Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
2016-07-24PingHeBoZengXiaoLiZhangDianLiangFangXiaQiaZhouKeQiangWanWenGuangTianDepartmentofGastroenterologyYongchuanHospitalofChongqingMedicalUniversityChongqingChinaDepartmentofInfectiousDiseasesYongchuanHospitalofChongqingMedic
Ping He, Bo Zeng, Xiao-Li Zhang, Dian-Liang Fang, Xia-Qia Zhou, Ke-Qiang Wan, Wen-Guang Tian*Department of Gastroenterology, Yongchuan Hospital of Chongqing Medical University, Chongqing, ChinaDepartment of Infectious Diseases, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
Contents lists available at ScienceDirect
Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
Ping He1, Bo Zeng1, Xiao-Li Zhang1, Dian-Liang Fang1, Xia-Qia Zhou2, Ke-Qiang Wan2, Wen-Guang Tian2*
1Department of Gastroenterology, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
2Department of Infectious Diseases, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
ABSTRACT
Objective: To explore the protective eff ect and its molecular mechanism of apoptosis signalregulating kinase1 (ASK1) inhibitor (GS-459679) on acetaminophen-induced liver injury in mice. Methods: The model of liver injury was established by administration of acetaminophen (APAP) (300 mg/kg, i.p.) on C57BL/6 mice. Forty-eight male C57BL/6 mice were randomly divided into four groups, consisting of control group, GS group (GS-459679, 30 mg/kg, i.p.), APAP-induced group, and GS combined with APAP-induced group. For GS combined with APAP-induced group, mice were treated with GS 30 min prior to administration of APAP. After mice were euthanized at 6 h or 12 h, respectively, serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were analyzed, and mRNA levels of TNF-αα, IL-6 and IL-1βwere tested. The activity of glutathione (GSH), oxidized GSH (GSSG) and malondialdehyde were quantifi ed. In addition, ASK1,P-ASK1, JNK and P-JNK protein levels were tested in all groups. Results: The ASK1 and P-ASK1 levels were up-regulated in APAP-induced group. Compared to the control group, serum levels of ALT and AST, and mRNA levels of TNF-αα, IL-6 and IL-1βwere increased in APAP-induced group. Meanwhile, the levels of MAD and GSSG, and the ratio of GSSG/GSH were higher and the JNK was activatedin APAP-induced group compared with that in control group. However, compared to APAP-induced group, GS combined with APAP-induced group displayed a decrease of protein expression levels of ASK1, P-ASK1 and P-JNK, a reduction of serum levels of ALT and AST, a decrease in TNF-αα, IL-6 and IL-1β mRNA levels, and a low ration of GSSG/GSH. Conclusions: GS-459679 treatment eff ectively down-regulates ASK1 and P-ASK1 expression. Addition of GS-459679 decreases the generation of liver metabolites and infl ammatory factors, reduces oxidative stress reaction, inhibits JNK activation, and then protects the responsiveness to APAP-induced liver injury.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Apoptosis signal-regulating kinase 1
Acetaminophen
Liver injury
JNK
1. Introduction
In recent years, drug-induced liver injury has become an important factor aff ecting the treatment eff ect and prognosis of patients in clinic, with paracetamol [acetaminophen (APAP)] induced liver injury as a typical representative[1-4]. High doses or frequent cumulative use of APAP can induce acute and severe liver tissue necrosis, and even cause liver failure and death. APAP-induced liver injury is mainly due to that metabolism of cytochrome P450 enzyme system produces excessive N-acetyl-p-benzo-quinone imine (NAPQI), leading to peroxidatic reaction of hepatic cellular mitochondrioneventually inducing the activation of MAP and JNK signal path[5,6], thus causing cell apoptosis or necrosis. Meanwhile, APAP activates the body’s immune cells to produce infl ammatory factors, thus activating immune system[2,7-10]. Therefore, to explore the molecular mechanism of APAP-induced liver injury and fi nd inhibiting effect of APAP-induced liver injury will provide animportant basis for development of drugsto prevent and control APAP-induced liver injury.
Apoptosis signal-regulating kinase 1 (ASK1) is one of the family members of mitogen-activated protein kinase kinase kinase. Multiple inflammatory factors and oxidative stress can activate ASK1 and its Ser-83 and Thr-845 phosphorylation. Activated ASK1 induces the activation of JNK downstream signaling pathways through MKK3 and MKK4[11], and finally leads to cell apoptosis by caspase 3 pathway[12,13]. Nakagawa et al found that the APAP-induced liver injury was decreased in ASK1 knockout mice through inhibiting JNK pathway activation, reducing serum level of alanine aminotransferase (ALT) and oxidative stress level and decreasing the numbers of APAP-induced liver cell apoptosis[9]. Xie et al reported that pretreatment of ASK1 inhibitor (GS-459679) lowered APAP-induced peroxide stress of cellular mitochondria and inhibited mitochondrial JNK activation[14]. However, the defensive function of ASK1 inhibitor on APAP-induced liver injury is still unclear. The present study aimed to discuss the protective effect of GS-459679 on APAP-induced liver injury and the preliminary research of its mechanism, which will provide new drug molecules for the exploitation of treatment of APAP-induced liver injury.
2. Materials and methods
2.1. Materials
Forty-eight male C57/BL6 mice of clean grade, aged 8 wk and weighted 20-25 g were purchased from Laboratory Animal Centre of Nanjing University. GS-459679 (ASK1 inhibitor) was bought from Gilead Sciences, Inc. Anti-bodies of ASK1, P-ASK1, JNK1 and P-JNK were all purchased from Abcam. cDNA reverse transcription kit (PrimeScript 1st Strand cDNA Synthesis Kit) and fl uorescence quantitative PCR kit (SYBR Green PCR Master Mix Kit) were bought from Takara Bio. Protein lysis buffer (RIPA) and protein quantitative kit were purchased from Thermo Fish. Glutathione (GSH) and malondialdehyde (MAD) assay kit were bought from Beyotime.
2.2. Methods
2.2.1. Acute liver injury model
Forty-eight male C57/BL6 mice were randomly divided into four groups (12 mice in each group), consisting of control group, GS group (GS-459679), APAP-induced group, and GS combined with APAP-induced group. Animals were fasting for 12 h before experiment and water ad libitum. Mice in control group were given intraperitoneal injection of PBS. GS group was treated with 30 mg/ kg GS-459679 dissolved in PBS. In APAP-induced group, 300 mg/ kg APAP was injected intraperitoneally. For GS combined with APAP-induced group, mice were treated with GS 30 min prior to administration of APAP. After administration of 6 h or 12 h, all the animals were sacrificed (3 mice each time). Blood was collected and livers were separated. Various indexes were detected to estimate the liver injury status. All the animal experiments complied with the animal ethics standards.
2.2.2. Effects on biochemical index
Blood was collected from eyeball of mice in all groups. After coagulation and keeping for 30 min, it was centrifuged at 3 000 rpm/min, 4℃ for (15-20) min. Precipitation was discarded and supernatant was taken. Contents of ALT and aspartate aminotransferase (AST) were determined through 7170A automatic biochemical analyzer. Liver tissues were taken to make into homogenate, and contents of GSH, oxidized GSH (GSSG) and MDA were tested according to kit instructions. MDA content was detected according to the MAD kit operational procedure.
2.2.3. Q-PCR method
Mice in all the groups were sacrificed under anesthesia. Livers were separated, and (50-100) mg liver issues were made into homogenate. Total RNA was extracted by phenol chloroform extracting method as follows: 1 mL Trizol was added in liver issue and kept for 50 min, and then 200 μL chloroform was added and mixed to stand for 10 min; after centrifugation at 10 000 rpm/min, 4℃ for 10 min, upper water phase was taken to add 1-fold volume of isopropyl alcohol and keep for 10 min; after centrifugation at 4℃ for 10 min, supernatant was discarded, and precipitation was washed with 75% ethanol and dried at room temperature. The extracted RNA was reversed to cDNA by two steps method according to the kit instruction, and the concentration of cDNA was detected by UV spectrophotometer. TNF-αα primer sequence: forward primer: GACGTGGAACTGGCAGAAGAG, reverse primer: TTGGTGGTTTGTGAGTGTGAG; IL-6 primer sequence: forward primer: CCAAGAGGTGAGTGCTTCCC, reverse primer: CTGTTGTTCAGACTCTCTCCCT; IL - 1β primer sequence: forward primer: GCAACTGTTCCTGAACTCAACT, reverse primer: ATCTTTTGGGGTCCGTCAACT. GAPDH was considered as internal reference. GAPDH primer sequence: forward primer: AGGTCGGTGTGAACGGATTTG, reverse primer: TGTAGACCATGTAGTTGAGGTCA. After standardization of cDNA, Q-PCR reaction system amplification was as follow: predegeneration at 94℃ for 5 min, degeneration at 94℃ for 30 s, anneal at 65℃ for 50 s, extension at 72℃ for 1 min, 30 cycles; extension at 70℃ for 10 min. mRNA levels of TNF-αα, IL-6 and IL-1β were detected by ABI 7900 HT Fast software.
2.2.4. Western blot method
About (100-200) g liver issue was taken and pyrolyzed by RIPA. After centrifugation at 12 000 rpm/min, 4℃ for 10 min, supernatantwas obtained. Total protein content was acquired according to protein quantitative kit steps. A total of (40-60) μg protein was taken to perform SDS-PAGE electrophoresis, trarsmembran, sealing, hatching primary antibodies (anti-ASK1, 1:1 500; anti-PASK1, 1:1 000; anti-JNK, 1:2 000; anti-P-JNK, 1:1 000; anti-ASK1, 1:1 500; anti-P-ASK1, 1:1 000; anti-JNK, 1:2 000; anti-P-JNK, 1:1 000), washing, hatching second antibodies and developing], expression levels of ASK1, P-ASK1, JNK and P-JNK were detected. GAPDH was the internal reference.
2.3. Statistical analysis
All the data were processed with Graphpad prism 5.0. Data were expressed as mean±SEM. t-test was used for the comparisons of ALT, AST, GSH, GSSD levels and mRNA levels of TNF-α, IL-6 and IL-1β between APAP-induced group and GS combined with APAP-induced group. P<0.05 was considered as statistically signifi cant.
3. Results
3.1. Effects of APAP-induced liver injury on ASK1 expression
As shown in Table 1, through western blot test, expression level of ASK1 and P-ASK1 in APAP-induced liver injury group increased; at the same time, GS-459679 signifi cantly decreased the protein levels of ASK1 and P-ASK1, which was consistent with the previous report[9]. Hence, GS-459679 could be used as an effective ASK1 inhibitor.

Table 1Inhibition of ASK1 and P-ASK1 levels by addition of GS-459679 [n (repeat count)= 3].
3.2. Effects of ASK1 inhibitor on serums of ALT and AST
Compared to control group, after 12 h of APAP-induced liver injury, levels of ALT and AST increased signifi cantly, while those in GS combined with APAP-induced group and APAP-induced group after 12 h of administration signifi cantly decreased (ALT: P<0.05; AST: P<0.05) (Table2).

Table 2Effects of ASK1 inhibitor on serum ALT and AST levels in the mice of APAP-induced liver injury [n (repeat count)=3].
3.3. Effects of ASK1 inhibitor on the contents of GSH, GSSG and MAD
As shown in Table 3, compared to normal control group, after 12 h of APAP induction, GSH level changed, GSSG level increased (P<0.05), GSSG/GSH ratio increased significantly (P<0.05), and MAD level increased (P<0.05). In GS combined with APAP-induced group, compared to APAP-induced group, there were no signifi cant difference in terms of GSH, GSSG and MAD levels; at the same time, GSSG/GSH ratio recovered signifi cantly (P<0.05). The results showed that ASK1 decreased the APAP-induced oxidative stress level.
3.4. Effects of ASK1 inhibitor on the mRNA level of inflammatory factors
Q-PCR detection showed that compared to control group (Table 4), the mRNA levels of TNF-αα, IL-6 and IL-1β were signifi cantly increased after 6 h of APAP induction (TNF-α: P<0.01; IL-6: P<0.01; IL-1β: P<0.01). Compared to APAP-induced group, the mRNA levels of TNF-ααand IL-1β in GS combined with APAP-induced group were signifi cantly decreased (TNF-αα: P<0.05; IL-1β: P<0.01), but were higher than those in control group. But, there was no significant differences in mRNA levels of IL-6 (P=0.052). Therefore, the decrease of ASK1 inhibitor was produced by APAP-induced infl ammatory factors.

Table 3Eff ects of ASK1 inhibitor on the levels of GSH, GSSG and MAD in the mice of APAP-induced liver injury [n (repeat count)=3].

Table 4Eff ects of ASK1 inhibitor on the mRNA levels of TNF-α, IL-6 and IL-1β in the mice of APAP-induced liver injury [n (repeat count)=3].
3.5. Effects of ASK1 inhibitor on JNK expression level
Table 5 showed that P-JNK level in GS group was slightly lower than that in control group, and it was higher in APAP-induced group compared to that in control group. However, P-JNK level in GS combined with APAP-induced group was signifi cantly lower than that in APAP-induced group, but was higher than that in control and GS groups. There were no significant differences in JNK expression levels among control, APAP-induced and GS combined with APAP-induced groups. The results showed that ASK1 inhibitor decreased the phosphorylation of JNK. What’s more, ASK1 inhibitor significantly decreased the JNK activation which was caused by APAP induction.
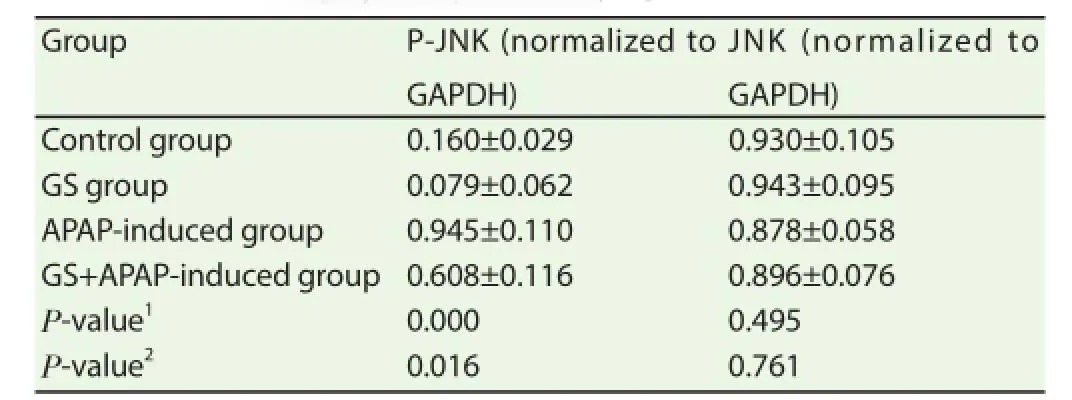
Table 5Eff ects of ASK1 inhibitor on protein levels of JNK and P-JNK in the mice of APAP-induced liver injury [n (repeat count)=3].
4. Discussion
ASK1 is serine/threonine-protein kinase, which activates P38 and JNK molecule through MAPK pathway activation[15]. Under resting state, thioredoxin (Trx) bonded the N-terminal domains of ASK1 to form Trx-ASK1 compound, thus inhibiting ASK1 activation. Under the external stimulation (such as oxidative stress and infl ammatory factors), Trx and ASK1 were separated to cause ASK1 activation, thus inducing the MAP2 phosphorylation and downstream JNK activation[9,16]. Study of Nakagawa et al showed that ASK1-/- mice eff ectively protect APAP-induced liver injury through inhibiting JNK activation[9]. Xie et al found that pretreatment of ASK1 inhibitor (GS-459679) lowered APAP-induced peroxide stress of cellular mitochondria and inhibited mitochondrial JNK activation[14]. On the basis of the result, the present study further illuminated the mechanism of effect of ASK1 inhibitor on APAP-induced liver injury. Our study showed that GS-459679 can decrease the APAP-induced serum transaminase levels (ALT and AST), reduce oxidative stress product (GSSG), inhibit the expressions of inflammatory factors (TNF-αα, IL-6 and IL-1β), and JNK activation, and thus protect APAP-induced liver injury.
When excessive APAP is ingested, most of the drugs are combined with glucuronic acid and expelled from the body, while a small part of drugs generate NAPQI by oxidation reaction of cytochrome P450 enzyme system; after exhausting GSH, NAPQI is combined with sulfydryl-containing proteins, thus causing oxidative stress and dysfunction of mitochondria and fi nally inducing afunction of liver cells[7,8,17,18]. Hence, inhibition of oxidative stress is the key to protect APAP-induced liver injury. It was found that the oxidant and antioxidant balance (GSSG/GSH) of liver cells was broken in APAP-induced liver injury. After the pretreatment of ASK1 inhibitor, GSSG level and GSSG/GSH ratio were decreased, that is to say, the balance of the redox reaction of liver cells got certain recovery. Hyun also reported that ASK1 inhibitor can decrease the ROS level of liver cells, reduce the output of lipid oxidation products and inhibit oxidative stress[19].
Many literatures reported that APAP-induced liver cell apoptosis and necrosis can further activate immune cells such as NK and neutrophile granulocyte which secrete a variety of infl ammatory factors, and then accelerate the failure of liver function[9,10,20]. Nakagawa et al reported that in APAP-induced liver injury model of ASK1-/- mice, expression levels of IL-1αα and IL-1β were all reduced[9]. It is found in our study that compared to APAP-induced group, TNF-α, IL-6 and IL-1β in GS combined with APAP-induced group decreased by diff erent degrees, which indicated that ASK1 inhibitor can also reduce products of infl ammatory factors and lower the body’s immune response.
Mitochondrial oxidative stress in APAP-induced liver injury eventually activates JNK pathway induced liver cell apoptosis or necrosis[5]. ASK1 is the upstream signal molecule of JNK, which can activate JNK into the nucleus by the activation of MKK3 and MKK4, thus causing apoptosis[11]. Hayakawa et al reported that ASK1 inhibitor (K188) decreased the proliferation and tumor size of gastric carcinoma cells through inhibiting JNK phosphorylation[12]. Xie etal found that GS-459679 reduced the JNK activator positioning of mitochondria, and GS-459679 protected APAP-induced liver injury through inhibiting JNK pathway[14]. Our study also showed that GS-459679 inhibited JNK phosphorylation, which further illustrates that the protective eff ect of ASK1 inhibitor on APAP-induced liver injury depends on activation inhibition of JNK.
In conclusion, ASK1 inhibitor (GS-459679) can protect the APAP-induced liver injury through eliminating toxic metabolite of liver, inhibiting oxidative stress, removing immune response and inhibiting JNK activation at the molecular level. However, the therapeutical eff ect of GS-459679 still needs to be further confi rmed, and therapeutic window of GS-459679 use needs to be intensively studied.
Confilict of interst statement
We declare that we have no confl ict of interest.
References
[1] Grant LM, Rockey DC. Drug-induced liver injury. Curr Opin Gastroenterol 2012; 28(3): 198-202.
[2] Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol 2011; 8(4): 202-211.
[3] Lilly LB. Drug-induced liver disease. In: Heathcote EJ, editor. Hepatol diagnosis and clinical management. Oxford: Wiley-Blackwell; 2012. p. 235-243.
[4] Yuan L, Kaplowitz N. Mechanisms of drug-induced liver injury. Clin Liver Dis 2013; 17(4): 507-518.
[5] Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 2010; 246(1-2): 8-17.
[6] Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci 2013; 34(4): 243-253.
[7] Xie Y, Mcgill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, et al. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol 2014; 279(3): 266-274.
[8] Jaeschke H, Mcgill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 2012; 44(1): 88-106.
[9] Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 2008; 135(4): 1311-1321.
[10] Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 2011; 26(Suppl 1): 173-179.
[11] Liang T, Zhang X, Xue W, Zhao S, Zhang X, Pei J. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int JMol Sci 2014; 15(9): 15754-15765.
[12] Hayakawa Y, Hirata Y, Sakitani K, Nakagawa H, Nakata W, Kinoshita H, et al. Apoptosis signal-regulating kinase-1 inhibitor as a potent therapeutic drug for the treatment of gastric cancer. Cancer Sci 2012; 103(12): 2181-2185.
[13] Niso-Santano M, González-Polo RA, Bravo-San Pedro JM, Gómez-Sánchez R, Lastres-Becker I, Ortiz-Ortiz MA, et al. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radic Biol Med 2010; 48(10): 1370-1381.
[14] Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, et al. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol 2015; 286(1): 1-9.
[15] Watanabe T, Sekine S, Naguro I, Sekine Y, Ichijo H. Apoptosis signalregulating kinase 1 (ASK1)-p38 pathway-dependent cytoplasmic translocation of the orphan nuclear receptor NR4A2 is required for oxidative stress-induced necrosis. J Biol Chem 2015; 290(17): 10791-10803.
[16] Yu Y, Richardson DR. Cellular iron depletion stimulates the JNK and p38 MAPK signaling transduction pathways, dissociation of ASK1-thioredoxin, and activation of ASK1. J Biol Chem 2011; 286(17): 15413-15427.
[17] Mcgill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 2012; 264(3): 387-394.
[18] Xie Y, Williams CD, Mcgill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by infl ammasome activation. Toxicol Sci 2013; 131(1): 325-335.
[19] Hyun MS, Hur JM, Mun YJ, Kim D, Woo WH. BBR induces apoptosis in HepG2 cell through an Akt-ASK1-ROS-p38MAPKs-linked cascade. J Cell Biochem 2010; 109(2): 329-338.
[20] Pires DA, Marques PE, Pereira RV, David BA, Gomides LF, Dias ACF, et al. Interleukin-4 defi ciency protects mice from acetaminophen-induced liver injury and infl ammation by prevention of glutathione depletion. Inflamm Res 2014; 63(1): 61-69.
Document heading 10.1016/j.apjtm.2016.01.029
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Wen-Guang Tian, Master, Associate Chief Physician, Associate Professor, master's supervisor, Associate Head of Department, Department of Infectious Diseases, Yongchuan Hospital of Chongqing Medical University, No. 439, XuanHua Road, Yongchuan District, Chongqing 402160, China.
Tel: 023-85381658; 13883210666
E-mail: twg9366@163.com
Foundation project: It is supported by Soft Science Foundation of Yongchuan District of Chongqing City (Grant No.YCSTC, 2011BE5015).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Immune formulation-assisted conventional therapy on anti-infective effectness of multidrug-resistant Mycobacterium tuberculosis infection mice
- Effect of dimethyl fumarate on rats with chronic pancreatitis
- Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Effect of miR-467b on atherosclerosis of rats
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
