金黄色葡萄球菌耐消毒剂基因的检测
2016-08-08吴建国黄余清刘成伟黄文祥
吴建国, 黄余清, 严 明, 刘成伟, 黄文祥, 贾 蓓
金黄色葡萄球菌耐消毒剂基因的检测
吴建国1, 黄余清1, 严明2, 刘成伟2, 黄文祥2, 贾 蓓2
摘要:目的 对金黄色葡萄球菌(金葡萄)的耐消毒剂基因qacA/B进行检测分析。方法 收集重庆市奉节县人民医院2014年1—12月住院患者中分离的金葡菌51株,采用药敏试验、PCR技术进行mecA和qacA/B检测,筛选出的携带有qacA/B基因的金葡菌进行spa分型,并分析相应的临床情况和对16种抗菌药物的耐药模式。结果 金葡菌中mecA和qacA/ B检测率分别为21.6%(11/51)和13.7%(7/51);耐甲氧西林金葡菌(MRSA)菌株中qacA/B阳性率为54.5%(6/11),甲氧西林敏感金葡菌(MSSA)菌株中qacA/B阳性率为2.5%(1/40),阳性率差异有统计学意义(P<0.05)。7株qacA/B经spa分型发现4个型,分别为t037、t091、t932、t895,其中t037有4株来源于儿科病房,其余菌株各1株,以t037型别为主。结论 该院分离出携带耐消毒剂qacA/B基因的金葡菌,MRSA携带率明显高于MSSA,并且可能至少存在1个来自院内的克隆株在儿科病房流行。
关键词:金黄色葡萄球菌; 耐消毒剂基因; qacA/B; mecA; spa分型
金黄色葡萄球菌(金葡菌)是医院感染的重要病原菌之一,尤其是耐甲氧西林金葡菌(MRSA),因其严重的多重耐药,而引起当今世界范围内的广泛关注。由于消毒剂大量使用,导致细菌对消毒剂的敏感性下降[1-2]。与抗菌药物耐药菌株一样,抗消毒剂菌株的出现可能导致医院消毒的失败,可能引起医院内耐药菌如MRSA感染的流行和传播。金葡菌耐消毒剂基因的种类至少有12种如(qacA~qacJ, smr, 及norA)[3-8],对消毒剂的耐药与细菌携带qacA/B密切相关,尤其在MRSA菌株中广泛流行[9-12]。奉节县人民医院为地处重庆市偏远山区的县级二甲医院,本研究通过对我院2014 年1—12月分离的51株金葡菌进行mecA和耐消毒剂qacA/B基因的检测,并进行spa分型和耐药模式分析,以了解该基因在我院MRSA和金葡菌甲氧西林敏感株(MSSA)中的流行情况,有利于对金葡菌所致医院感染的控制。
1 材料与方法
1.1材料
收集奉节县人民医院2014年1—12月临床各科室分离的金葡菌51株,标本分别来自于痰液、伤口分泌物、脓液、血液等。使用法国生物梅里埃公司生产的葡萄球菌和微球菌鉴定系统API STAPH,以及乳胶凝集法快速检测金葡菌试剂Slidex Staph Plus对所收集的菌株进行鉴定。质控菌株为金葡菌ATCC25923、ATCC29213、ATCC43300,qacA/B阳性菌株TS77。
1.2方法
1.2.1 药敏试验 药敏试验采用纸片法或稀释法测定MIC,以2014年CLSI[13]评判标准,检测51株细菌对16种抗菌药物以及4种消毒剂的药敏。
1.2.2 细菌DNA提取及模板制备 取MH平皿上新鲜过夜单个菌落2~3个,混悬于浓度为10 g/ mL溶菌酶的50 μL灭菌TE液中 (pH=8.0),37℃水浴1 h,100℃煮沸10 min,14 000 r/min离心10 min后取上清液即含细菌DNA。
1.2.3 mecA基因扩增 mecA引物参照JONAS等[14]设计,上游引物 P1:5'-AAAAT-CGATGGTAAAGGTTGGG-3'(22 bp);下游引物P2:5'-AGTTCTGCAGTACCG GATTTGC-3'(22 bp)。PCR反应体系(50 μL):10×PCR反应缓冲液5 μL,MgCl23 μL,dNTP4 μL,DNA模板2 μL,Taq酶0.5 μL,灭菌蒸馏水加至50 μL。PCR反应条件:94 ℃预变性2 min,94 ℃变性30 s,50 ℃退火45 s,72 ℃延伸60 s,进行30次循环,最后延伸10 min,反应结束后,将PCR产物于4 ℃保存。取5 μL PCR反应产物样品在1.5%琼脂糖凝胶中电泳,紫外凝胶电泳成像并保存。PCR扩增产物由上海生工生物工程有限公司测序,将结果与GenBank中提供的序列进行比对。
1.2.4 qacA/B基因扩增 qacA引物根据Pubmed基因库中公布的基因序列用primer 5.0设计:上游引物 P1:5'-GCTGCATTTATGACAATGTT-TG-3' (22 bp);下游引物P2:5'-AATCCCACCTACTAAAGCAG-3'(20 bp)。
1.2.5 spa分型 对16株qacA阳性的金葡菌进行spa分型,spa分型引物参照参考文献[15-16]:上游引物 P1:5'-GACGATCCTTCAGTGAGCAAAG-3’(22 bp);下游引物P2:5'-GCAGCAATTTTGTCGGCAGTAG -3'(20 bp)。10×PCR反应缓冲液5 μL,MgCl23 μL,dNTP 4 μL,DNA模板2 μL,Taq酶0.5 μL,灭菌蒸馏水加至50 μL。PCR反应条件:95 ℃预变性4 min,95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸45 s,共30次循环,然后72 ℃延伸10 min, 4 ℃保存。取5 μLPCR反应产物样品加入1.5%琼脂糖凝胶加样孔中,在1×TBE中,电压110 V,时间25 min,紫外凝胶电泳成像并保存。PCR扩增产物由上海生工生物工程有限公司测序。测序结果的重复编码判读参照文献[17],并通过spa分型的数据库(http:// www.ridom.de/spaserver)进行分型。
2 结果
2.1基因的检测
表明mecA的携带率为21.6%(11/51),部分检测结果见图1。
2.2 MIC测定

图1 mecA基因电泳图Figure 1 Electrophoretogram of the PCR products for mecA gene
7株携带有qacA/B基因的金葡菌和参考菌株对4种常用消毒剂和头孢西丁的药敏试验见表1。
2.3基因在金葡菌中的检出率
金葡菌中qacA/B检测阳性率为13.7%(7/51);MRSA菌株中qacA/B阳性率为54.5%(6/11),MSSA菌株中qacA/B阳性率为2.5%(1/40),差异有统计学意义(P<0.05);在7株qacA/B阳性菌株中6株为MRSA,1株为MSSA,阳性率差异有统计学意义(Fisher精确概率法,P<0.05),见图2。
2.4spa 分型
7株携带有qacA/B的金葡菌的spa分型电泳图见图3。如表2所示,可见spa基因重复序列数为6~10个。将测序结果提交spa重复序列的数据库进行比对,分别定为t037、t091、t932、t895,其中t037有4株,其余菌株各1株,以t037型别为主,占的4/7。
2.5细菌分布
分析了7株携带qacA/B基因金葡菌患者的临床资料显示,感染均来自于医院内,科室分布以儿科为主,标本来源为痰,呼吸道感染传播为主,见表3。
2.6耐药模式
7株qacA/B阳性菌株对16种常用抗金葡菌药物的耐药表型进行分型,其中507、509、511和520均为A型,534、551和557分别为B、C、D 型。A型的菌株表现为对氨苄西林-舒巴坦、氯霉素、环丙沙星、红霉素、磷霉素、夫西地酸、庆大霉素、亚胺培南、莫西沙星、四环素耐药,对利奈唑胺、利福平、替考拉宁和奎奴普丁-达福普汀敏感,B型则除了对氯霉素、红霉素和四环素耐药外对其他检测抗菌药物均敏感,而C型和D型除了对磷霉素和莫西沙星敏感性不一致外其余都耐药[10],见表4。
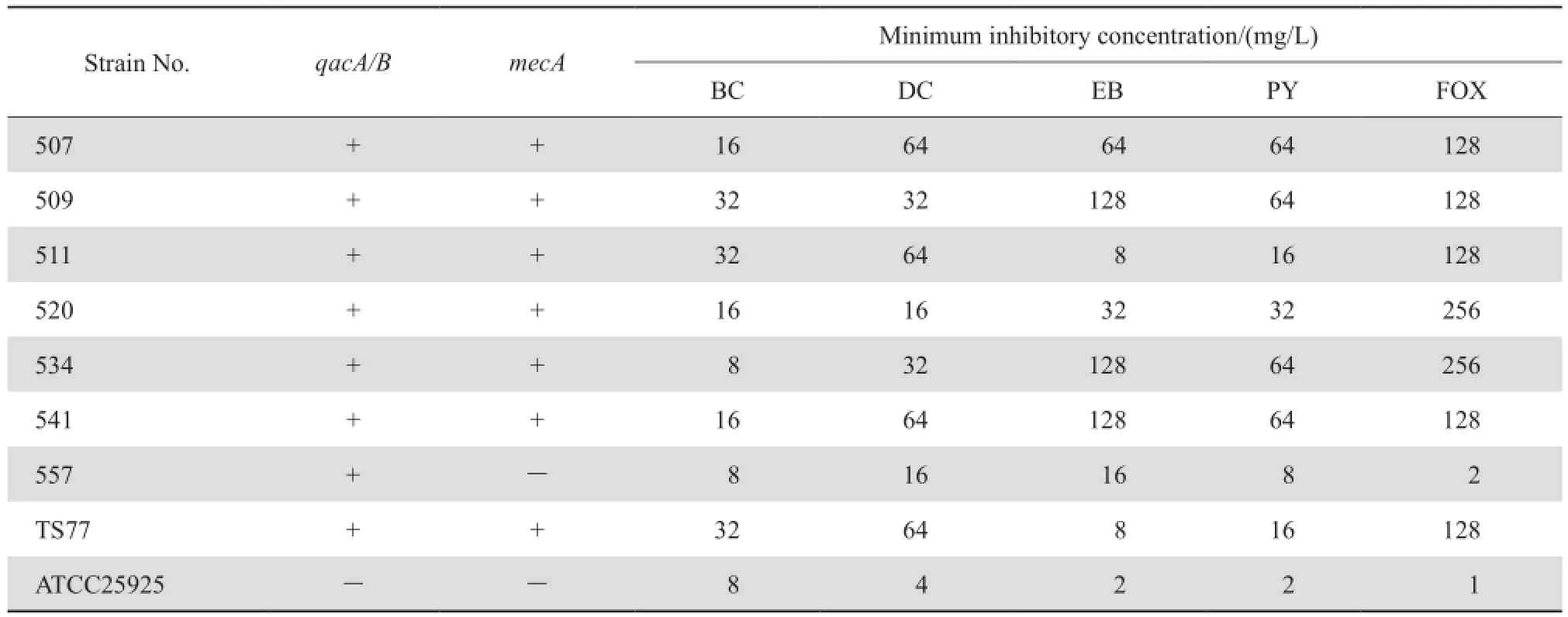
表1 7株qacA/B阳性株和参考菌株mecA检出率及对4种消毒剂及头孢西丁的MICTable 1 The susceptibility of 7 qacA-positive S. aureus strains and 2 reference strains to 4 disinfectants and cefoxitin in terms of minimum inhibitory concentrations and mecA status
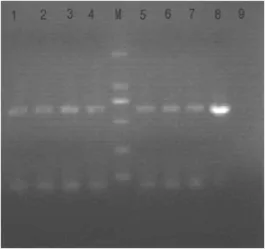
图2 qacA/B基因检测电泳图Figure 2 Electrophoretogram of the PCR products for qacA/B gene M, marker; Lane 1-4, Lane5-7: 507, 509, 511, 520, 534, 541, 557 test strains. Lane 8: TS77, qacA/B-positive strain; Lane 9: negative stain.

图3 spa分型电泳图Figure 3 Electrophoresis for spa typing of S. aureus strains M, marker; Lane 1-7: 507, 509, 511, 520, 534, 541, 557 test strains.

表2 7株qacA/B阳性细菌SPA重复序列的特征及分型Table 2 Spa typing of the 7 strains of qacA/B-positive S. aureus based on the features of spa repetitive sequences

表3 qacA/B阳性的金葡菌临床资料Table 3 Clinical details of the qacA/B-positive S. aureus strains

表4 qacA/B阳性金葡菌对常用抗菌药物耐药模式Table 4 Resistant pattern of qacA/B-positive S. aureus strains to commonly used antimicrobial agents
3 讨论
mecA基因的检出是确证MRSA的金标准,研究结果显示mecA基因的检出率与金葡菌对头孢西丁的耐药率是一致的,药敏结果提示7株携带有qacA/B基因的金葡菌6株为mecA基因阳性菌株,消毒防腐剂类对带有qacA/B基因菌株的MIC均高于未携带该基因菌株,菌号557未检测出mecA基因,尽管消毒剂对该菌的MIC低于mecA阳性菌株,但也明显高于阴性对照菌株,表明MSSA也可携带耐消毒剂基因,提示可能增加社区金葡菌感染的传播。
我院的qacA/B检出率明显高于WEI等[18]报道的qacA/B的检测率9.1%,MRSA中qacA/B的检出率为17.1%,2006年黄支密等[19]首次报道国内MRSA菌株检出qacA/B基因,其后国内其他地区报道的检出率为38%~92%[20],本研究与日本及2001年欧洲报道的qacA/B在MRSA的检出率44%~63%大致相当[12,21], MIYAZAKI等[22]2007报道MRSA中qacA/B的检出率达到80%,巴西3所医院收集的74株MRSA中qacA/ B检出率为80%[22],这些检出率的差异可能与地域不同,用药的频次、习惯及收集标本的多少有关;MRSA 中qacA/B基因的阳性检出率高,目前已经从MRSA菌株中检出多种消毒剂耐药基因(qacA/B及qacC/D家族),这可能是qacA/B等消毒剂耐药基因多位于多重耐药基因位点上,故导致MRSA对某些消毒剂的耐药性与对抗菌药物的耐药性的关联性,我们的药敏实验中也可看到MRSA菌株的耐消毒剂水平也更高于MSSA菌株,更有利于耐药菌的生存与传播,因此应当高度重视并调查耐消毒剂基因在MRSA中的传播,以降低医院感染的发生。
spa分型显示,国外MRSA主要以t001、t002为主,国内其他地区发现有t030及t037流行[23],此次我们的研究中并未发现新的spa分型菌株,本研究对象是携带耐消毒剂基因的MRSA,但也与国内流行趋势一致,另外也说明spa分型存在很强的地域性,有助于不同地区实验室的比较。临床资料结合spa分型可以看到我院儿科可能存在医院携带有qacA/B MRSA克隆株的流行。
本研究的不足之处主要为我院存在临床标本送检率及检出率不高,因此1年时间仅收集51株,样本量小,统计结果可能出现偏倚。
参考文献:
[1]RUSSELL AD. Do biocides select for antibiotic resistance? [J].J Pharm Pharmaco, 2000, 52(2):227-233.
[2]REVERDY ME, BES M, BRUN Y, et al. Evolution of resistance to antibiotics and antiseptics of hospital Staphylococcus aureus strains isolated from 1980 to 1991 [J]. Pathol Biol (Paris), 1993,41(9):897-904.
[3]BJORLAND J, STEINUM T, SUNDE M, et al. Novel plasmidbornegene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus intermedius[J]. Antimicrob Agents Chemother, 2003, 47(10):3046-3052.
[4]GRINIUS L, DREDUNIENE G, GOLDBERG EB, et al. A staphylococcal multidrug resistance gene product is a member of a new protein family [J]. Plasmid, 1992, 27(2):119-129.
[5]HEIR E, SUNDHEIM G, HOLCK AL. TheqacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry [J]. J Appl Microbiol, 1999, 86(3):378-388.
[6]HEIR E, SUNDHEIM G, HOLCK AL. TheStaphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance [J]. FEMS Microbiol Letters, 1998,163(1):49-56.
[7]PAULSEN IT, BROWN MH, LITTLEJOHN TG, et al. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus:membrane topology and identification of residues involved in substrate specificity[J]. Proc Nat Acad Sci, USA, 1996,93(8):3630-3635.
[8]ROUCH DA, CRAM DS, DIBERARDINO D, et al. Effluxmediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline-and sugar-transport proteins[J]. Mol Microbiol, 1990, 4(12):2051-2062.
[9]ALAM MM, KOBAYASHI N, UEHARA N, et al. Analysis on distribution and genomic diversity of high-level antiseptic resistance genes qacA and qacB in human clinical isolates of Staphylococcus aureus[J]. Microb Drug Resist, 2003, 9(2):109-121.
[10]MAYER S, BOOS M, BEYER A, et al. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and-susceptible European isolates of Staphylococcus aureus[J]. J Antimicrob Chemother, 2001, 47(6):896-897.
[11]NOGUCHI N, HASE M, KITTA M, et al. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus[J]. FEMS Microbiol Letters, 1999, 172(2):247-253.
[12]NOGUCHI N, SUWA J, NARUI K, et al. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus aureus isolated in Asia during 1998 and 1999[J]. Med Microbiol, 2005,54(Pt 6):557-565.
[13]Clinical and Laboratory Standards Institute. Performance standards for Antimicrobial Suscreptibility testing, 19th Informational Supplement [S]. 2014, M100-S19.
[14]JONAS D, SPECK M, DASCHNER FD, et al. Rapid PCR-based identifcation of methicillin-resistant Staphylococcus aureus from screening swabs [J]. Clin Microbiol, 2002, 40(5):1821-1823.
[15]TANG YW, WADDINGTON MG, SMITH DH, et al.Comparison of protein A gene sequencing with pulsed-feld gel electrophoresis and epidemiologic data for molecular typing of methicillin-resistant Staphylococcus aureus[J]. Clin Microbiol,2000, 38(4):1347-1351.
[16]OLIVERIRA DC, CRISOSTOMO I, SANTOS-SANCHES I,et al. Comparison of DNA sequencing of the protein A gene polymorphic region with other molecular typing techniques for typing two epidemiologically diverse collections of methicillinresistant Staphylococcus aureus[J]. Clin Microbiol, 2001,39(2):574-580.
[17]HARMSEN D, CLAUS H, WITTE W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management[J]. Clin Microbiol,2003, 41(12):5442-5448.
[18]WEI X, JIA B. Prevalence and molecular typing of the antiseptic resistance genes qacA/B among Stphylococcus aureus strains isolated in a teaching hospital[J]. African Biotechnol, 2012, 11 (47):1078-1079
[19]黄支密,仵蕾,吴林松,等. 耐甲氧西林金黄色葡萄球菌对消毒剂和常用抗菌药物的耐药性及相关基因的研究[J]. 中华微生物学和免疫学杂,2006,(12):1116-1117.
[20]SONG Y, DU X, LI T, et al. Phenotypic and molecular characterization of Staphylococcus aureus recovered from different clinical specimens of inpatients at a teaching hospital in Shanghai between 2005 and 2010[J]. Med Microbiol, 2013, 62(Pt 2):274-282.
[21]FLUIT AC, WIELDERS CL, VERHOEF J, et al. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study[J]. Clin Microbiol, 2001, 39(10):3727-3732.
[22]MIYAZAKI NH, ABREU AO, MARIN VA, et al. The presence of qacA/B gene in Brazilian methicillin-resistant Staphylococcus aureus[J]. Mem Inst Oswaldo Cruz, 2007, 102(4):539-540.
[23]毛镭篥,肖盟,王贺,等. 全国多中心细菌耐药监测网中血流感染相关金黄色葡萄球菌的分子流行病学研究[J]. 中国感染与化疗杂,2015,15(2):120-125.
2.重庆医科大学附属第一医院感染科,重庆市传染病寄生虫病学重点实验室。
中图分类号:R378.11
文献标识码:A
文章编号:1009-7708(2016)03-0340-06
DOI:10.16718/j.1009-7708.2016.03.016
收稿日期:2015-06-15 修回日期:2015-08-15
基金项目:重庆市卫生计生委医学科研面上项目(2013-2-286)。
作者简介:1.重庆市奉节县人民医院感染科,重庆 404600; 吴建国(1973—),男,副主任医师,主要从事细菌耐药性分析及致病机制研究。
通信作者:贾蓓,E-mail:beijia2008@yeah.net。
Detection of disinfectant-resistant gene in Staphylococcus aureus
WU Jianguo, HUANG Yuqing, YAN Ming, LIU Chengwei, HUANG Wenxiang, JIA Bei. (Department of Infectious Diseases, Chongqing Fengjie County People's Hospital, Chongqing 404600, China)
Abstract:Objective To detect the disinfectant-resistant gene qacA/B in the strains of Staphylococcous aureus isolated from January 2014 to December 2014. Methods Fifty-one isolates were collected. PCR assay was used to detect mecA gene and qacA/ B gene in the isolates followed by Staphylococcus protein A (spa) typing. Antimicrobial-resistant phenotypic typing was conducted to analyze the homology of these qacA/B positive strains. The clinical information of the patients from whom the strains were isolated was collected to further understand the clinical background of qacA/B-carrying S. aureus. Results The prevalence of mecA and qacA/B genes was 21.6% (11/51) and 13.7% (7/51), respectively in the strains. The prevalence of qacA/B gene in the methicillinresistant S. aureus strains (54.5%, 6/11) was signifcantly higher than that in the methicillin-sensitive S. aureus strains (2.50%, 1/40). The prevalence of mecA gene in qacA/B gene positive strains (6/7) was signifcantly higher than that in qacA/B gene negative strains (1/7). These qacA/B positive strains were classifed into 4 spa types (t037, t091, t932 and t895). The main type was t037 (4/7), which was from the pediatric ward. Conclusions The prevalence of qacA/B gene is low in the S. aureus strains. However, the prevalence of this gene in methicillin-resistant S. aureus strains is far higher than that in methicillin-sensitive S. aureus. spa type t037 may be a prevalent clone in pediatric ward.
Key words:Staphylococcus aureus; disinfectant-resistant gene; qacA/B; mecA; spa typing
