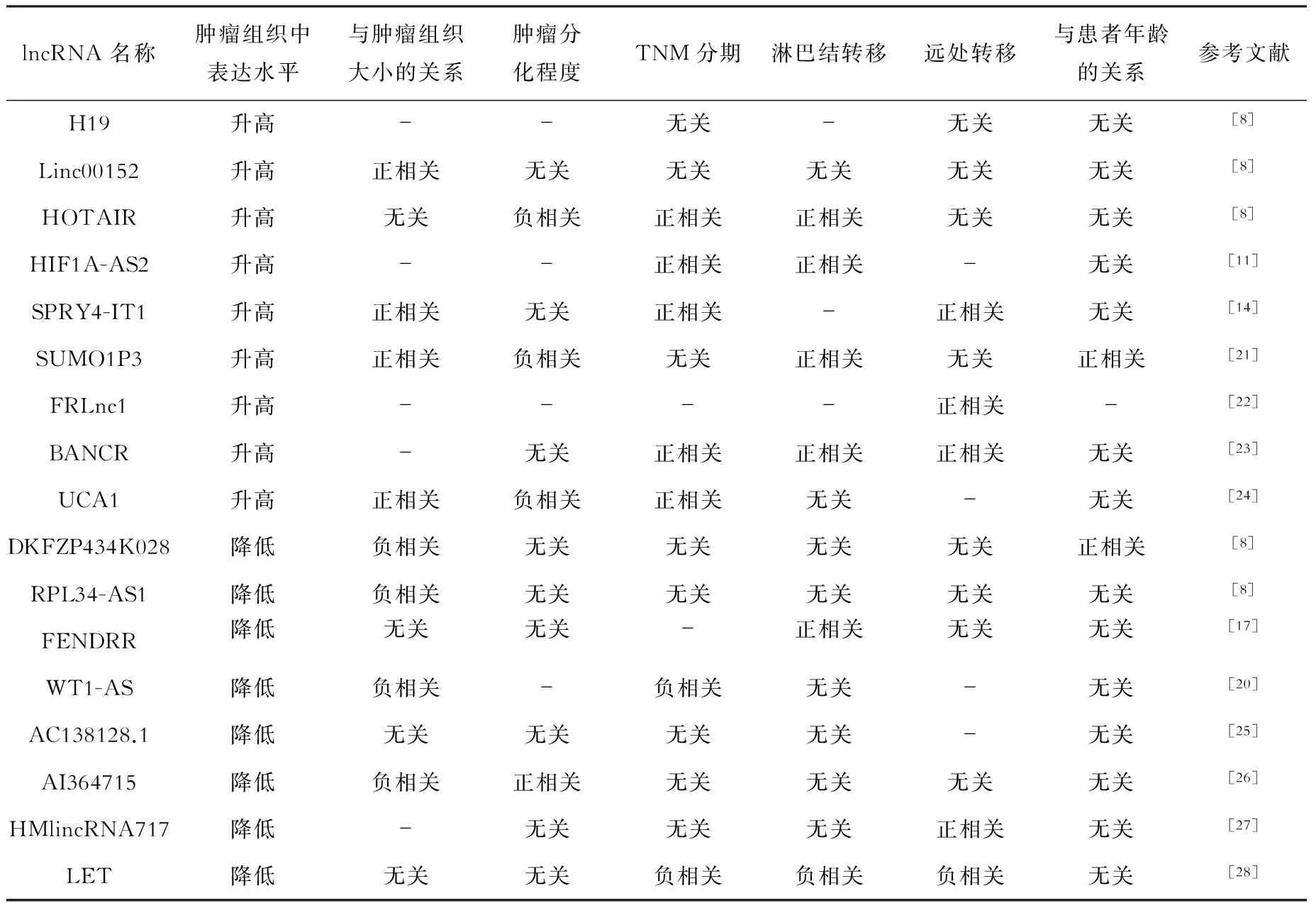
表1 lncRNA的表达与胃癌临床病理特征的关系
2.3lncRNA在胃癌侵袭转移中的作用
侵袭和转移是恶性肿瘤的重要特征之一。越来越多的证据表明,上皮-间质转化(EMT)及肿瘤细胞间的黏附能力下降,可能参与了肿瘤转移的重要环节。Zhao等[8]的研究发现,lncRNA Linc00152能抑制胃癌细胞中EMT的进程。Han等[32]研究了lncRNA LEIGC在胃癌细胞中的作用,敲低其表达水平后,胃癌细胞发生了EMT。该研究进一步检测了与EMT相关的一些蛋白,研究结果与此一致,因此可以认为lncRNA LEIGC在胃癌细胞中是一个潜在的EMT抑制剂。Zhao等[7]的研究发现,lncRNA HULC可抑制胃癌细胞凋亡、促进胃癌细胞增殖,其作用机制可能与诱导细胞自噬,促进EMT有关。转化生长因子-β(TGF-β)信号通过miR-200家族和ZEB1/2之间的双负反馈循环调节肿瘤EMT[33],Saito等[34]的研究发现,lncRNA ATB通过TGF-β诱导胃癌EMT变化以促进转移发生。Xu等[17]发现过表达lncRNA FENDRR能诱导胃癌肺转移结节增多,并使肿瘤细胞间的黏附分子FN1表达下降,证明了lncRNA FENDRR可能通过影响FN1来调节胃癌细胞的转移。
2.4lncRNA与胃癌的诊断
lncRNA可存在于血浆、胃液及组织中,目前部分血浆中的lncRNA已被作为潜在的肿瘤标志物。Zhou等[35]的研究发现血浆中lncRNA H19能为早期胃癌的诊断提供可靠依据。胃液中lncRNA的获得相对容易,并能够为胃癌提供有效的诊断。Zheng等[36]收集了49份胃液标本(26份胃癌患者标本和23份健康者标本),发现胃癌标本中lncRNA UCA1的水平高于健康标本。Yang等[37]研究了130份胃液标本(包括胃良性病变、胃不典型增生、胃癌癌前病变和胃癌患者),发现胃癌患者的胃液中lncRNA ABHD11-AS1水平显著高于正常胃、萎缩性胃炎、胃溃疡,并与胃癌患者的性别、肿瘤大小、分期、劳伦分型及血清中癌胚蛋白(CEA)水平相关。值得注意的是,胃液中lncRNA ABHD11-AS1作为诊断胃癌的标志物时,早期胃癌的诊断率提高到71.4%。
2.5lncRNA可能的作用机制
lncRNA的调节机制复杂且多样化:可调控下游基因转录或作为分子阻断剂阻断该分子发生作用;亦可通过与蛋白结合,定位到特定的DNA序列上;也可与多个相关转录因子结合发挥作用;还可竞争性结合miRNA以调控基因表达。Cai等[22]发现lncRNA FRLnc1可通过调节FOXM1参与肿瘤生长及血管生成。Wang等[13]研究了lncRNA MALAT1的功能,发现其可能通过调节SF2/ASF来促进胃癌细胞增殖。有研究表明,lncRNA GAS5通过下调E2F1和p21的表达来诱导胃癌细胞增殖[16],而lncRNA WT1-AS则通过抑制细胞外信号调节激酶(ERK)蛋白磷酸化水平来阻止胃癌发生[20]。
竞争性内源RNA(ceRNA)在转录后调节及肿瘤的发生发展中具有重要的作用[38]。ceRNA通过竞争性结合miRNA以调节基因表达,是RNA之间相互作用的一种新机制。如Liu等[39]发现胃癌中lncRNA HOTAIR可通过竞争性抑制miR-331-3p来调节表皮生长因子受体2(HER2)的表达,增加了转录后调控的水平。lncRNA H19可通过调控miR-675抑制RUNX1而促进肿瘤细胞增殖[40]。lncRNA H1F1A-AS2在胃癌发展中发挥了重要的作用,通过调节与肿瘤相关的缺氧诱导因子-1α(HIF-1α)途径促进肿瘤发展。lncRNA ANRIL在胃癌组织中表达上调,通过表观沉默miR-99a/miR-449a促进肿瘤生长[11]。
3问题与展望
lncRNA在组织生理及病理过程中发挥了重要的调节作用,其作用机制仍有待进一步探索。lncRNA在疾病发生发展中的作用是错综复杂的,一方面可能是由于lncRNA在物种间的低保守性,使得物种中lncRNA的功能不确定[41];另一方面由于缺乏相关的研究工具,lncRNA的数据库还不够充足,很难全面揭示lncRNA的生物学功能;此外,lncRNA与肿瘤转移或耐药的机制研究尚不成熟[42-43]。无论当前研究显示lncRNA是抑癌基因或是促癌基因,可以确定的是lncRNA与胃癌有着密切的联系,以lncRNA为靶点的药物有望在胃癌的临床治疗中起到重要的作用。因此,明确lncRNA在胃癌及其他疾病中的具体作用尤为重要,有助于全面深入地认识胃癌的发生发展机制,并为临床治疗胃癌提供更多的途径。
参考文献
1 Niazi F, Valadkhan S. Computational analysis of functional long noncoding RNAs reveals lack of peptide-coding capacity and parallels with 3′ UTRs[J]. RNA, 2012, 18: 825-843.
2 Kaikkonen MU, Lam MT, Glass CK, et al. Non-coding RNAs as regulators of geneexpression and epigenetics[J]. Cardiovasc Res, 2011, 90: 430-440.
3 Kim J, Kim KM, Noh JH, et al. Long noncoding RNAs in diseases of aging[J]. Biochim Biophys Acta, 2016, 1859: 209-221.
4 Xiao B, Guo J. Long noncoding RNA AC096655.1-002 has been officially named as gastric cancer-associated transcript 1, GACAT1[J]. Tumour Biol, 2013, 34: 2697-2701.
5 Yang F, Bi J, Xue X, et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells[J]. FEBS J, 2012, 279: 3159-3165.
6 Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer[J]. Oncotarget, 2014, 5: 2318-2329.
7 Zhao Y, Guo Q, Chen J, et al. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation[J]. Oncol Rep, 2014, 31: 358-364.
8 Zhao J, Liu Y, Zhang W, et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer[J]. Cell Cycle, 2015, 14: 3112-3123.
9 Endo H, Shiroki T, Nakagawa T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer[J]. PLoS One, 2013, 8: e77070.
10 Kong R, Zhang EB, Yin DD, et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16[J]. Mol Cancer, 2015, 14: 82.
11 Chen WM, Huang MD, Kong R,et al. Antisense long noncoding RNA HIF1A-AS2 is upregulated in gastric cancer and associated with poor prognosis[J]. Dig Dis Sci, 2015, 60: 1655-1662.
12 Fei ZH, Yu XJ, Zhou M, et al. Upregulated expression of long non-coding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer[J]. Tumour Biol, 2016, 37: 1983-1993.
13 Wang J, Su L, Chen X, et al. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF[J]. Biomed Pharmacother, 2014, 68: 557-564.
14 Peng W, Wu G, Fan H, et al. Long noncoding RNA SPRY4-IT1 predicts poor patient prognosisand promotes tumorigenesis in gastric cancer[J]. Tumor Biol, 2015, 36: 6751-6758.
15 Peng W, Si S, Zhang Q, et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression[J]. J Exp Clin Cancer Res, 2015, 34: 79.
16 Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer[J]. BMC Cancer, 2014, 14: 319.
17 Xu TP, Huang MD, Xia R, et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression[J]. J Hematol Oncol, 2014, 7: 63.
18 Han Y, Ye J, Wu D, et al. LEIGC long non-coding RNA acts as a tumor suppressor in gastric carcinomaby inhibiting the epithelial-to-mesenchymaltransition[J]. BMC Cancer, 2014, 14: 932.
19 Xia T, Chen S, Jiang Z, et al. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression[J]. Sci Rep, 2015, 5: 13445.
20 Du T, Zhang B, Zhang S, et al. Decreased expression of long non-coding RNA WT1-AS promotes cell proliferation and invasion in gastriccancer[J]. Biochim Biophys Acta, 2015, 1862: 12-19.
21 Mei D, Song H, Wang K, et al. Up-regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association[J]. Med Oncol, 2013, 30: 709.
22 Cai H, Chen J, He B, et al. A FOXM1 related long non-coding RNA contributes to gastric cancer cell migration[J]. Mol Cell Biochem, 2015, 406: 31-41.
23 Li L, Zhang L, Zhang Y, et al. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer[J]. Biomed Pharmacother, 2015, 72: 109-112.
24 Zheng Q, Wu F, Dai WY, et al. Aberrant expression of UCA1 in gastric cancer and its clinical significance[J]. Clin Transl Oncol, 2015, 17: 640-646.
25 Chen X, Sun J, Song Y, et al. The novel long noncoding RNA AC138128.1 may be a predictive biomarkerin gastric cancer[J]. Med Oncol, 2014, 31: 262.
26 Zhu S, Mao J, Shao Y, et al. Reduced expression of the long non-coding RNA AI364715 in gastric cancer and its clinical significance[J]. Tumour Biol, 2015, 36: 8041-8045.
27 Shao Y, Chen H, Jiang X, et al. Low expression of lncRNA-HMlincRNA717 in human gastric cancer and its clinical significances[J]. Tumor Biol, 2014, 35: 9591-9595.
28 Zhou B, Jing XY, Wu JQ, et al. Down-regulation of long non-coding RNA LET is associated with poor prognosis in gastric cancer[J]. Int J Clin Exp Pathol, 2014, 7: 8893-8898.
29 Zhang XW, Bu P, Liu L, et al. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance[J]. Biochem Biophys Res Commun, 2015, 462: 227-232.
30 Ding J, Li D, Gong M, et al. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer[J]. Onco Targets Ther, 2014, 7: 1625-1630.
31 Wang Y, Zhang D, Wu K, et al. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines[J]. Mol Cell Biol, 2014, 34: 3182-3193.
32 Han Y, Ye J, Wu D, et al. LEIGC long non-coding RNA acts as a tumor suppressor in gastric carcinoma by inhibiting the epithelial-to-mesenchymal transition[J]. BMC Cancer, 2014, 14: 932.
33 Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells[J]. EMBO Rep, 2008, 9: 582-589.
34 Saito T, Kurashige J, Nambara S, et al. A long non-coding RNA activated by transforming growth factor-beta is an independent prognostic marker of gastric cancer[J]. Ann Surg Oncol, 2015, 22: 915-922.
35 Zhou X, Yin C, Dang Y, et al. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer[J]. Sci Rep, 2015, 5: 11516.
36 Zheng Q, Wu F, Dai WY, et al. Aberrant expression of UCA1 in gastric cancer and its clinical significance[J]. Clin Transl Oncol, 2015, 17: 640-646.
37 Yang Y, Shao Y, Zhu M, et al. Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer[J]. Tumour Biol, 2016, 37: 1183-1188.
38 Tay Y, Kats L, Salmena L, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs[J]. Cell, 2011, 147: 344-357.
39 Liu X, Sun M, Nie F, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer[J]. Mol Cancer, 2014, 13: 92.
40 Zhuang M, Gao W, Xu J, et al. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1[J]. Biochem Biophys Res Commun, 2014, 448: 315-322.
41 Struhl K.Transcriptional noise and the fidelity of initiation by RNA polymerase Ⅱ[J]. Nat Struct Mol Biol, 2007, 14: 103-105.
42 Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transitionis not required for lung metastasis but contributes to chemoresistance[J]. Nature, 2015, 527: 472-476.
43Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transitionis dispensable for metastasis but induces chemoresistance in pancreatic cancer[J]. Nature, 2015, 527: 525-530.
(本文编辑:林磊)
基金项目:国家自然科学基金(81573747,81302093,81272752)
通信作者:朱金水,Email: zhujs1803@163.com
DOI:10.3969/j.issn.1673-534X.2016.03.005
(收稿日期:2015-12-20)
