The oncofetal protein IMP3 is an indicator of early recurrence and poor outcome in mucoepidermoid carcinoma of salivary glands
2016-08-10MohamedElshafeyRehabAhmedMohamedMouradEssamGaballah
Mohamed R. Elshafey, Rehab A. Ahmed, Mohamed I Mourad, Essam T. Gaballah
The oncofetal protein IMP3 is an indicator of early recurrence and poor outcome in mucoepidermoid carcinoma of salivary glands
Mohamed R. Elshafey1, Rehab A. Ahmed2, Mohamed I Mourad1, Essam T. Gaballah1
1Oral Pathology Department, Faculty of Dentistry, Mansoura University, Mansoura 35516, Egypt;2Pathology Department,Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt
ABSTRACTObjective: Mucoepidermoid carcinoma (MEC) is the most common primary malignancy of the salivary glands. Insulin-like growth factor-II mRNA-binding protein-3 (IMP3) is an important prognostic factor in some cancers and a tool that differentiates between benign and malignant pancreatic lesions. This study aimed to identify a relationship between the expression of IMP3 and the outcome of salivary gland MEC, as well as to differentiate MEC from pleomorphic adenoma (PA).Methods: Tissue specimens from 70 cases of salivary gland MEC, 40 cases of PA, and 10 cases with normal salivary gland were examined immunohistochemically for IMP3. The association among the expression of IMP3, clinicopathological characteristics and patient's survival was assessed.Results: IMP3 was present in 51.4% of MEC but absent in PA and normal salivary gland tissues. IMP3 expression was associated with age > 60 years, submandibular gland tumors, tumor size > 4 cm, high-grade tumors, lymph node metastasis, involvement of surgical margins, perineural invasion, distant metastasis, advanced TNM stage, tumor relapse, and death (P<0.05). Increased expression of IMP3, tumors of the submandibular gland, and lymph node metastasis were independent prognostic factors of disease-free survival (DFS). In addition, IMP3 was a strong predictor of overall survival (OS) together with distant metastasis and intermediate and high-grade tumors.Conclusions: IMP3 expression is highly important in evaluating the outcome of MEC. IMP3 can be used to differentiate MEC from PA of salivary glands.
KEYWORDSIMP3; mucoepidermoid carcinoma; salivary glands; prognosis
Introduction
Mucoepidermoid carcinoma (MEC) is the most common primary malignancy of salivary glands in both children and adults; MEC accounts for 29% to 34% of malignant tumors of major and minor salivary glands1.
The impact of the clinicopathological features on the outcome of MEC of salivary glands has been studied. Features with poor impact on the outcome include highgrade tumors, positive lymph node metastasis, perineural invasion, advanced TNM stage, distant metastasis, positive surgical margins, and submandibular location. Convenient prognostic biomarkers remain mostly irrelevant2.
IMP3 is an oncofetal protein that regulates the growth of cells in the early stages of embryogenesis. IMP3 is also important for the adhesion, migration, and widespread of malignancies3,4. IMP3 is known as K-homologous domain containing protein overexpressed in cancer (KOC) or L523S5,6. The gene of IMP3 is placed on chromosome 7p11.2 and its protein is revealed in the developing muscle,epithelium, and placenta. However, the expression of IMP3 is absent or low in mature tissues7,8. Several studies have found IMP3 overexpression in various malignancies, which is a relevant prognostic biomarker in these cancers9. Among the cancers that showed the poor impact of IMP3 on their outcome were renal cell carcinoma, oral squamous cell carcinoma, hepatocellular carcinoma, and pancreatic adenocarcinoma8,10-12. IMP3 was also found to be a useful tool that distinguishes benign from malignant pancreatic lesions6.
MEC being misdiagnosed as pleomorphic adenoma (PA) is an extremely rare case because of the cytologically blandepithelial component of the latter, which is characterized by its vacuolated nuclei, inconspicuous nucleoli, and few mitotic figures. However, occasionally pleomorphic adenoma shows prominent clear cell change or mucous metaplasia. In addition, extensive inflammation, marked necrosis,squamous metaplasia, and keratin pearls can occur in its epithelial components following fine needle aspiration or spontaneous infarction. These conditions may exhibit cellular atypia and frequent mitotic figures. These changes can lead to a wrong diagnosis of PA as low-grade MEC or squamous cell carcinoma13,14.
To our knowledge, no study has evaluated the expression of IMP3 in salivary gland MEC. Therefore, we aim to investigate the expression of IMP3 by immunohistochemistry and its relationship with the clinicopathological characteristics and outcome of salivary glands MEC in our locality (Egypt). We can select high-risk patients to facilitate intensive therapy and strict follow-up. We also assessed the expression of IMP3 in PA and its utility to differentiate the latter from MEC.
Materials and methods Data retrieval
This study was conducted retrospectively on selected 70 cases of salivary gland MEC with available paraffin blocks,complete clinicopathological, and follow-up data without distant metastasis at the initial diagnosis from January 2000 to December 2014. All cases in this study underwent surgical resection. Some cases underwent neck lymph node dissection. Patients with close margin, multiple lymph node metastases, perineural invasion, high grade lesions,extracapsular invasion, or advanced stage received postoperative radiotherapy (RT). None of the patients received preoperative RT. The study also included 10 cases with normal salivary glands and 40 cases with PA.
The patients’ clinicopathological data were recovered from the folders of the pathology laboratory of the Oncology Center, Faculty of Medicine, Mansoura University, Egypt. These data included the patients's age and sex, primary tumor site, primary tumor size, status of the surgical margin,lymph node status, and the presence or absence of perineural invasion. The study included 29 males and 41 females with a ratio of 1:1.4. The patients' age ranged from16 to 78 years with a mean of 51.6±13.8 years. The size of primary tumors ranged from 1 to 7 cm with a mean of 4.2±1.6 cm. The clinicopathological characteristics of other patients are shown in Table 1.
Patient follow-up was done every 3 to 6 months for the first 2 years then once a year. The follow-up comprised clinical examination and periodic ultrasonography of the head and neck. In addition, chest X-ray, bone scan, and abdominal ultrasonography were performed when relapse was suspected. Overall survival (OS) and disease-free survival (DFS) data were retrieved from the archive of the Oncology Center. Previous clinicopathological and follow-up data were obtained after getting an approval from the institutional review board. This study was approved by the ethics committee of Mansoura University.
The original H&E sections were brought back from the archives of the pathology laboratory in the Oncology Center for reassessment of previous pathological data in addition to the adequacy of specimen. The diagnosis of mucoepidermoid carcinoma was conducted as stated in the WHO classification15. The tumors were graded according to the system proposed by the AFIP16,17. TNM staging was performed for major salivary gland tumors in accordance with the AJCC system18. TNM staging of minor salivary gland tumors was conducted similar to the method of squamous cell carcinomas of the site of origin19. Thereafter,the paraffin blocks of the selected cases were retrieved from the archives. Sections from the blocks of some high-grade cases with mucous cells that are difficult to identify were stained by PAS and Alcian blue stains.
Immunohistochemical staining
All selected blocks were recut at the thickness of 3 to 4 um. The slices were placed on coated slides. After xylene deparaffinization, the sections were rehydrated in descending grades of alcohol then in water. Antigen retrieval was performed by using 0.01 M citric acid buffer (pH 6.0) and heated for 10 minutes in the microwave. The sections were then incubated in a blocking medium (3% H2O2) for five minutes followed by washing with distilled water. Rabbit monoclonal antihuman antibody (clone; EPR 5111, 1:50,dilution, Abcam, 1 Kendall Square, Suite B2304, Cambridge,MA 02139-1517, USA) was used against IMP3 antigen.
A positive control using a slide from a pancreatic ductal adenocarcinoma was tested per run of immunostaining. The IMP3 antibody was incubated at room temperature for 60 min. Immunodetection was executed using Power-stain TM 1.0 poly HRP DAB kit for mouse+rabbit (Cat No 52-0017,Genemed Biotechnologies, Inc., 458 Carlton Ct., South San Francisco, CA 94080, USA). Immune staining was performed based on manufacturer's instructions. Immunoreaction was visualized by adding DAB. Counterstaining of slides was performed with the Mayer hematoxylin. A negative control was tested in each series by omitting the primary antibody.

Table 1 Clinicopathological features of salivary gland MEC and its association with IMP3 expression
Immunostaining evaluation
Sections of the studied cases were scored according to the percentage of positive tumor cells as follows: 0, <10%; 1,10%-25%; 2, 26%-50%; and 3, >50%. Tumor cells were stained with staining pattern as either nuclear and/or cytoplasmic. Cases with positive cells >10 % were considered positive. We also divided the positive cases into focal or heterogenous (10%-50% of tumor cells were stained) and diffuse (>50% of tumor cells were stained).
Statistical analysis
Data analysis was conducted using SPSS program version 17 (Inc., Chicago, IL, USA). Descriptive data were presented in number and percentage format. Quantitative statistics were calculated in the form of mean ± standard deviation (SD). The association between the different clinicopathological parameters and IMP3 expression was tested using Chi square (χ2) and Fisher’s exact probability test. The independent sample t-test (compare continuous variable in 2 groups) and one-way ANOVA (compare continuous variable in three groups) were applied to compare the duration of survival between factors. The construction of survival curves was conducted using the Kaplan-Meier method, and the significance was assessed with the log-rank test. Univariate and multivariate survival analyses were performed with the Cox proportional hazards model to detect any independent prognostic factor. A P-value lesser than 0.05 was considered statistically significant. DFS was estimated from the time of surgery until the time of local or distant recurrence. OS was estimated from the time of diagnosis to the time of the last follow-up or the time of patient's death.
Results
IMP3 immunohistochemical expression and its association with clinicopathological characteristics
In this study, 36 (51.4%) out of 70 MEC cases were positive for IMP3. Among the positive cases, 13 (36.2%), 15 (41.6%),and 8 (22.2%) cases were scored 1, 2, and 3, respectively (Figures 1 and 2). Scores 1 and 2 represented cases with heterogeneous expression, whereas a score of 3 represented cases with diffused expression.

Figure 1 Cytoplasmic expression of IMP3 (score 2) in intermediate grade MEC of salivary gland (immunohistochemical staining, 200 x).

Figure 2 Cytoplasmic expression of IMP3 (score 3) in high grade MEC of salivary gland with perineural invasion (arrow)(immunohistochemical staining, 200 x).
Based on Table 1, IMP3 expression is significantly associated with the old age group, where 72.7% of cases older than 60 years were positive for IMP3 expression versus 41.6% of patients younger than 60 years (P=0.01). Primary tumor site (the highest expression in submandibular gland), tumor size more than 4 cm, high-grade tumors, lymph node metastasis, involvement of surgical margin, perineural invasion, distant metastasis, advanced TNM stage, tumor recurrence, and death of patient were significantly associated with IMP3 positive expression (P<0.05).
All studied cases of pleomorphic adenoma and normal salivary gland tissues were negative for IMP3 expression witha significant difference when compared to MEC cases (P=0.000).
Follow-up and survival analysis of patients with salivary gland MEC
In this study, 36 (51.4%) out of 70 cases of salivary gland MEC developed recurrence. The mean time of recurrence was 47.65±15.8 months, which ranged from 10 to 74 months. Patients with IMP3 overexpression had significantly lower DFS rates than those with absent IMP3 expression (P=0.000). The Kaplan-Meier plots are shown in Figure 3.
We also found that 29 (41.4%) cases died during their follow-up. Mean time to death was 51.9±11.5 months, which ranged from 20 to 74 months. Patients with IMP3 overexpression had significantly lower OS rates than those with absent expression (P=0.001). The Kaplan-Meier plots are shown in Figure 4.

Figure 3 DFS rates in months according to IMP3 expression status in salivary gland MEC.
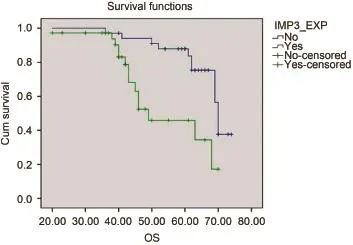
Figure 4 OS in months according to IMP3 expression status in salivary gland MEC.
The duration of OS and DFS in patients with MEC and the factors that affected them are summarized in Table 2. The means of OS in IMP3 positive and negative cases were 40.8 ±12.6 and 64.1±22.7 months, respectively, with a statistically significant difference (P<0.001). We also found that the means of DFS in IMP3 negative and positive cases were 59.14±9.5 and 36.8±12.7 months, respectively. The difference between the cases was statistically significant (P<0.001). However, a significant difference was not observed in the duration of DFS or OS between cases with focal IMP3 and those with diffused IMP3 expression at P=0.9, as shown in Table 2.
The univariate and multivariate Cox proportional hazards model analysis of various prognostic factors in salivary gland MEC in relation to DFS are shown in Table 3. IMP3 expression (P=0.004, odds ratio: 8.2 and 95% CI: 1.96 to 34.6), tumors of the submandibular gland (P=0.03, odds ratio: 2.75 and 95% CI: 1.07 to 7.05), and lymph node metastasis (P=0.003, odds ratio: 3.9 and 95% CI: 1.6 to 9.9)remained as independent prognostic factors.
The factors that predicted OS using the univariate and multivariate Cox proportional hazards model analysis are shown in Table 4. IMP3 expression (P=0.001, odds ratio: 7.17, and 95% CI: 2.3 to 27.08), distant metastasis (P=0.01,odds ratio: 6.7 and 95% CI: 1.37 to 35.3), tumors with intermediate grade (P=0.02, odds ratio: 5.6 and 95% CI: 1.3 to 24.46) and tumors with high grade (P=0.0.04, odds ratios: 1.26 and 95% CI: 1.002 to 1.59) remained as independent prognostic factors.
Discussion
Many studies investigated the clinicopathological factors that affect the prognosis of salivary gland MEC2,17,19. Nguyen et al.20stated that some low-grade MEC behaves aggressively. Thus, they evaluated the use of HER2/neu and Ki-67 as prognostic biomarkers of salivary gland MEC.
Other researchers studied the expression of MUC1 and its effect on MEC outcome21. However, no studies evaluated the expression of IMP3 in this type of cancer even if it was examined in several types of cancers such as pulmonary carcinoma, carcinoma of the pancreas, renal cell carcinoma,hepatocellular carcinoma, gastric adenocarcinoma,endometrial serous carcinoma, and urothelial carcinoma5,6,8,9,11,12,22-24.

Table 2 Association of clinicopathological factors and IMP3 expression with the duration of survival in patients with MEC

Table 3 Cox proportional hazard models showing the predictors of DFS of salivary gland MEC
In the study of Li et al.10, no significant association was found between IMP3 expression in the squamous cell carcinoma of the oral cavity (SCCOC) and the patient's age, primary tumor site, or distant metastasis. However, they found that the expression of IMP3 in SCCOC was significantly associated with the high histological grade,lymph node metastasis, and advanced tumor stages, which is consistent with our results. These results indicate theimportance of IMP3 for adhesion, migration, cell growth,and differentiation. We also found a significant association between IMP3 and the old age category, tumors of the submandibular gland, large tumor size, and involvement of the surgical margin, perineural invasion, recurrence of the tumor, patient's death, and distant metastasis. The latter two findings are in accordance with research results of Hoffmann et al. who stated that the expression of the IMP3 protein in the clear cell carcinoma of the kidney was a significant predictor of metastasis and patient's death22.
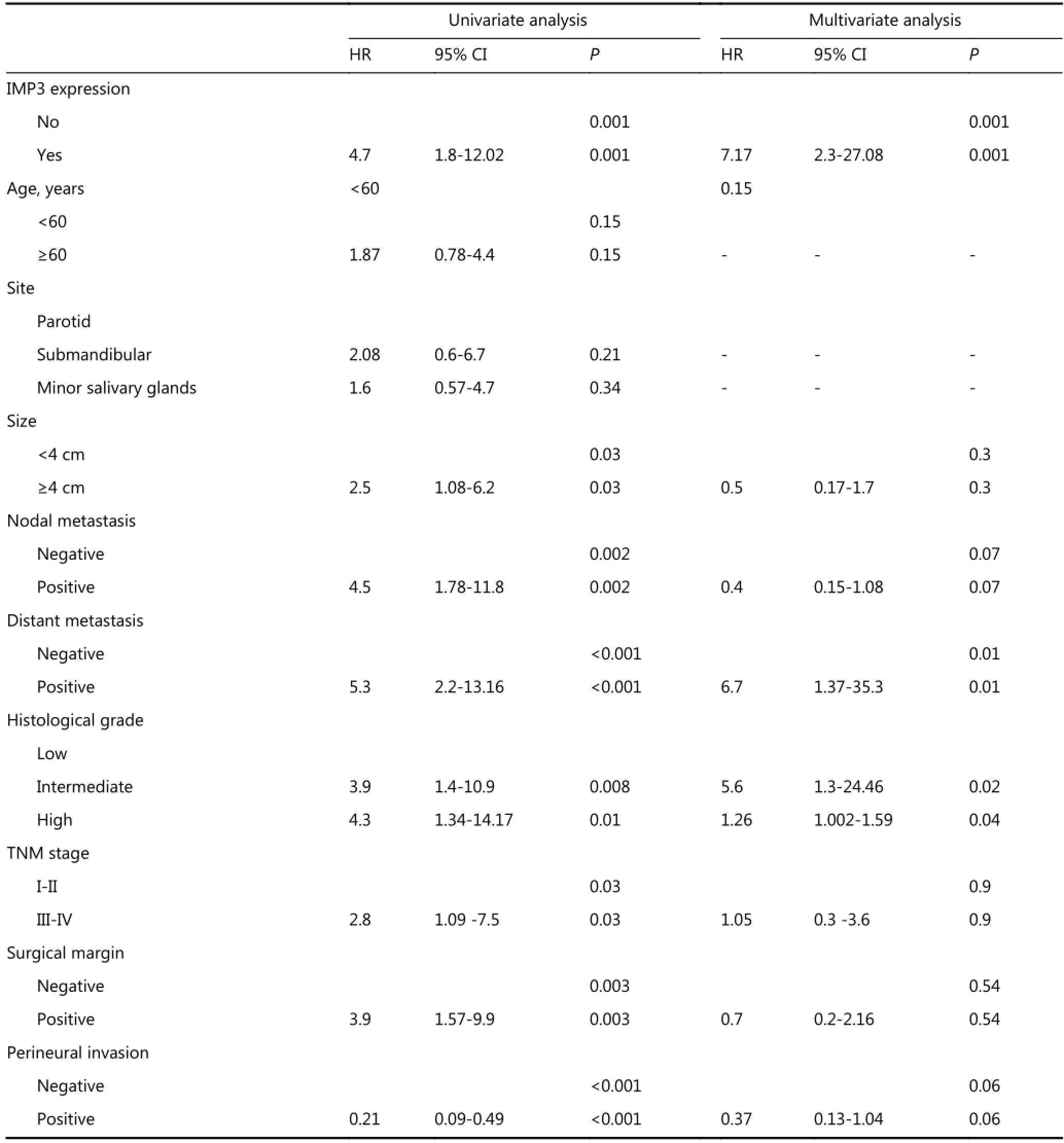
Table 4 Cox proportional hazard models showing the predictors of OS of salivary gland MEC
In the present study, IMP3 expression was found in MEC cells, but it was absent in normal salivary gland tissue and PAwith a significant difference. Thus, this molecular marker may be a useful tool for differentiating between benign and malignant lesions in difficult cases. This finding is similar to preoperative biopsies or fine needle aspiration cytology where MEC may be misinterpreted as benign tumors such as PA and is excised without safety surgical margins, which increases recurrence and poor outcome19.
Schaeffer et al.12found IMP3 expression in ductal adenocarcinoma of the pancreas, but not in normal pancreatic tissue; this finding simplifies the interpretation of staining. Schaeffer et al. explained their finding based on the expression of IMP3 in the embryonic tissue as mentioned by Mueller et al.4; however, this expression was not found in adult tissue, which suggests that IMP3 is epigenetically silenced in adult tissues. Thus, they hypothesized that the cause of IMP3 gene re-expression in pancreatic ductal adenocarcinoma could be the result of promoter hypomethylation and not due to gene amplification12. The same finding may be true for salivary MEC. Thus, further studies on hypomethylation hypothesis of the IMP3 gene are suggested. The findings can be used as a target for remethylating enzymes.
Ozawa et al.19found that the five-year OS rate of MEC accounted for 62.3%, whereas the five-year recurrence free survival (RFS) rate accounted for 57.2%. Our results were worse than the previous results, which accounted for 42.8% and 35.7% of OS and DFS, respectively. This finding can be attributed to the difference in treatment modalities in different countries, high percentage of patients with stage III and IV tumors, and ignorance of patients of regular followup in our locality. Thus, relevant survival data are insufficient in our study.
The present study shows that IMP3 positivity, tumors of the submandibular gland, and lymph node metastasis are independent poor prognostic factors of DFS in MEC. Ghosh-Laskar et al.25found that lymph node metastasis predicted poor locoregional control and DFS, which is consistent with our results. They also found that the high grade of tumor affects DFS, which does not agree with our findings. This result can be explained by the fact that some patients with low-grade tumors exhibit advanced tumor stage, lymph node, and distant metastasis19.
3.1 术后第48h症状视觉模拟量表 (visual analogue scale,VAS)评分:用 VAS 评分,使用 0~10 表示对患者生活质量的影响程度,0为不影响生活质量,10为最大极限影响到了生活质量,术后48h予患者行VAS评分,分别根据鼻塞、鼻部疼痛、烦躁不安、溢泪、鼻痒、打喷嚏、头面部疼痛及压迫感、头疼、咳嗽、吞咽困难、睡眠困难、耳闷胀、耳痛、夜间睡眠质量、晨起注意力不集中、沮丧、焦虑、易怒、忧虑等19项进行症状问卷调查,统计分别出现的例数。
Guzzo et al.17and Ozawa et al.19reported that the primary site does not have an impact on the RFS of MEC. Other studies reported that tumors of the submandibular gland had poor prognosis compared with those of other sites, which is consistent with our findings26. Given the contradictory findings in different studies, future studies on a large number of cases should investigate the impact of the primary site on MEC outcome.
Regarding the OS in our study, we found that increased IMP3, distant metastasis, and tumors with intermediate and high grades were independent prognostic factors. Ozawa et al.19found that the grade of MEC does not affect OS, but they found that the age of >56 years was an independent prognostic factor, which disagrees with our findings.
Surgical margin has no significant effect on either OS or DFS based on the multivariate analysis in our study. However, a significant effect was observed on univariate analysis, which is consistent with Ghosh-Laskar et al.'s research results25that positive cut margins show poor outcomes. This finding must be taken into consideration because cases of positive margin need additional treatment,such as postoperative radiotherapy, to decrease local recurrence27.
Schaeffer et al.12reported that, based on multivariate analysis, OS in patients with pancreatic ductal adenocarcinoma was significantly affected by the overexpression of IMP3; shorter survival was found in patients with IMP3 expressing tumors than that with IMP3 negative tumors, which is consistent with our findings. A similar finding was also noticed in renal cell carcinoma and SCCOC8,10. Thus, this biomarker could be a therapeutic target for such cancers as indicated by Wang et al.5in lung carcinoma.
Conclusions
Our study is the first to investigate IMP3 expression in salivary gland MEC. IMP3 was related to tumors of the submandibular gland, large tumor size, tumor differentiation, perineural invasion, advanced TNM stage,positive surgical margins, lymph node, and distant metastasis. The expression of IMP3 was also an independent predictor for the outcome of MEC in terms of DFS and OS. We suggest conducting future studies on a wide scales and over a long duration of follow-up to enable IMP3 to become a target of MEC therapy. The absence of IMP3 in PA and its presence in 51.4% of MEC indicates that this biomarker should be studied prospectively on preoperative biopsies with diagnostic difficulties. The results should be compared with excisional biopsies to determine their diagnostic validity.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Vargas PA, Gerhard R, Araújo Filho VJ, De Castro IV. Salivary gland tumors in a Brazilian population: a retrospective study of 124 cases. Rev Hosp Clin Fac Med Sao Paulo. 2002; 57: 271-6.
2.Mchugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS,Kies MS, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012; 118: 3928-36.
3.Li W, Liu D, Chang W, Lu X, Wang YL, Wang H, et al. Role of IGF2BP3 in trophoblast cell invasion and migration. Cell Death Dis. 2014; 5: e1025.
4.Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C,et al. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999; 88: 95-9.
5.Wang T, Fan L, Watanabe Y, Mcneill PD, Moulton GG, Bangur C,et al. L523S, an RNA-binding protein as a potential therapeutic target for lung cancer. Br J Cancer. 2003; 88: 887-94.
6.Yantiss RK, Woda BA, Fanger GR, Kalos M, Whalen GF, Tada H, et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005; 29: 188-95.
7.Monk D, Bentley L, Beechey C, Hitchins M, Peters J, Preece MA, et al. Characterisation of the growth regulating gene IMP3, a candidate for Silver-Russell syndrome. J Med Genet. 2002; 39: 575-81.
8.Jiang Z, Chu PG, Woda BA, Rock KL, Liu Q, Hsieh CC, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006; 7: 556-64.
9.Zheng W, Yi X, Fadare O, Liang SX, Martel M, Schwartz PE, et al. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol. 2008; 32: 304-15.
10.Li S, Cha J, Kim J, Kim KY, Kim HJ, Nam W, et al. Insulin-like growth factor mRNA-binding protein 3: a novel prognostic biomarker for oral squamous cell carcinoma. Head Neck. 2011; 33: 368-74.
11.Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL, Wang TH, et al. RNA-binding protein insulin-like growth factor mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008; 48: 1118-27.
12.Schaeffer DF, Owen DR, Lim HJ,Buczkowski AK, Chung SW,Scudamore CH, et al. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer 2010;10:59.
13.Eveson JW, Kusafuka K, Stenman G. Pleomorphic adenoma. In Barnes L, Eveson JW, Reichart P. World Health Organization classification of tumours. Pathology and genetics of head and neck tumors. Lyon: IARC Press. 2005; 254-8.
14.Li S, Baloch ZW, Tomaszewski JE, Livolsi VA. Worrisome histologic alterations following fine-needle aspiration of benign parotid lesions. Arch Pathol Lab Med. 2000; 124: 87-91.
15.Goode RK, El-Naggar AK. Mucoepidermoid carcinoma In Barnes L, Eveson JW, Reichart P. World Health Organization classification of tumours. Pathology and genetics of head and neck tumors. Lyon: IARC Press. 2005; 219-20.
16.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathological analysis of 234 cases with evaluation of grading criteria. Cancer. 1998; 82:1217-24.
17.Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002; 9: 688-95.
18.Sobin LH, Gospodarowicz M, Wittekind C. UICC TNM Classification of malignant tumors, 7th edition. Hoboken, New Jersy: John Wiley & Sons, Ltd. 2009; 54-5.
19.Ozawa H, Tomita T, Sakamoto K, Tagawa T, Fujii R, Kanzaki S, et al. Mucoepidermoid carcinoma of the head and neck: clinical analysis of 43 patients. Jpn J Clin Oncol. 2008; 38: 414-8.
20.Nguyen LH, Black MJ, Hier M, Chauvin P, Rochon L. HER2/neu and Ki-67 as prognostic indicators in mucoepidermoid carcinoma of salivary glands. J Otolaryngol. 2003; 32: 328-31.
21.Liu S, Ruan M, Li S, Wang L, Yang W. Increased expression of MUC1 predicts poor survival in salivary gland mucoepidermoid carcinoma. J Craniomaxillofac Surg. 2014; 42: 1891-6.
22.Hoffmann NE, Sheinin Y, Lohse CM, Parker AS, Leibovich BC,Jiang Z, et al. External validation of IMP3 expression as an Independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112: 1471-9.
23.Jeng YM, Wang TH, Lu SH, Yuan RH, Hsu HC. Prognostic significance of insulin-like growth factor mRNA-binding protein 3 expression in gastric adenocarcinoma. Br J Surg. 2009; 96: 66-73.
24.Sitnikova L, Mendese G, Liu Q, Woda BA, Lu D, Dresser K, et al. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res. 2008; 14: 1701-6.
25.Ghosh-Laskar S, Murthy V, Wadasadawala T, Agarwal J, Budrukkar A, Patil N, et al. Mucoepidermoid carcinoma of the parotid gland: factors affecting outcome. Head Neck. 2011; 33: 497-503.
26.Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol. 1984; 81: 696-701.
27.Rapidis AD, Givalos N, Gakiopoulou H,Stavrianos SD, Faratzis G,Lagogiannis GA, et al. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol 2007; 43:130-6.
Cite this article as: Elshafey MR, Ahmed RA, Mourad MI, Gaballah ET. The oncofetal protein IMP3 is an indicator of early recurrence and poor outcome in mucoepidermoid carcinoma of salivary glands. Cancer Biol Med. 2016; 13: 286-295. doi: 10.20892/j.issn.2095-3941.2016.0037
Correspondence to: Rehab A. Ahmed
E-mail: rehaballah1975@gmail.com
Received April 7, 2016; accepted April 25, 2016. Available at www.cancerbiomed.org
Copyright © 2016 by Cancer Biology & Medicine
猜你喜欢
杂志排行
Cancer Biology & Medicine的其它文章
- Modulation of B-cell receptor and microenvironment signaling by a guanine exchange factor in B-cell malignancies
- Influence of obesity and bariatric surgery on gastric cancer
- Characterization of FGFR signaling pathway as therapeutic targets for sarcoma patients
- Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang
- Genetic and molecular changes in ovarian cancer
- New generation of breast cancer clinical trials implementing molecular profiling
