Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
2016-07-24ZiRanWangYuXinLiHongYanLeiDaiQunYangLiQuanWangMingYuLuoDepartmentofCerebralSurgeryLinyiPeopleHospitalShandong276000China
Zi-Ran Wang, Yu-Xin Li, Hong-Yan Lei, Dai-Qun Yang, Li-Quan Wang, Ming-Yu LuoDepartment of Cerebral Surgery, Linyi People’s Hospital, Shandong 276000, China
Contents lists available at ScienceDirect
Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
Zi-Ran Wang, Yu-Xin Li, Hong-Yan Lei, Dai-Qun Yang, Li-Quan Wang, Ming-Yu Luo*
Department of Cerebral Surgery, Linyi People’s Hospital, Shandong 276000, China
ABSTRACT
Objective: To observe the effect of nuclear transcription factor-KB (NF-KB) on cerebral edema in rats with traumatic brain injury (TBI). Methods: Male SD rats with fl uid percussion injury (FPI) were selected. After separation and culture, rats’ astrocytes all suff ered FPI. The expression of NF-κB and the water content were detected at the animal and cellular levels, while the activity of NOX was evaluated at the cellular level. Results: According to the results, the positive expression of NF-κB and expression of mRNA were signifi cantly increased and the water content was increased for rats after TBI, while NF-κB inhibitor BAY11-7082 could signifi cantly reduce the eff ect of TBI. 1 and 3h after FPI of astrocytes, the activation of NF-κB was increased and BAY 11-7082 could signifi cantly improve the injury-induced swelling of astrocytes. After the injury of astrocytes, the activity of NOX was also increased, while BAY 11-7082 could reduce the activity of NOX. Conclusions: The results show that the activation of NF-KB in astrocytes is a key factor in the process of cerebral edema after TBI of rats.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Nuclear transcription factor-KB
Traumatic brain injury
Astrocytes
Rat
1. Introduction
Cerebral edema is major complication of nervous system for traumatic brain injury (TBI), and the swelling of astrocytes is the important part of early cerebral edema after the trauma[1,2]. The mechanism of astrocytes swelling after trauma has not been clear yet. It is considered that oxidative stress plays a key role in the pathogenesis of TBI[3-5]. According to the previous researches, the injury of astrocytes cultured in vitro could signifi cantly increase the free radical[6].
The active oxygen free radical could stimulate the activation of different kinds of transcription factors, especially nuclear transcription factor-KB (NF-KB)[7,8]. The activated NF-KB might contribute to the swelling of astrocytes after being exposed to ammonia[9], and it also plays a role during cerebral edema after hepatic failure[10]. Meanwhile, the activated NF-KB is found after TBI[11,12]. However, the function of NF-KB in the swelling of astrocytes and cerebral edema after TBI has not been explained. This study is to discuss the activation of NF-KB after the injury of astrocytes in vitro and in vivo and whether the activated NF-KB would contribute to the swelling of astrocytes and cerebral edema after TBI.
2. Materials and methods
2.1. Animals
Male SD rats with the weight of 250-350 g were provided by the laboratory of Shandong University. All rats were fed under sterile environment with the temperature of (22±2)℃. Rats were given free diet and drinking.
2.2. Instruments and reagents
The fl uid percussion injury device was purchased from NatureGene Corp.; the rabbit polyclonal NF-KB and GFAP antibodies from Santa; DMEM and fetal bovine serum from Gibco; 3-O-methyl-D-glucopyranose from Y-Y Chemical Reagents; NOX activity assay kit from Shanghai Suolaibao Bio-technology Co., Ltd.; BAY 11–7082 and SN50 from Sigma.
2.3. Methods
2.3.1. Establishment of rat model of moderate brain injury with lateral fluid percussion
The method proposed by Sinke et al[9] was used to establish the rat model of moderate brain injury with lateral fl uid percussion (TBI). The part of percussion was the right parietal cortex. Rats in Sham group were given the same procedure except the injury. Rats in the treatment group were given the intravenous injection of BAY 11-7082 (1, 5 and 10 mg/kg) and NF-KB inhibitor 5-10 min after TBI. Rats in other groups were given the equal volume of normal saline. Afterwards, rats were randomly divided into the control group, sham group, TBI group and BAY 11-7082+TBI group.
2.3.2. Detection of cerebral water content
Three hours after TBI, the head of rats was cut off and the brain was collected. The fi lter paper was used to absorb the water on the surface of brain at fi rst. Then the brain was placed on the electronic analytical balance to analyze the wet weight. Afterwards, it was placed in the oven for the baking at 110℃ for 24 h and then the dry weight was measured. The cerebral water content (%)= (wet weight - dry weight )/wet weight ×100%.
2.3.3. Immunohistochemistry
The brain tissues were fixed with 4% paraformaldehyde for 24 h and then stored in the cold 30% sucrose for 12 h. Afterwards, they were cut into 20 μm slices. After being sealed with the fetal bovine serum for 30 min, they were given the DAPI staining and then cultured with rabbit polyclonal NF-KB and GFAP antibodies for 3 h at the room temperature. After being washed with PBS for 3 times, they were incubated in the fl uorescein-labeled horseradish peroxidase secondary antibody for 30 min. Then the slides were prepared and observed under the microscope.
2.3.4. RT-PCR to detect the expression of NF-KB in brain tissue
Trizol kit (Invitrogen) was employed to extract the total RNA from the brain tissue. RNA was reversely transcribed into cDNA through one-step RT-PCR kit and then PCR amplification was performed. The collected 5 μL amplifi cation products were used for the testing in 2% agarose gel.
2.3.5. Culture of primary astrocytes
After collection of the head from 1-day rat, the cortex was separated and sliced into pieces in HBSS. It was then digested with the pancreatin. The digestion was ended after adding 10% FBS. The supernatant was removed after centrifugation and then seeded on DMEM with the double-antibody and fetal bovine serum. Then it was cultured in the incubator at 37℃ and 5% CO2.
2.3.6. Establishment of fluid percussion injury (FPI) of astrocytes
The fl uid was replaced according to the routine procedure 24 h before the fl uid percussion. After eliminating the metabolic product of cells, the culture medium was removed. The alcohol was used for the disinfection around the Petri dish. It was then placed in the injury chamber that was full of Hanks. The falling angle of hammer was adjusted to set the impact force as 0.2 MPa. After the percussion, the culture medium was added and it was placed in the incubator. NF-KB inhibitor BAY 11-7082 or SN50 was added in astrocytes after FPI. After being cultured for 3 h, the related detection was performed. Cells were divided into control group, sham group, FPI group, BAY 11-7082+FPI group and SN50+FPI group.
2.3.7. Detection of cell volume
One milliliter 3-O-methyl-D-glucopyranose was added in the culture medium that contained the injured cells. After the culture, the culture medium was collected and stored separately for the radioactivity measurement. Cells were washed rapidly. A total of 0.5 mL 1N NaOH was added to determine the radioactivity of cell extracts.
2.3.8. Western blot assay
To specify the changes in the expression of NF-KB after TBI, the moderate fluid percussion injury was applied to the right parietal cortex of rats. The cerebral cortex was cut into sections for the examination 3 h after TBI. To explore the changes of NF-KB in the astrocytes, the sections were labeled with GFAF and DAPI at the same time. The whole-cell lysis buffer was added in the cultured astrocytes according to the proportion of 10 mL/g. After the homogenization, cells were broken with the ultrasound. After all cells that had been lysed, BCA method was employed for the protein quantifi cation. The gel electrophoresis and membrane transfer were performed. The equal samples were collected for 10% gel electrophoresis. Afterwards, PVDF membrane was taken out and sealed with 10% skim milk. The membrane was put in NF-KB antibody with the concentration of 1:1 000 and then incubated at the room temperature for 3 h. Afterwards, the membrane was put in 1:5 000 horseradish peroxidase labeled goat anti-rabbit or goat antimouse IgG to be incubated for 1 h. According to the reaction steps of ECL kit, it was exposed in the darkroom to obtain the X-ray fi lm that indicated the specifi c protein bands.
2.3.9. Detection of NOX activity
After the lysis of cells, the activity of NOX in the supernatant was detected according to the steps of NOX activity assay kit.
2.4. Statistical analysis
The data was processed with SPSS 18.0. All data was expressed by the mean ± standard deviation. The tukey post-hoc comparison was performed among groups. P<0.05 indicated the statistically signifi cant diff erence.
3. Results
3.1. Expression of NF-KB in cerebral cortex of rats after TBI
According to the results of immunohistochemistry in Table 1, compared with Control group and Sham group, the positive expression of NF-KB was increased in TBI group, while 1, 5,and 10 mg/kg BAY11-7082 could inhibit the positive expression of NF-KB in the cortex of TBI. According to the results of RTPCR in Table 1, compared with Control group and Sham group, the expression of NF-KB mRNA was increased in TBI group, while 1, 5, and 10 mg/kg BAY11-7082 could reduce the expression of NF-KB in the cortex of TBI.

Table 1Expression of NF-KB and mRNA in cerebral cortex of rats after TBI.
3.2. Inhibition of NF-KB to reduce cerebral edema of rats after TBI
As shown in Table 2, compared with the control group and sham group, the degree of cerebral edema in TBI group was signifi cantly increased, while the treatment of 1, 5 and 10mg/kg BAY 11-7082 could reduce the cerebral edema after TBI, while the treatment of BAY 11-7082 (5 mg/kg) had the most signifi cant eff ect.
2.3. Activation of NF-KB after FPI of astrocytes
According to the results of western blot shown in Table 3, compared with the control group, the expression of NF-KB protein was increased in FBI group and decreased in BAY11-7082+FPI group and SN50+FPI group. According to the results of RT-PCR shown in Table 1, compared with the control group, the expression of NF-KB mRNA was increased in TBI group and decreased in BAY11-7082+FPI group and SN50+FPI group.

Table 2Eff ect of BAY 1107082 on cerebral edema of rats after TBI.

Table 3Expression of NF-KB after FPI of astrocytes.
2.4. Inhibition of NF-KB to reduce the swelling of astrocytes after FPI
As shown in Table 4, compared with the control group, the cell swelling was significant in FPI group, while the degree of cell swelling was decreased in BAY11-7082+FPI group and SN50+FPI group.
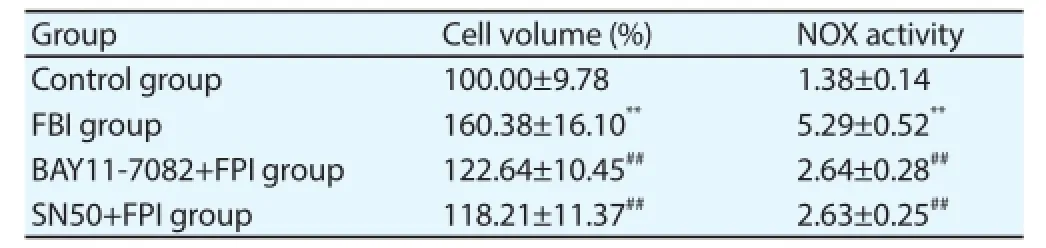
Table 4Inhibition of NF-KB to reduce the swelling and NOX activity of astrocytes after FPI.
2.5. Inhibition of NF-KB to reduce the NOX activity of FPI-induced cells
As shown in Table 4, compared with the control group, the NOX activity was signifi cantly increased in FPI group and decreased in BAY 11-7082 + FPI group and SN50+FPI group.
4. Discussion
According to the results of this study, the positive expression of NF-KB, the expression of mRNA and cerebral water content were increased in rats after TBI, while BAY11-7082 could significantly reduce the cerebral water content after TBI. The FPI of astrocytes could also induce the activation of NF-KB and the inhibition against the activation of NF-KB could reduce the swelling degree of astrocytes caused by the injury. Furthermore, the inhibition of NF-KB could also reduce NOX activity of astrocytes that is caused by FPI. The results indicate that the activation of NF-KB in astrocytes is a key factor in the formation of cerebral edema after TBI.
The oxidative stress and infl ammatory mediator could also activate NF-KB[13-15]. The activated NF-KB could induce the infl ammation / oxidative stress response genes, which would play the key role in some neurological injuries[16]. The activated NF-KB is also found in the brain after TBI[17]. But it has not been clear that whether NF-KB is involved in the astrocyte swelling/cerebral edema after TBI of brain. The results of this study also indicates that FPI of astrocytes could cause the activation of NF-KB, while BAY11-7082, a kindof NF-KB inhibitor, could significantly reduce the FPI-induced swelling of astrocytes. The mechanism for the mechanical injury of astrocytes to activate NF-KB and the further cell swelling has not been clear yet. According to a study, the oxygen free radicals are increased after the injury of astrocytes and the mitochondria permeability transition pore (mPT) and MAPKs are activated. The inhibition of mPT and MAPKs could relieve the swelling of astrocytes[6]. It is because the increased oxygen free radicals could activate NF-KB and induce the activation of mPT and MAPKs[18]. There are some other factors that contributed to the activation of NF-KB of course.
The mechanism of activated NF-KB in the astrocytes that are induced by the mechanical injury has not been clear. The oxidative stress is the potential factor for the activation of NF-KB. The activation of NF-KB could stimulate the generation of NOX, while NOX is one of major sources for the superoxides[19,20]. It has been proved that the activated NOX could contribute to the swelling of astrocytes after the treatment of ammonia[9], which is in accordance with the fi ndings of this study. It’s found that the activity of NOX is increased after the cultured astrocytes suff ere from the mechanical injury, while BAY11-7082 could signifi cantly reduce the activity of NOX. All above results showed that the cell swelling after NF-KB-induced FPI is mediated by increasing the activity of NOX to the certain extent.
In conclusion, the activation of NF-KB and cerebral edema are significantly increased after TBI and the inhibition of NF-KB could signifi cantly reduce the cerebral edema after TBI. The FPI of astrocytes could induce the activation of NF-KB and increase the activity of NOX. BAY11-7082 could signifi cantly reduce the activity of NOX and the swelling of astrocytes. The results indicate that the activation of NF-KB in astrocytes play a key role in the formation of cytotoxic cerebral edema after TBI. The inhibition against the activation of NF-KB might be applied in the treatment of early cerebral edema for patients with TBI.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Wang CG. Research advances in the pathogenesis of traumatic cerebral edema. Chin J Brain Dis Reh 2012; 2(4): 30-32.
[2] Kou Z, VandeVord PJ. Traumatic white matter injury and glial activation: from basic science to clinics. Glia 2014; 62(11):1831-1855.
[3] Bai LX, Song JN. Research advances in the mechanism of oxidative stress injury after traumatic brain injury. Chin J Brain Dis Reh 2013; 3(5): 35-38.
[4] Lu XY, Wang HD, Xu JG. Pretreatment with tert-butylhydroquinone attenuates cerebral oxidative stress in mice after traumatic brain injury. J Surg Res 2014; 188(1): 206-212.
[5] Rodríguez-Rodríguez A, Egea-Guerrero JJ, Murillo-Cabezas F. Oxidative stress in traumatic brain injury. Curr Med Chem 2014; 21(10): 1201-1211.
[6] Jayakumar AR, Rao KV, Panickar KS. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol 2008; 67: 417–427.
[7] Yu HL, Zhao TF, Wu H. Pinellia ternata lectin exerts a pro-infl ammatory eff ect on macrophages by inducing the release of pro-infl ammatory cytokines, the activation of the nuclear factor-KB signaling pathway and the overproduction of reactive oxygen species. Int J Mol Med 2015; 36(4): 1127-1135.
[8] Hong SH, Jeong HK, Han MH. Esculetin suppresses lipopolysaccharideinduced infl ammatory mediators and cytokines by inhibiting nuclear factor-KB translocation in RAW 264.7 macrophages. Mol Med Rep 2014; 10(6): 3241-3246.
[9] Sinke AP, Jayakumar AR, Panickar KS. NF-kappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem 2008; 106: 2302–2311.
[10] Jayakumar R, Bethea JR, Tong XY. NF-KB in the mechanism of brain edema in acute liver failure: studies in transgenic mice. Neurobiol Dis 2011; 41: 498–507.
[11] Laird MD, Sukumari-Ramesh S, Swift AE. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4. J Neurochem 2010; 113: 637–648.
[12] Zhang YL, Wang BK, Liao ZG. The establishment of a modifi ed lateral fl uid percussion model of brain injury in rat and the pertinent pathologic changes. J West Chin Univ Med Sci 1999; 4(4): 363-367.
[13] Tóbon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinfl ammation and oxidative stress. CNS Neurol Disord Drug Targets 2014; 13(9): 1615-1626.
[14] Siomek A. NF-KB signaling pathway and free radical impact. Acta Biochim Pol 2012; 59(3): 323-331.
[15] Yu XJ, Zhang DM, Jia LL. Inhibition of NF-KB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol Appl Pharmacol 2015; 284(3): 315-322.
[16] Jiang P, Li C, Xiang Z. Tanshinone IIA reduces the risk of Alzheimer's disease by inhibiting iNOS, MMP 2 and NFKBp65 transcription and translation in the temporal lobes of rat models of Alzheimer's disease. Mol Med Rep 2014; 10(2): 689-694.
[17] Sticozzi C, Belmonte G, Meini A. L-1β induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFKB/Ca+-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience 2013; 12(252): 367-383.
[18] Bubici, C., Papa, S., Dean, K. Mutual crosstalk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological signifi cance. Oncogene 2006; 25(51): 6731–6748.
[19] Luengo-Blanco M, Prando C., Bustamante J. Essential role of nuclear factor-kappaB for NADPH oxidase activity in normal and anhidrotic ectodermal dysplasia leukocytes. Blood 2008; 112(4): 1453–1460.
[20] Manea A, Manea SA, Gafencu AV. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 2007; 113(4-5): 163–172.
Document heading 10.1016/j.apjtm.2016.01.027
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*Corresponding author: Ming-Yu Luo, Master’s Degree, Deputy Attending Physician, Department of Cerebral Surgery, Linyi People’s Hospital, Shandong 276000, China.
Tel.: 13954993801
Foundation project: It was supported by Technology Program for Development of Pharmacy of Shandong 2013 (No. 2013WS0351).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Effect of miR-467b on atherosclerosis of rats
- Effect of dimethyl fumarate on rats with chronic pancreatitis
