Characterization of FGFR signaling pathway as therapeutic targets for sarcoma patients
2016-08-10WenYaZhouHongZhengXiaoLingDuJiLongYang
Wen-Ya Zhou, Hong Zheng, Xiao-Ling Du, Ji-Long Yang
Characterization of FGFR signaling pathway as therapeutic targets for sarcoma patients
Wen-Ya Zhou1, Hong Zheng2, Xiao-Ling Du3, Ji-Long Yang1
1Department of Bone and Soft Tissue Tumor;2Department of Epidemiology and Biostatistics, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Tianjin 300060, China;3Department of Diagnostics,Tianjin Medical University, Tianjin 300061, China
ABSTRACTThe fibroblast growth factor receptor (FGFR) family plays important roles in regulating cell growth, proliferation, survival,differentiation and angiogenesis. Deregulation of the FGF/FGFR signaling pathway has been associated with multiple development syndromes and cancers, and thus therapeutic strategies targeting FGFs and FGFR in human cancer are currently being explored. However, few studies on the FGF/FGFR pathway have been conducted in sarcoma, which has a poor outcome with traditional treatments such as surgery, chemotherapy, and radiotherapy. Hence, in the present review, we provide an overview of the role of the FGF/FGFR pathway signal in sarcoma and FGFR inhibitors, which might be new targets for the treatment of sarcomas according to recent research.
KEYWORDSSarcoma; FGFR signaling pathway; FGFR inhibitors
Introduction
Sarcomas are tumors of putative mesenchymal origin, which can arise anywhere in the body. They are rare tumors,comprising less than 1% of adult cancers and nearly 21% of children cancers1. The vast majority of diagnosed sarcomas are soft tissue sarcomas, while malignant bone tumors make up just over 10% of sarcomas2. The rarity of the disease combined with the diverse number of subtypes make sarcomas very difficult to study and treat. Owing to the poor outcome of traditional treatments such as surgery,chemotherapy and radiotherapy, new treatments such as target therapy have been investigated and have shown great success.
The fibroblast growth factor receptor (FGFR) signaling pathway not only plays a ubiquitous role in a variety of biological processes, including cell growth, survival,differentiation and angiogenesis, but has also been implicated in tumor development3,4. Identification of the roles and relationships within the fibroblast growth factor (FGF)/FGFR family and their links to tumor growth and progression will be critical in designing new drug therapies to target FGFR pathways3. This review highlights the growing knowledge of FGFs and FGFR in tumor cell growth and survival, and provides an overview of FGF intracellular signaling pathways,the role of FGFR signaling pathway in sarcomas, and current research on FGFR inhibitors in sarcoma.
FGFs
FGFs are expressed in almost all tissues and play important roles in development, wound healing and neoplastic transformation by promoting mitosis of both epithelial and mesenchymal cell types5. To date, 23 different FGFs have been identified in human, among which 18 (FGF1-10 and 16-23) serve as mitogenic signaling molecules that bind to four high-affinity FGFRs (FGFR1-4). FGFs also bind the heparan sulfate glycosaminoglycans (HSGAGs), which facilitates dimerization (activation) of FGFRs and protect the ligands from degradation6. Thus, FGF signaling is activated through FGF binding to FGFRs in an HSGAG-dependent manner.
The FGFs are highly dependent on specific FGFR signaling to promote tumor progression. Deregulation of FGFs, such as FGF1, -2, -3, -4, -5, -10, -17, -18, and -19, have been verified in a variety of human tumors5. In particular, FGF2 is expressed in many malignant tumors including breast cancer,pancreatic cancer, non-small cell lung cancer (NSCLC),bladder cancer, head and neck cancer, prostate cancer,hepatocellular carcinoma (HCC), melanomas and astrocytomas7-15. FGF1 is also related with tumorigenesis and epithelial-to-mesenchymal transition, as well as invasion and metastasis16,17. In contrast, the expression of other FGFs such as FGF6-9, FGF11 and FGF12-16 in malignant tumors has been less extensively investigated. Recent evidence has shown that FGF7 and FGF10 are involved in the proliferation of ameloblastoma cells through the MAPK pathway18, while FGF8 might contribute to the proliferative and metastatic capacity of colorectal cancer (CRC) cells through Yesassociated protein 1 (YAP1)19. Sun et al.20found that FGF9 secreted by cancer-associated fibroblasts (CAFs) might play an important role in promoting the anti-apoptosis and invasive capability of gastric cancer cells. In cisplatin-resistant HeLa cisR cells, FGF13 plays a pivotal role in regulating resistance to platinum drugs, possibly through a mechanism shared by platinum and copper21. FGF15 may contribute to HCC development in the context of chronic liver injury and fibrosis22. FGF16 might contribute to the cancer phenotype of ovarian cells and might be a therapeutic strategy for treating invasive ovarian cancers23. FGF22 might play a potential pro-oncogenic role in the skin24. However, available data were mainly from preclinical work on cell models, and further clinical confirmation is required.
Previous studies also showed that FGF1 (acidic FGF) and FGF2 (basic FGF) and their receptors can promote autocrine and paracrine growth control of cancers5,25,25. These autocrine and paracrine loops involving FGFRs and FGFs have been found in NSCLC, HCC, breast and prostate cancer, and CRC4,26. Once these loops have become disrupted, resulting from increased release of FGFs in cancer or stromal cells, they can promote the proliferation, survival, and angiogenesis abilities of cancer cells. FGFs, and especially FGF2, are among the earliest identified pro-angiogenic factors in tumors, and they have a direct effect on tumor angiogenesis27. A recent study showed that FGF2 and its receptor FGFR3 as well as apurinic/apyrimidinic endonuclease 1 (APE1) play an important role in angiogenesis in human osteosarcoma cells28. Another report demonstrated that in vitro and in vivo, miR-503 can reduce tumor angiogenesis by downregulating FGF2 and vascular endothelial growth factor-A (VEGFA)29.

Table 1 Ligand specificities of the FGFR family
FGFRs
The FGFRs are receptors that bind to members of the FGF family of proteins. The receptors consist of an extracellular ligand domain with three immunoglobulin-like domains (IIII), a transmembrane domain, and an intracellular tyrosine kinase domain that transmits the signal to the interior of the cell. An acid box, located between the domains Ig-I and Ig-II,plays a role, together with the Ig-I-like domain, in receptor auto-inhibition. Ig-II and Ig-III compose the ligand-binding site30. There are only four FGFRs (FGFR1, FGFR2, FGFR3 and FGFR4) in the cell surface with seven isoforms FGFR-(1b, 1c, 2b, 2c, 3b, 3c and 4) owing to alternative splicing in the Ig-III-like domain, with different ligand-binding specificities6,31(Table 1). Each receptor can be activated by several FGFs, and the FGFs can also activate more than one receptor in many cases. For example, FGF1 can bind all seven principal FGFRs44, while FGF7 can only activate FGFR2b45.
The gene encoding FGFR5 was recently identified. The FGFR5 protein lacks a cytoplasmic tyrosine kinase domain and one isoform compared with FGFR1-4, and FGFR5γ only includes the D1 and D2 extracellular domains46. FGFR5 has been assumed to be a decoy receptor to inhibit ligand-induced responses of other FGFRs. However, researchers demonstrated that FGFR5 does not act as a decoy receptor but directly affects ligand-independent and -dependent mechanisms of extracellular signal-regulated kinase 1/2 (ERK1/2) activity by interacting with known substrate proteins35,46,47.
Endothelial cells express FGFR1 more often than FGFR2,while the expression of FGFR3 or FGFR4 has been less frequently reported3. Immunohistochemical results showed a statistically significant increase of FGFR2 in epithelial dysplasias and squamous cell carcinomas compared with normal oral epithelium, and FGFR3 expression was statistically significantly increased in dysplastic and carcinomatous tissues compared with normal oral epithelium. While staining for FGF1 is decreased or lost in the development of epithelial dysplasia and carcinoma, FGF2 also showed increased intensity in squamous cell carcinomas48. FGFR3 is downregulated in CRC cells, while FGFR1 is overexpressed in CRC cells. Interestingly, by introducing of FGFR1 siRNA to disrupt the expression of FGFR1 in cells, the expression of FGFR3 was effectively increased as well as the tumor suppressive activities. These results showed that the reciprocal relationship between FGFR1 and FGFR3 in colorectal tissue may act as an important role in the progression of tumor formation49.
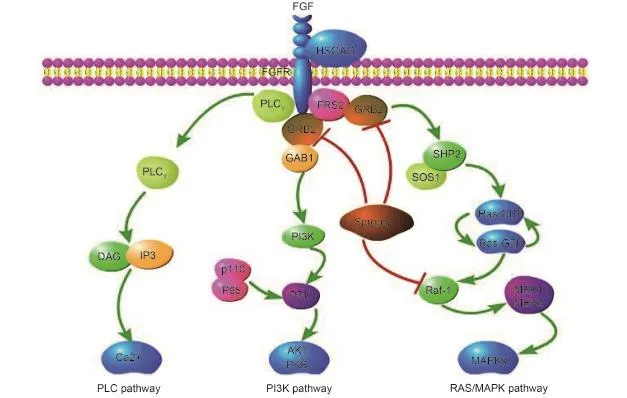
Figure 1 FGF/FGFR signaling pathways.
FGF/FGFR signaling pathway
The binding of FGFs to FGFRs (aided by HSPGs) induces receptor dimerization, which triggers the activation of the FGFRs. The activation of FGFRs brings the intracellular kinases into close proximity, enabling them to transphosphorylate each other. This sets in motion a cascade of downstream signals, finally affecting mitogenesis and differentiation50,51(Figure 1). Several intracellular proteins have been implicated in promoting FGF-mediated signaling,such as phospholipase Cγ (PLCγ), fibroblast growth factor receptor substrate 2 (FRS2), src homology 2 domaincontaining transforming protein B (Shb), Src kinase,ribosomal S6 protein kinase (RSK), signal transducers and activators of transcription (STATs), and CT10 regulator of kinase (Crk)6,52,40. Specifically, the adaptor growth-factorreceptor-bound protein 2 (GRB2) triggers the Ras/MAPK pathway and PI3K/Akt intracellular signaling cascades by binding to phosphorylated FRS252. Rab5 small GTPase, a binding partner of activated FGFRs, is involved in maintaining the RAS-MAPK signaling but not PI3K-AKT signaling53.
The FGFR signaling pathway plays important biological roles in multiple processes include pro-survival signals as well as anti-apoptotic signals and stimulation of cell proliferation
and cell migration6. There are two kinds of regulators that control the signaling output from activated FGFRs: one includes the negative regulators, such as Sprouty proteins,mitogen-activated protein kinase phosphatase 3 (MKP3) and the similar expression to the FGF (SEF)54-57; and the other includes the positive regulators, such as fibronectin-leucinerich transmembrane protein (FLRT1), FLRT2 and FLRT358,59. Sprouty proteins can decouple the downstream signaling of FGF by binding to GRB254. Sprouty related enabled/vasodilator-stimulated phosphoprotein homology1 domain-containing protein (SPRED2), a Sprouty-related protein, was also shown to attenuate FGF signaling by redirecting FGFRs to lysosomes by interacting with the late endosomal protein neighbor of breast cancer early-onset 1 (NBR1)57.
The role and clinical significance of FGFR signaling pathway in sarcomas
Osteosarcoma
Osteosarcoma (OS), the most common primary bone tumor,is a genetically complex disease with a high incidence of pulmonary metastasis and poor prognosis, which mainly affects children and adolescents60. The amplification of several FGFR genes has been observed in OS, such as FGFR1,FGFR2 and FGFR361. However, a study on the expression of cell surface receptors revealed low expression of FGFR-2 and FGFR-3 across ten OS cell lines (OS229, OS232, OS231,OS238, OS242, OS252, OS290, OS293, OS308, and OS311)62. In addition, amplification of FGFR-1 is disproportionately observed in the rare histological variants of OS. The amplification of FGFR-1 was observed in 18.5% of patients who showed a poor response to chemotherapy, and no FGFR-1 gene amplification was detected in the patients who responded well to therapy63. High expression levels of APE1,FGF2 and FGFR3 have been found in OS and are significantly related with poor prognosis. High expression of FGF2 and its receptor 3 FGFR3 was an adverse prognostic factor. We found that APE1 can promote angiogenesis by upregulating FGF2 and FGFR3 in vitro and in vivo64. Thus,the use of FGFR kinase inhibitors may be a promising treatment for patients with OS.
Chondrosarcoma
Chondrosarcoma, the second most common primary malignancy of the bone, is difficult to treat. Wide excision is the only treatment owing to its resistance to both
chemotherapy and radiation65. FGFs and FGFRs are thought to be negative regulators of chondrocytic growth, such as achondroplasia and related chondrodysplasias, which are caused by constitutively active mutations in FGFR366. Sahni et al.67reported that FGF inhibited chondrocytic growth through activating the STAT1 pathway, in which p21 might also play a role in inhibiting chondrocytic proliferation67. In rat chondrosarcoma cells, FGF2 arrested the cell cycle at G1phase and inhibited proliferation, which seemed to be mediated at least partially through p21 induction, the inactivation of cyclin E-Cdk2 and activation of pRb68. In addition, immunohistochemistry revealed high expression of FGFR3 and aberrant cellular localization of heparan sulfate proteoglycansin in 42 dedifferentiated, 23 clear cell, and 23 mesenchymal chondrosarcoma tissues of human69. Taken together, FGF and FGFR may be a potential therapeutic target in chondrosarcoma.
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a malignancy with features of skeletal muscle and the most frequent soft tissue sarcoma in children. There are two main kinds of variants of RMS: embryonal rhabdomyosarcoma (eRMS) and alveolar rhabdomyosarcoma (aRMS)70. Recently, several FGFR4 tyrosine kinase domain mutations were found in 7.5% primary human RMS tumors, and the mutants K535 and E550 showed autophosphorylation of the receptor. In RMS tumors, overexpression of FGFR4 was associated with advanced stage cancer and poor survival, while FGFR4 knockdown in a human RMS cell line reduced tumor growth71. FGFR4 protein is expressed in the two main variants of RMS (eRMS and aRMS), but protein expression is higher in aRMS. FGFR4 loss-of-function reduced cell proliferation in both subtypes in vitro. Interestingly, FGFR4 was necessary and sufficient for expression of the antiapoptotic protein B-cell CLL/lymphoma 2-like 1 (BCL2L1)in aRMS; this was not observed in eRMS, indicating that FGFR4 may play dichotomous roles in RMS subtypes72. The fusion gene FGFR1-FOXO1 has also been verified in RMS35. Inhibiting FGFR signaling might represent an important strategy to enhance the efficacy of current RMS treatments. FGFR4 signaling rescues only subgroups of alveolar RMS cells from apoptosis induced by compounds targeting the IGF1R-PI3K-mTOR pathway, and FGFR4-activated signaling is involved in the different behaviors of the phenotypes73. Together, these data suggest that FGFR4 may act as an oncogene in RMS, and these findings support the potential therapeutic targeting of FGFR4 in RMS.
Liposarcomas
The fibroblast growth factor receptor substrate 2 (FRS2) is an adaptor protein that plays a critical role in FGFR signaling. Several studies showed the amplification of FRS2 gene as well as the overexpression of FRS2 protein in liposarcoma, and the FGFR/FRS2 signaling was activated in about 75% of FRS2-positive high-grade liposarcomas. It was also observed in three high-grade liposarcoma cell lines: FU-DDLS-1, LiSa-2, and SW87274,75. Importantly, use of the FGFR selective inhibitor NVP-BGJ-398 can significantly inhibit the growth of two cell lines and suppressed the FGFR signal transduction. These findings indicate that FRS2 may serve as a potential therapeutic target in liposarcomas75. Comparative expression analyses using whole-genome microarrays were conducted in myxoid liposarcomas and fat tissue samples,and the results showed that FGFR2 was overexpressed and were validated by qPCR, immunohistochemistry and Western blot analysis in primary tumor samples. In vitro,inhibition of FGFR showed effects on proliferation and cell migration and induced apoptosis, which indicated that the FGFR might be a therapy target for myxoid liposarcoma76.
Synovial sarcoma
Synovial sarcoma is a soft tissue malignancy that typically arises in young adults. The growth regulatory mechanisms are unknown2. A recent study shows that multiple FGF genes including FGF2, FGF8, FGF9, FGF11 and FGF18 were expressed in synovial sarcoma cell lines, and FGF8 showed growth stimulatory effects in all synovial sarcoma cell lines77. The growth stimulatory effect of FGF were transmitted through ERK1/2, and FGF signals in synovial sarcoma induced the phosphorylation of ERK1/2. While FGFR inhibitors induced significant growth inhibition in vitro and in vivo was only associated with a downregulation of phosphorylated ERK1/2 and an ERK kinase inhibitor showed growth inhibitory effects for synovial sarcoma77. Hence, FGF signals have an important role in the growth of synovial sarcoma, and inhibitory molecules will be of potential use for molecular target therapy in synovial sarcoma.
Other sarcomas
Girnita et al.78showed that the bFGF pathway may be important for the maintenance of a malignant phenotype of Ewing's sarcoma cells through upregulating the EWS/FLI-1 protein. Another study found that bFGF-induced cell death was associated with upregulation of p21 and p53, downregulation of PCNA and cyclin A and a decrease in active pRb1, consistent with accumulation of cells in G1. These data demonstrate that the bFGF pathway may be a therapeutic target in Ewing’s sarcoma79. Overexpression of FGFR3, FGF2 and FGFR4 has been identified in the epidermal regions of dermatofibroma while the expression of FGF1 and FGF9 has not been found in dermatofibroma80. However, more studies are required. FGF2, alone or coexpressed with platelet-derived growth factor-B (PDGF-B)and vascular endothelial growth factor receptor-3 (VEGFR-3), involved in angiogenesis, is a significant independent negative prognosticator in widely tumor resected nongastrointestinal stromal tumor soft-tissue sarcomas81.
FGFR inhibitors and their role in sarcomas
Small-molecule tyrosine kinase inhibitors targeting the ATP-binding site of the intracellular tyrosine kinase domain in a number of different receptor tyrosine kinases (RTKs) have been successfully used for therapy for cancers such as NSCLC and breast cancer82,83. However, most of these inhibitors show broad specificity and target not only FGFRs, but also VEGFRs and/or PDGFRs, as they share structural similarities and have similar kinase domains. Several clinical trials targeting FGFRs have also been conducted in cancer. A phase 1 clinical trial of BAY1187982, an anti-FGFR2 antibody, in subjects with advanced solid tumors known to express FGFR2 has been conducted (http://ClinicalTrials.gov,NCT02368951). BGJ398 will be tested in phase 2 clinical trials in patients with advanced cholangiocarcinoma with FGFR2 gene fusions or other FGFR genetic alterations (http://ClinicalTrials.gov, NCT02150967). Lenvatinib,another example, will be tested in phase 1/2 clinical trials in children and adolescents with refractory or relapsed solid malignancies (http://ClinicalTrials.gov, NCT02432274). Other tyrosine kinase inhibitors such as BIBF1120, TKI258,nintedanib and ponatinib are also in phase 1 and/or 2 clinical trials (http://ClinicalTrials.gov)84-87.
Aberrations in FGFR signaling are involved in the pathophysiology of several malignancies and disorders. FGFR inhibitors such as small-molecule FGFR inhibitors could be of potential use for targeted therapy in sarcomas. A recent study showed that xenogenic mesenchymal stem cells (MSCs) can selectively migrate to the tumor site and may be under the guidance of FGF2/FGFR pathways, while no MSCs were found in other organs in a fibrosarcoma-bearing C3H/HeN mice model. In addition, nitric oxide synthase (iNOS) protein that was delivered by genetically modifiediNOS-MSC showed a significant anti-tumor effect both in vitro and in vivo88. Regorafenib (BAY 73-4506, Stivarga®) is an oral diphenylurea multi-kinase inhibitor that targets VEGFR1-3, TIE2, PDGFR-beta, and FGFR as well as KIT,RET, and RAF. Currently, a multinational, randomized,placebo controlled phase 2 trial of regorafenib in metastatic soft tissue sarcoma (REGOSARC, NCT01900743) has been initiated. The study recruited patients with metastatic soft tissue sarcoma (STS) that received at least doxorubicin (or another anthracyclin) as a previous treatment. No results are currently available89. FGFR4 is a tractable therapeutic target for patients with RMS. In a recent study, ponatinib (AP24534) was determined as the most potent FGFR4 inhibitor because it was shown to inhibit the growth of RMS cells expressing wild-type or mutated FGFR4 by increasing apoptosis. Similar results were observed in a RMS mouse model expressing mutated FGFR4. Therefore, it suggests that ponatinib might be a potentially effective therapeutic agent for RMS tumors90.
Conclusions
The FGF/FGFR family has been linked to mechanisms underlying tumor progression. Several intracellular proteins such as PLCγ, FRS2, Shb, Src kinase, RSK, STATs and Crk, as well as several regulators including Sprouty proteins,SPRED2, MKP3, SEF and FLRT1-3, play important roles in FGFR signaling pathways. The genetic alterations of FGF/FGFR are involved in sarcomas such as OS,chondrosarcoma, RMS, liposarcomas, and synovial sarcoma. In addition, several drugs have been researched in sarcoma such as rogorafenib and ponatinib. Recent FGF/FGFR signaling pathway research suggests that the FGFR inhibitor combination with surgery, radiation and chemotherapy might enhance the therapy responses for sarcoma patients.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant No. 81372872 and 81402215),funds from the University Cancer Foundation via the Sister Institution Network Fund (SINF) at the Tianjin Medical University Cancer Institute & Hospital (TMUCIH), Fudan University Shanghai Cancer Center (FUSCC), and University of Texas MD Anderson Cancer Center (UT MDACC).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012; 2: 14.
2.Christopher D.M. Fletcher JAB, Pancras C.W. Hogendoorn,Frodrik Mertens. Who classification of tumours of soft tissue and bone. Lyon: IARC. 2013.
3.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009; 9: 639-51.
4.Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011; 437: 199-213.
5.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004; 11: 709-24.
6.Beenken A, Mohammadi M. The FGF family: Biology,pathophysiology and therapy. Nat Rev Drug Discov. 2009; 8: 235-53.
7.Lindner V, Majack RA, Reidy MA. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990; 85: 2004-8.
8.Halaban R. Growth factors and melanomas. Semin Oncol. 1996; 23: 673-81.
9.Bian XW, Du LL, Shi JQ, Cheng YS, Liu FX. Correlation of BFGF,FGFR-1 and VEGF expression with vascularity and malignancy of human astrocytomas. Anal Quant Cytol Histol. 2000; 22: 267-74.
10.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor,placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997; 57: 963-9.
11.Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, et al. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993; 53: 5289-96.
12.Berger W, Setinek U, Mohr T, Kindas-Mugge I, Vetterlein M,Dekan G, et al. Evidence for a role of FGF-2 and FGF receptors in the proliferation of non-small cell lung cancer cells. Int J Cancer. 1999; 83: 415-23.
13.Gazzaniga P, Gandini O, Gradilone A, Silvestri I, Giuliani L,Magnanti M, et al. Detection of basic fibroblast growth factor MRNA in urinary bladder cancer: Correlation with local relapses. Int J Oncol. 1999; 14: 1123-7.
14.Dellacono FR, Spiro J, Eisma R, Kreutzer D. Expression of basic fibroblast growth factor and its receptors by head and neck squamous carcinoma tumor and vascular endothelial cells. Am J Surg. 1997; 174: 540-4.
15.Huang X, Yu C, Jin C, Yang C, Xie R, Cao D, et al. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol Carcinog. 2006; 45: 934-42.
16.Ramos C, Becerril C, Montano M, Garcia-De-Alba C, Ramirez R,Checa M, et al. FGF-1 reverts epithelial-mesenchymal transitioninduced by TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol. 2010; 299: L222-31.
17.Jouanneau J, Plouet J, Moens G, Thiery JP. FGF-2 and FGF-1 expressed in rat bladder carcinoma cells have similar angiogenic potential but different tumorigenic properties in vivo. Oncogene. 1997; 14: 671-6.
18.Nakao Y, Mitsuyasu T, Kawano S, Nakamura N, Kanda S,Nakamura S. Fibroblast growth factors 7 and 10 are involved in ameloblastoma proliferation via the mitogen-activated protein kinase pathway. Int J Oncol. 2013; 43: 1377-84.
19.Liu R, Huang S, Lei Y, Zhang T, Wang K, Liu B, et al. FGF8 promotes colorectal cancer growth and metastasis by activating YAP1. Oncotarget. 2015; 6: 935-52.
20.Sun C, Fukui H, Hara K, Zhang X, Kitayama Y, Eda H, et al. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Cancer. 2015; 15: 333.
21.Okada T, Murata K, Hirose R, Matsuda C, Komatsu T, Ikekita M,et al. Upregulated expression of FGF13/FHF2 mediates resistance to platinum drugs in cervical cancer cells. Sci Rep. 2013; 3: 2899.
22.Uriarte I, Latasa MU, Carotti S, Fernandez-Barrena MG, Garcia-Irigoyen O, Elizalde M, et al. Ileal FGF15 contributes to fibrosisassociated hepatocellular carcinoma development. Int J Cancer. 2015; 136: 2469-75.
23.Basu M, Mukhopadhyay S, Chatterjee U, Roy SS. FGF16 promotes invasive behavior of skov-3 ovarian cancer cells through activation of mitogen-activated protein kinase (MAPK) signaling pathway. J Biol Chem. 2014; 289: 1415-28.
24.Jarosz M, Robbez-Masson L, Chioni AM, Cross B, Rosewell I,Grose R. Fibroblast growth factor 22 is not essential for skin development and repair but plays a role in tumorigenesis. PLoS One. 2012; 7: e39436.
25.Takahashi JA, Fukumoto M, Igarashi K, Oda Y, Kikuchi H,Hatanaka M. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J Neurosurg. 1992; 76: 792-8.
26.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009; 8: 580-8.
27.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002; 8: 483-9.
28.Xu Q, Gao S, Liu J. [the expression of caspase-3, BFGF and MVD in laryngeal squamous cell carcinoma and the relationship among them]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27: 1084-7.
29.Zhou B, Ma R, Si W, Li S, Xu Y, Tu X, et al. Microrna-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013; 333: 159-69.
30.Olsen SK, Ibrahimi OA, Raucci A, Zhang F, Eliseenkova AV, Yayon A, et al. Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc Natl Acad Sci U S A. 2004; 101: 935-40.
31.Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993; 60: 1-41.
32.Itoh N, Ornitz DM. Evolution of the FGF and FGFR gene families. Trends Genet. 2004; 20: 563-9.
33.Matsumoto E, Sasaki S, Kinoshita H, Kito T, Ohta H, Konishi M, et al. Angiotensin II-induced cardiac hypertrophy and fibrosis are promoted in mice lacking FGF16. Genes Cells. 2013; 18: 544-53.
34.Hamidouche Z, Fromigue O, Nuber U, Vaudin P, Pages JC, Ebert R, et al. Autocrine fibroblast growth factor 18 mediates dexamethasone-induced osteogenic differentiation of murine mesenchymal stem cells. J Cell Physiol. 2010; 224: 509-15.
35.Itoh N, Ohta H. Roles of FGF20 in dopaminergic neurons and parkinson's disease. Front Mol Neurosci. 2013; 6: 15.
36.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaklotho. J Cell Physiol. 2008; 215: 1-7.
37.Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Regulation of renal phosphate transport by FGF23 is mediated by fgfr1 and FGFR4. Am J Physiol Renal Physiol. 2014; 306: F351-8.
38.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004; 118: 257-70.
39.Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J Neurosci. 2005;25: 7470-9.
40.Sonvilla G, Allerstorfer S, Heinzle C, Stattner S, Karner J,Klimpfinger M, et al. Fibroblast growth factor receptor 3-IIIC mediates colorectal cancer growth and migration. Br J Cancer. 2010; 102: 1145-56.
41.Fu T, Kim YC, Byun S, Kim DH, Seok S, Suino-Powell K, et al. FXR primes the liver for intestinal FGF15 signaling by transient induction of beta-klotho. Mol Endocrinol. 2016; 30: 92-103.
42.Cinque L, Forrester A, Bartolomeo R, Svelto M, Venditti R,Montefusco S, et al. FGF signalling regulates bone growth through autophagy. Nature. 2015; 528: 272-5.
43.Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, et al. Fgf-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999; 11: 729-35.
44.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996; 271: 15292-7.
45.Duchesne L, Tissot B, Rudd TR, Dell A, Fernig DG. N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J Biol Chem. 2006; 281: 27178-89.
46.Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P,et al. Identification of a new fibroblast growth factor receptor,FGFR5. Gene. 2001; 271: 171-82.
47.Silva PN, Altamentova SM, Kilkenny DM, Rocheleau JV. Fibroblast growth factor receptor like-1 (FGFRL1) interacts with shp-1 phosphatase at insulin secretory granules and induces beta-cell ERK1/2 protein activation. J Biol Chem. 2013; 288: 17859-70.
48.Wakulich C, Jackson-Boeters L, Daley TD, Wysocki GP. Immunohistochemical localization of growth factors fibroblast growth factor-1 and fibroblast growth factor-2 and receptorsfibroblast growth factor receptor-2 and fibroblast growth factor receptor-3 in normal oral epithelium, epithelial dysplasias, and squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93: 573-9.
49.Jang JH. Reciprocal relationship in gene expression between FGFR1 and FGFR3: Implication for tumorigenesis. Oncogene. 2005; 24: 945-8.
50.Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res. 2010; 8: 1439-52.
51.Moosa S, Wollnik B. Altered FGF signalling in congenital craniofacial and skeletal disorders. Semin Cell Dev Biol. 2015;
52.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005; 16: 139-49.
53.Vecchione A, Cooper HJ, Trim KJ, Akbarzadeh S, Heath JK,Wheldon LM. Protein partners in the life history of activated fibroblast growth factor receptors. Proteomics. 2007; 7: 4565-78.
54.Martinez N, Garcia-Dominguez CA, Domingo B, Oliva JL, Zarich N, Sanchez A, et al. Sprouty2 binds GRB2 at two different prolinerich regions, and the mechanism of erk inhibition is independent of this interaction. Cell Signal. 2007; 19: 2277-85.
55.Li C, Scott DA, Hatch E, Tian X, Mansour SL. Dusp6 (MKP3) is a negative feedback regulator of fgf-stimulated erk signaling during mouse development. Development. 2007; 134: 167-76.
56.Kovalenko D, Yang X, Nadeau RJ, Harkins LK, Friesel R. Sef inhibits fibroblast growth factor signaling by inhibiting FGFR1 tyrosine phosphorylation and subsequent erk activation. J Biol Chem. 2003; 278: 14087-91.
57.Mardakheh FK, Yekezare M, Machesky LM, Heath JK. Spred2 interaction with the late endosomal protein nbr1 down-regulates fibroblast growth factor receptor signaling. J Cell Biol. 2009; 187: 265-77.
58.Bottcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol. 2004; 6: 38-44.
59.Haines BP, Wheldon LM, Summerbell D, Heath JK, Rigby PW. Regulated expression of FLRT genes implies a functional role in the regulation of FGF signalling during mouse development. Dev Biol. 2006; 297: 14-25.
60.Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009; 152: 15-32.
61.Baird K, Davis S, Antonescu CR, Harper UL, Walker RL, Chen Y, et al. Gene expression profiling of human sarcomas: Insights into sarcoma biology. Cancer Res. 2005; 65: 9226-35.
62.Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, et al. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012; 118: 740-9.
63.Fernanda Amary M, Ye H, Berisha F, Khatri B, Forbes G, Lehovsky K, et al. Fibroblastic growth factor receptor 1 amplification in osteosarcoma is associated with poor response to neo-adjuvant chemotherapy. Cancer Med. 2014; 3: 980-7.
64.Ren T, Qing Y, Dai N, Li M, Qian C, Yang Y, et al. Apurinic/apyrimidinic endonuclease 1 induced upregulation of fibroblast growth factor 2 and its receptor 3 induces angiogenesis in human osteosarcoma cells. Cancer Sci. 2014; 105: 186-94.
65.Leddy LR, Holmes RE. Chondrosarcoma of bone. Cancer Treat Res. 2014; 162: 117-30.
66.Webster MK, Donoghue DJ. Fgfr activation in skeletal disorders: Too much of a good thing. Trends Genet. 1997; 13: 178-82.
67.Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D,Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999; 13: 1361-6.
68.Aikawa T, Segre GV, Lee K. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-CDK2. J Biol Chem. 2001; 276: 29347-52.
69.van Oosterwijk JG, Meijer D, van Ruler MA, van den Akker BE,Oosting J, Krenacs T, et al. Screening for potential targets for therapy in mesenchymal, clear cell, and dedifferentiated chondrosarcoma reveals Bcl-2 family members and TGFbeta as potential targets. Am J Pathol. 2013; 182: 1347-56.
70.De Giovanni C, Landuzzi L, Nicoletti G, Lollini PL, Nanni P. Molecular and cellular biology of rhabdomyosarcoma. Future Oncol. 2009; 5: 1449-75.
71.Taylor JGt, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009; 119: 3395-407.
72.Crose LE, Etheridge KT, Chen C, Belyea B, Talbot LJ, Bentley RC,et al. FGFR4 blockade exerts distinct antitumorigenic effects in human embryonal versus alveolar rhabdomyosarcoma. Clin Cancer Res. 2012; 18: 3780-90.
73.Wachtel M, Rakic J, Okoniewski M, Bode P, Niggli F, Schafer BW. FGFR4 signaling couples to bim and not bmf to discriminate subsets of alveolar rhabdomyosarcoma cells. Int J Cancer. 2014;135: 1543-52.
74.Wang X, Asmann YW, Erickson-Johnson MR, Oliveira JL, Zhang H, Moura RD, et al. High-resolution genomic mapping reveals consistent amplification of the fibroblast growth factor receptor substrate 2 gene in well-differentiated and dedifferentiated liposarcoma. Genes Chromosomes Cancer. 2011; 50: 849-58.
75.Zhang K, Chu K, Wu X, Gao H, Wang J, Yuan YC, et al. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 2013; 73: 1298-307.
76.Kunstlinger H, Fassunke J, Schildhaus HU, Brors B, Heydt C, Ihle MA, et al. FGFR2 is overexpressed in myxoid liposarcoma and inhibition of FGFR signaling impairs tumor growth in vitro. Oncotarget. 2015; 6: 20215-30.
77.Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR, et al. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: Potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11: 2702-12.
78.Girnita L, Girnita A, Wang M, Meis-Kindblom JM, Kindblom LG,Larsson O. A link between basic fibroblast growth factor (BFGF)and EWS/FLI-1 in ewing's sarcoma cells. Oncogene. 2000; 19: 4298-301.
79.Westwood G, Dibling BC, Cuthbert-Heavens D, Burchill SA. Basic fibroblast growth factor (BFGF)-induced cell death is mediated through a caspase-dependent and p53-independent cell death receptor pathway. Oncogene. 2002; 21: 809-24.
80.Ishigami T, Hida Y, Matsudate Y, Murao K, Kubo Y. The involvement of fibroblast growth factor receptor signaling pathways in dermatofibroma and dermatofibrosarcoma protuberans. J Med Invest. 2013; 60: 106-13.
81.Kilvaer TK, Valkov A, Sorbye SW, Smeland E, Bremnes RM,Busund LT, et al. Fibroblast growth factor 2 orchestrates angiogenic networking in non-GIST STS patients. J Transl Med. 2011; 9: 104.
82.Noonan S, Man Wong K, Jimeno A. Nintedanib, a novel triple angiokinase inhibitor for the treatment of non-small cell lung cancer. Drugs Today (Barc). 2015; 51: 357-66.
83.Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dienstmann R,et al. Phase I/IIa study evaluating the safety, efficacy,pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014; 25: 2244-51.
84.Eisen T, Loembe AB, Shparyk Y, MacLeod N, Jones RJ,Mazurkiewicz M, et al. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results. Br J Cancer. 2015; 113: 1140-7.
85.Konecny GE, Finkler N, Garcia AA, Lorusso D, Lee PS, Rocconi RP, et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: A non-randomised, open-label, two-group,two-stage, phase 2 study. Lancet Oncol. 2015; 16: 686-94.
86.du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S,Colombo N, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (ago-ovar 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016; 17: 78-89.
87.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in philadelphia chromosomepositive leukemias. N Engl J Med. 2013; 369: 1783-96.
88.Xiang J, Tang J, Song C, Yang Z, Hirst DG, Zheng QJ, et al. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009; 11: 516-26.
89.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res. 2014; 201: 185-96.
90.Li SQ, Cheuk AT, Shern JF, Song YK, Hurd L, Liao H, et al. Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (ap24534). PLoS One. 2013; 8: e76551.
Cite this article as: Zhou W, Zheng H, Du X, Yang J. Characterization of FGFR signaling pathway as therapeutic targets for sarcoma patients. Cancer Biol Med. 2016; 13: 260-268. doi: 10.20892/j.issn.2095-3941.2015.0102
Correspondence to: Ji-Long Yang
E-mail: yangjilong@tjmuch.com
Received January 18, 2015; accepted January 8, 2016. Available at www.cancerbiomed.org
Copyright © 2016 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- The oncofetal protein IMP3 is an indicator of early recurrence and poor outcome in mucoepidermoid carcinoma of salivary glands
- Modulation of B-cell receptor and microenvironment signaling by a guanine exchange factor in B-cell malignancies
- Influence of obesity and bariatric surgery on gastric cancer
- Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang
- Genetic and molecular changes in ovarian cancer
- New generation of breast cancer clinical trials implementing molecular profiling
