Effect of dimethyl fumarate on rats with chronic pancreatitis
2016-07-24WenXianZhangJianHongZhaoFuMinPingZhiJunLiuJunXiaGuXinQingLuAffiliatedHospitalofHebeiUniversityofEngineeringHandanHebei056002China
Wen-Xian Zhang, Jian-Hong Zhao, Fu-Min Ping, Zhi-Jun Liu, Jun-Xia Gu, Xin-Qing LuAffiliated Hospital of Hebei University of Engineering, Handan, Hebei 056002, China
Contents lists available at ScienceDirect
Effect of dimethyl fumarate on rats with chronic pancreatitis
Wen-Xian Zhang, Jian-Hong Zhao, Fu-Min Ping*, Zhi-Jun Liu, Jun-Xia Gu, Xin-Qing Lu
Affiliated Hospital of Hebei University of Engineering, Handan, Hebei 056002, China
ABSTRACT
Objective: To discuss the eff ect of dimethyl fumarate (DMF) on rats with L-arginine induced chronic pancreatitis (CP). Methods: Male Wistar rats were given DMF treatment (25 mg/kg) by oral lavage method; then Wistar rats were given the intraperitoneal injection of L-arginine for 5 times (250 mg/100kg, twice per time, each interval of 1 h) for building of CP model. Rats were divided into control group, CP group, DMF group and CP+DMF group. Rats in CP+DMF group were given the oral intragastric administration of DMF (25 mg/kg), while rats in control group and CP group were given the equal volume of normal saline. The weight of rats was evaluated and the intraperitoneal glucose tolerance test was performed (IPGTT, 2 g/kg). The islet of rats was isolated and then fl ow cytometry was employed to evaluate the quality and activity of islets. Meanwhile, the histology of non-endocrine tissues and levels of myeloperoxidase (MPO) and malondialdehyde (MDA) were detected. Results: Compared with control group, the weight of rats in CP group was signifi cantly reduced at week 2, 4 and 6; the blood glucose signifi cantly increased, AUC increased, the histopathological scores of pancreatic atrophy, acinar injury, edema and cellular infi ltration increased, levels of MDA and MPO increased, the islet equivalent and islet activity decreased at 0, 30, 60, 120 and 180 min. Compared with CP group, the weight of rats in CP+DMF group signifi cantly increased at week 2, 4 and 6; the blood glucose signifi cantly decreased, AUC decreased, the histopathological scores of pancreatic atrophy, acinar injury, edema and cellular infi ltration decreased, levels of MDA and MPO decreased, the islet equivalent and islet activity increased at 0, 30, 60, 120 and 180 min. Conclusions: DMF treatment can improve CP induced by L-arginine and islet function in rats.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Dimethyl fumarate
Chronic pancreatitis
Rats
Mechanism
1. Introduction
Chronic pancreatitis (CP) is a kind of progressive infl ammatory diseases, which would cause the damage and fi brosis of pancreas parenchyma and eventually lead to endocrine and exocrine function obstacle[1-3]. Statistics indicated that CP incidence was still increasing[4]. In a structured questionnaire, CP patients indicated CP disease had the severe impact on their body, social and psychological health[5]. At present, the main therapeutic method for CP is supportive care, including nutrition and pain control[6,7]. But the specifi c pathogenesis has not been clear and thus the clinical eff ect of main treatments was poor[8,9].
Dimethyl fumarate (DMF) is used in the treatment of patients with multiple sclerosis[10,11]. The mechanism for DMF has not been clear yet. However, its unique antioxidative and anti-infl ammatory features have already been proved[12-14]. CP could induce the oxidative stress and infl ammation response in islet endocrine and non-endocrine cells. Thus in this study, it’s presumed that DMF might inhibit the development of CP through the antioxidant and anti-inflammatory response. By building the rat model of CP and feeding rats with the certain dose of DMF, it is to discuss the inhibition and mechanism of DMF on the infl ammation and oxidative stress of rats with CP.
2. Materials and methods
2.1. Materials
2.1.1. Animals
Male Wistar rats (250-300 g) were purchased from HebeiUniversity of Engineering laboratory, and all rats were raised in a sterile environment at (22±2)℃, free access to eating and drinking, with fasting for 12 h before experiment.
2.1.2. Reagents
L-arginine and DMF were purchased from Sigma; HE, TMRE and 7-AAD dye from Sigma; malondialdehyde (MDA) kit and myeloperoxidase (MPO) kit from Nanjing Jiansheng Biological Products Research Institute; collagenaseV from Sigma; dithizone (DTZ) from Shanghai No.3 Reagent Factory; rabbit-derived polyclonal insulin and glucagonantibody from Santa.
2.2. Methods
2.2.1. Building of CP model
L-arginine was dissolved in NS (20%), with the intraperitoneal injection of L-arginine (250 mg/100 kg, twice per time, every interval of 1 h) for 5 times (day 1, 5, 9, 13 and 17); after 7 weeks, rats were put to death. Feom 24 h before building of CP model, rats were given DMF treatment (25 mg/kg) by oral lavage method. Rats in the control group and CP group were given the equal volume of normal saline. After 7 weeks of administration, rats were put to death.
2.2.2. Intraperitoneal glucose tolerance (GT) test (IPGTT)
At week 7 of modeling, the intragastric administration of 2 g/kg glucose was performed and the blood was collected from the caudal vein. The trace glucose meter was employed to detect the blood glucose of rats at fasting, 30, 60, 90, 120 and 180 min after dining, as well as the area under the curve (AUC), AUC=1/2 fasting value + value at 1 h + value at 2 h + 1/2 value at 3 h.
2.2.3. Histological analysis
After 7 weeks, rats were put to death. Then the pancreas of rats was taken out for stabilization and the biochemical analysis was performed. Firstly, pancreas was fixed with 10% of formalin, embedded with paraffin, sliced into 5 mm pieces and then HE staining was conducted. Schmidt standard was adopted to evaluate the histological analysis based on the area of lesions, alveolus atrophy, acinar cell damage, fibrosis, interstitial edema and infl ammatory cell infi ltration.
2.2.4. Pancreas MDA and MPO detection
The partial pancreatic tissues of rats were collected for weighing. After that, the equivalent NS was added and l% of homogenate (made from low temperature homogenate machine) was used for being centrifuged at room temperature (3 000 g/min, 10 min) to obtain the supernatant. Finally, MDA content was detected according to the procedure of MDA kit. The extra partial pancreatic tissues of rats were collected for weighing. After that, the equivalent NS was added and 1% of homogenate (made from low temperature homogenate machine) was used for being centrifuged at room temperature (3 000 g/min, 10 min) to obtain the supernatant. Finally, MDA content was detected using the chemoenzymatic method. Operations should be strictly in accordance with the instruction manual.
2.2.5. Islet Isolation and Endocrine Function Evaluation
After taking out of the pancreas and weighing, common bile duct puncture and injection of cold collagen V (10 mL) digestive juices were conducted, which could make the pancreas adequate swelling. Afterwards, pancreas was put into cold collagen V (5 mL) digestive juices and digested in 37℃ water bath for 10 min; then Hanks solution (contained 10% of fetal calf serum) was used for digestion termination, mesh screen fi ltration, centrifugal collecting of the islet. 50 μL of DTZ working solution was mixed with 50 μL of islet for 5 min. The cell mass numbers of scarlet DTZ staining was counted under the microscope, which was equivalent to the (150 μm in diameter) islet equivalent quantity according to formula conversion and then the total obtained islet equivalent quantity was calculated.
2.2.6. Islet activity evaluation
Cultured islet was separated into single-cell suspension liquid. Cells were placed into flow cytometry instrument for TMRE staining after being washed. TMRE was selectively combined with mitochondrial membrane to detect the cell apoptosis. After being washed, cell 7-AAD was stained. 7-AAD combined with cell DNA indicated the cell membrane permeability changes after cell death.
2.2.7. Islet alpha/beta cell ratio
Single-cell suspension liquid was placed in 4% of paraformaldehyde and was fi xed for 10 min at room temperature. After continuous PBS washing, cell was incubated by polyclonal insulin in rabbit and anti-Glucagon. Afterwards, the goat-anti-rabbit secondary antibody was used for marking and the fl ow cytometry (FCM) was employed for the analysis.
2.2.8. Statistical analysis
Data was expressed by mean ± SD. The t test or one-way analysis of variance was employed for the comparison between groups, where P<0.05 indicated the statistical diff erence.
3. Results
3.1. Effect of DMF on weight of rats with CP
As shown in Table 1, as the time of modeling passed, the weight of rats in control group, CP group and CP+DMF group signifi cantlyincreased, but the weight of rats in CP group was signifi cantly lower than that in control group at week 2; the weight of rats in CP+DMF group was signifi cantly higher than that in CP group (P< 0.05). The same situation was found at week 4 and 6. It indicated that, as the time of modeling passes, the weight of CP rats increased slowly, which was lower than that in the normal group. After being given GMF, the weight of CP rats was recovered gradually.
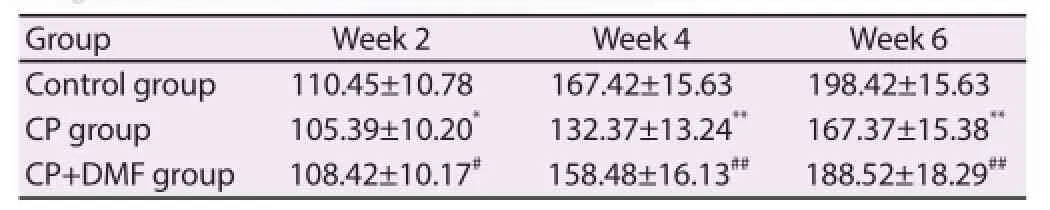
Table 1Weight changes in each group (g).
3.2. Improvement of DMF for GT in rats with CP
The results showed that the blood glucose level in CP group was signifi cantly higher than that in the model group; while the blood glucose level in CP+DMF group was signifi cantly lower than that in CP group (P<0.01). Meanwhile, the same situation was found at 30 and 60 min. But at 120 and 180 min, the blood glucose level in control group, CP group and CP+DMF group was decreased a bit, but the one in CP group was still signifi cantly higher than that in the model group and the one in CP+DMF group was signifi cantly lower than that in CP group (P<0.01) (Table 2).
The results showed that AUC value in CP group, (22.46±2.50) mmol/L•h-1, was signifi cantly higher than that in the control group, (15.70±1.45) mmol/L•h-1; while the one in CP+DMF group, (18.02±1.82) mmol/L•h-1, was significantly lower than that in CP group (P<0.01).

Table 2IPGTT results in each group (mmol/L).
3.3. Improvement of DMF for histopathology in rats with CP
L-arginine could cause pancreatic histologic changes in rats, including serious pancreatic acinar structure damages, edema and fatty infiltration. Oral administration of DMF treatment could significantly improve the above symptoms. As shown in Table 3, the histopathological score of the area of lesions, acinar cell damage, edema and inflammatory cell infiltration in CP group was signifi cantly higher than that in the control group; while the histopathological score in CP+DMF group was signifi cantly lower than that in CP group (P<0.01).

Table 3Histopathological score of rats in each group.
3.4. Improvement of DMF for biochemical parameter in rats with CP
Compared with the control group, the levels of MPO and MDA in CP group significantly increased. After the treatment of DMF, the levels of MPO and MDA in CP group signifi cantly decreased. The islet equivalent and islet activity in CP group were signifi cantly lower than those in the control group; while the islet equivalent and islet activity in CP+DMF group were signifi cantly higher than those in CP group (Table 4).
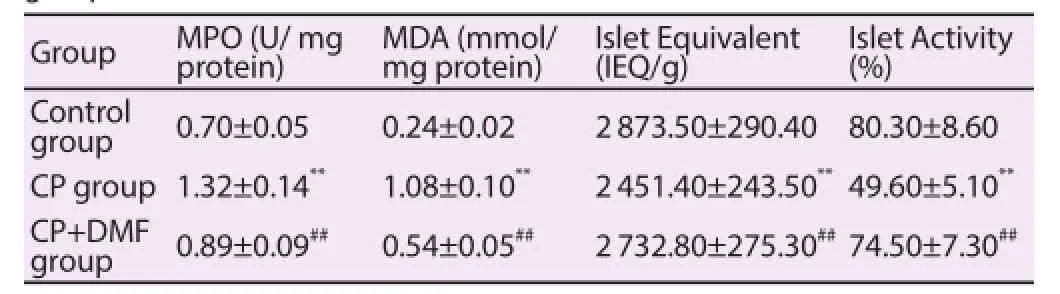
Table 4Levels of MPO and MDA and islet equivalent and islet activity of rats in each group.
4. Discussion
The results indicated the obvious diff erence of pathology, histology and pancreas biochemical markers between CP+DMF group and CP group. The weight of rats in CP+DMF group and CP group at week 2, 4 and 6 was signifi cantly higher than that in CP group; while the blood glucose level at 0, 30, 60, 120 and 180 min was signifi cantly lower than that in CP group and the histopathological score of the area of lesions, acinar cell damage, edema and infl ammatory cell infiltration was significantly lower than that in CP group, which indicated that DMF could significantly improve the pathological changes of pancreas, the blood glucose level and weight of CP rats. The results of comparison in the degree of fi brosis between CP group and control group showed no signifi cant diff erence, but it showed the certain tendency of damage. Besides, DMF could also improve the fibrosis of pancreas of L-arginine induced CP rats. The flow cytometry was employed to evaluate the quality and activity of islets. The results also indicated that DMF had the obvious protection forthe islet of L-arginine induced CP rats.
DMF had unique antioxidative and anti-inflammatory feature. Although mechanism of DMF was not clear, its side-effect was smaller, which made its use for more than 20 years. Fumarate was mainly used in the treatment of psoriasis, which was based on its inhibition of lymphocyte proliferation. Recent reports found that DMF selectively increased Th2 cytokines and inhibited Th1 responses[15]. There were also researches showing that DMF decreased the gene expression of pro-infammatory cytokines and chemotactic factors, and increased the expression of antioxidant molecules[16-18]. These findings suggested that DMF could be used in the treatment of autoimmune and infl ammatory diseases, including multiple sclerosis. Other scholars reported that DMF played its antioxygenation role through Nrf2 activator[14,15]. DMF was likely to have multiple eff ective anti-oxidation mechanisms.
MPO is the marker of inflammatory stress, while MDA is the by-product of lipid peroxidation, as the marker of cell injury and oxidative stress. The results of this study indicated that the levels of MPO and MDA was significantly increased in CP group. After the treatment of DMF for CP rats, the levels of MDA and MPO signifi cantly decreased, which indicated that DMF could improve the infl ammatory and oxidative stress of CP rats. Other researches also found that the antioxidant eff ect of DMF could protect the islet from the eff ect of L-arginine induced infl ammatory response[19,20]. It also indicated that DMF could reduce the levels of MPO and MDA to inhibit the infl ammatory and oxidative stress of CP rats and thus protect the islet.
In conclusion, long-term DMF treatment can eff ectively improve the histopathological changes, biochemical abnormalities and beta cell function in rats with CP. In the further studies, it is necessary to confi rm the effi cacy and side eff ects of DMF in the clinical practice.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Chiang KC, Chen TH, Hsu JT. Management of chronic pancreatitis complicated with a bleeding pseudoaneurysm. World J Gastroenterol 2014; 20(43): 16132-16137.
[2] Khristich TN. Persistence of chronic infl ammatory responses, role in the development of chronic pancreatitis, obesity and pancreatic cancer. Lik Sprava 2014; (11): 3-10.
[3] Sun XT, Li ZK. Research progress of CP cause and hazards and its pathogenesis. Chinese J Pancreatol 2013; 13(6): 430-432.
[4] Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010; 24(3): 219-231.
[5] Fitzsimmons D, Kahl S, Butturini G. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol 2005; 100(4): 918-926.
[6] Piciucchi M, Capurso G, Archibugi L. Exocrine pancreatic insuffi ciency in diabetic patients: prevalence, mechanisms, and treatment. Int J Endocrinol 2015; 20(15): 595649.
[7] Muniraj T, Aslanian HR, Farrell J. Chronic pancreatitis, a comprehensive review and update. Part Ⅱ: Diagnosis, complications, and management. Dis Mon 2015; 61(1): 5-37.
[8] Sharma V, Rana SS, Bhasin DK. Medical management of pain in chronic pancreatitis. Trop Gastroenterol 2014; 35(4): 205-211.
[9] Chen JM, Ferec C. Genetics and pathogenesis of chronic pancreatitis. Clin Res Hepatol Gastroenterol 2012; 36(5): 334-340.
[10] Sheremata W, Brown AD, Rammohan KW. Dimethyl fumarate for treating relapsing multiple sclerosis. Expert Opin Drug Saf 2015; 14(1): 161-170.
[11] Bomprezzi R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 2015; 8(1): 20-30.
[12] Ashrafi an H, Czibik G, Bellahcene M. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab 2012; 15(3): 361-371.
[13] Ghoreschi K, Bruck J, Kellerer C. Fumarates improve psoriasis and multiple sclerosis by inducing type Ⅱ dendritic cells. J Exp Med 2011; 208(11): 2291-2303.
[14] Kihara Y, Groves A, Rivera RR. Dimethyl fumarate inhibits integrin α4 expression in multiple sclerosis models. Ann Clin Transl Neurol 2015; 2(10): 978-983.
[15] Gill AJ, Kolson DL. Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol 2013; 33(4): 307-359.
[16] Seidel C, Bicker G. Developmental expression of nitric oxide/cyclic GMP signaling pathways in the brain of the embryonic grasshopper. Brain Res Dev Brain Res 2002; 138(1): 71-79.
[17] Kees F. Dimethyl fumarate: a Janus-faced substance. Expert Opin Pharmacother 2013; 14(11): 1559-1567.
[18] Zhao X, Sun G, Zhang J. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke 2015; 46(7): 1923-1928.
[19] Huang H, Taraboletti A, Shriver LP. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol 2015; 29(5): 169-175.
[20] Jing X, Shi H, Zhang C. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 2015; 28(6): 131-140.
Document heading 10.1016/j.apjtm.2016.01.023
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Fu-Min Ping, Master’s Degree, Affi liated Hospital of Hebei University of Engineering, No.81, Congtai Road, Handan, Hebei 056002, China.
Tel: 18031028169
E-mail: pfum@163.com
Foundation project: It was supported by the College Science Technology Research Project of Hebei Province (Grant No. ZD20131002).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of miR-467b on atherosclerosis of rats
- Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
