Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
2016-07-24JunLuYouTingJuChangLiFuZhouHuaGuoHaiXuYanHuiHuDepartmentofAnesthesiologyTheSecondAffiliatedHospitaltoNanchangUniversityNanchangJiangxi330006China
Jun Lu, You-Ting Ju, Chang Li, Fu-Zhou Hua, Guo-Hai Xu, Yan-Hui HuDepartment of Anesthesiology, The Second Affiliated Hospital to Nanchang University, Nanchang, Jiangxi 330006, China
Contents lists available at ScienceDirect
Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
Jun Lu*, You-Ting Ju, Chang Li, Fu-Zhou Hua, Guo-Hai Xu, Yan-Hui Hu
Department of Anesthesiology, The Second Affiliated Hospital to Nanchang University, Nanchang, Jiangxi 330006, China
ABSTRACT
Objective: To study the effects of Transient receptor potential cation channel, subfamily V, member 1 (TRPV1) combined with lidocaine on status and apoptosis of U87-MG glioma cell line, and explore whether local anesthetic produces neurotoxicity by TRPV1. Methods: U87-MG cells were divided into control group, gene silencing group, empty vector group and TRPV gene up-regulation group. For cells in each group, fl ow cytometry was employed to detect the intracellular calcium ion concentration and mitochondrial membrane potential at diff erent time point from cellular perspective. Cell apoptosis of U87-MG was assayed by fl ow cytometry and MTT from a holistic perspective. Results: Calcium ion concentration increased along with time. The concentration in TRPV1 gene up-regulation group was signifi cantly higher than those in other groups at each time point (P<0.05). After adding lidocaine, mitochondrial membrane potential in U87-MG signifi cantly increased (P<0.05). This increasing trend in TRPV1 gene up-regulation group was more signifi cant than other groups (P<0.05), while in TRPV1 gene silencing group, the trend signifi cantly decreased (P<0.05). Flow cytometry result and MTT result both showed that cell apoptosis in each group signifi cantly increased after lidocaine was added (P<0.05). This increasing trend in TRPV1 gene up-regulation group was more signifi cant than other groups (P<0.05), while in TRPV1 gene silencing group, the trend significantly decreased (P<0.05). Moreover, apoptosis was more severe along with the increasing concentration of lidocaine (P<0.05). Conclusions: In this study, it was proved that lidocaine could dose-dependently induce the increase of intracellular calcium ion concentration, mitochondrial membrane potential and apoptosis in U87-MG glioma cell line. The up-regulation of TRPV1 enhanced cytotoxicity of lidocaine, which revealed the correlations between them. Lidocaine might have increased intracellular calcium ion concentration by activating TRPV1 gene and induced apoptosis of U87-GM glioma cell line by up-regulating mitochondrial membrane potential.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
TRPV1
Lidocaine
U87-MG
Glioma
Cell state
Apoptosis
1. Introduction
Nerve block is often used in clinical anesthesia, which can keep patients awake, recover anesthesia smoothly, be easy of postoperative analgesia, and save medical costs. However, with the extensive application of nerve block anesthesia, the reports of local anesthetics (LA) causing neurotoxic effects gradually increased, and caused great concern[1]. A multi-center prospective study investigated 41 251 cases of spinal anesthesia patients, 35 379 cases of epidural anesthesia patients and 1 474 cases of patients with combination of epidural anesthesia, showing serious neurological complication rate of 0.018%[2,3]. Capdevila et al[4] reported that in 1 416 cases of patients treated with peripheral nerve block anesthesia, the occurrence of neuropathy was 0.21%. LA-caused nerve cell damage has correlations with dose, concentration and time, showing swelling of nerve cells, nerve demyelination, degeneration, mitochondrial swelling dissolution and apoptosis[5]. Currently, the ultrastructure of LA cause nerve tissue cells, metabolism, electrical physiological damage and how to prevent neurotoxic LA has become a hot topic in current clinical related disciplines.
Transient receptor potential cation channel, subfamily V, member 1 (TRPV1), one of the transient voltage receptor family members, is protein encoded by the TRPV1 gene[6]. TRPV1 is a ligand-gated non-selective cation channel protein mainly expressed in the dorsal root ganglion and trigeminal ganglion neurons. It is also present in sensory neurons, the aff erent nerve fi bers and the central nervous system, which can be activated by capsicin and inflammatory mediators, also known as the capsaicin receptor[7-9]. When TRPV1 channel proteins are activated, they mainly cause the calcium ion influx, adjusting corresponding physiological function or pathological mechanisms in the form of increasing the intracellular calcium ion concentration. Studies have shown that TRPV1 plays an important role in the pathological process of the generation of pain and enhancement of pain sensitivity[10]. Additionally, Leffl er et al[11,12] demonstrated that on behalf of local anesthesia drugslidocaine, activates TRPV1, and results in calcium ion concentration increasing in dorsal root ganglion cells and releasing of excitatory amino acids by interacting with TRPV1 capsaicin binding sites. However, in LA-induced nerve cell toxic damage, what factors started intracellular calcium overload? What is the role of TRPV1 channel protein in the apoptosis induced by LA? Whether inhibiting the expression of TRPV1 channel protein can prevent LA-induced nerve cell toxic damage? It is unclear.
Therefore, this study attempted to detect intracellular calcium ion concentration and other indicators when U87-MG glioma cell line was damaged by LA by using gene transfection and RNAi technique to up-regulate and silence TRPV1 gene, and then investigated its eff ect on mitochondrial membrane potential (JC-1) and apoptosis situation combining lidocaine, in order to explore whether LA producing neurotoxicity via TRPV1, aiming to discuss the molecular mechanism of LA neurotoxicity for providing theoretical basis to LA neurotoxicity prevention in depth.
2. Material and methods
2.1. Cell obtaining and culture conditions
The glioma U87-MG cell lines were kindly purchased from Hanbio Company (Shanghai, China). The cell lines were cultured and maintained in RPMI 1640 medium (Gibco, Life Technologies, UK) with 10% fetal bovine serum (FBS, Gibco) and containing 10% calf serum, 100 U/mL, 100 g/mL of penicillin streptomycin selection in the culture solution. The culture condition was set in 37℃ with 5% CO2in a humidifi ed atmosphere and the melanoma stem cell lines were routinely checked in case of mycoplasma infection.
2.2. Grouping and treatment
Glioma U87-MG cells were cultured in DMEM medium containing 10% fetal bovine serum at 37℃, 5% CO2and in saturated humidity condition. The plasmid was established and preliminarily identifi ed according to previous articles. Cells in logarithmic growth phase were seeded in 24-well culture plate. The cells was grouped and treated when the bottom were covered 70%.
Then glioma U87-MG cells were randomized into 8 groups: (1) U87-MG-scramble: control group; (2) U87-MG-shTRPV1 group: the cells transfected with shTRPV1; (3) U87-MG-Vector group: the cells transfected with empty vector; (4) U87-MG-TRPV1 group: the cells transfected with TRPV1; (5) U87-MG-scramble-lidocaine: control group along with lidocaine treatment; (6) U87-MG-shTRPV1-lidocaine group: the cells transfected with shTRPV1 along with lidocaine treatment; (7) U87-MG-Vector-lidocaine group: the cells transfected with empty vector along with lidocaine treatment; (8) U87-MG-TRPV1-lidocaine group: the cells transfected with TRPV1 along with lidocaine treatment. The specific transfecting steps refer to Lipofectamine 2000 instructions.
2.3. Measurement of intracellular calcium ions by flow cytometry
Fluo-3 is a fl uorescein-based calcium probe which allows fl ow cytometric measurement of calcium on instruments that are not equipped with a UV light source. Glioma U87-MG cells collected from every group at time point of 0, 1, 2, 3, 4 and 5 h were centrifuged at 180××g (950 rpm in Beckman TJ-6) in 12 mm×75 mm polypropylene tube for 6 min at room temperature, and resuspended in cell loading medium of (106-107) cells/mL. Then 10 mg/mL fluo-3 AM at (3-4) μg/mL final was added. Cells were centrifuged for 6 min at 180×g, room temperature. Pellet was gently resuspended in cell loading medium at cell concentration (about 3×106/mL). Cells were stored at room temperature and protected from light until analysis. Flow cytometer was set up according to previous protocols.
2.4. Detection of mitochondrial transmembrane potential
JC-1 is an ideal cationic lipid fl uorescent dye which can detect mitochondrial membrane potential. JC-1 can accumulate in cell at the state of monosome through normal cell membrane. Healthy mitochondrial membrane potential has polarity, so JC-1 is taken up rapidly into the mitochondria, and polymer formed in the mitochondria because of increasing concentration. The emitted light of the polymer is red fl uorescence that can be detected by red channel of flow cytometry. When apoptosis occurs to cell, mitochondrial membrane potential is depolarized. JC-1 is released from the mitochondria, lumining green fl uorescence in the form of monosome in the cytoplasm. And red light intensity decreased. Flow cytometry can be used to detect changes in mitochondrial membrane potential in U87-MG glioma cells of diff erent groups based on this feature.
2.5. Apoptosis calculation by flow cytometry with PI staining
PI staining was employed to quantify the apoptosis of gliomaU87-MG cells in each group. Briefly, cells were seeded in 6-well plates (2×105cells/mL) then stained using PI (Kaiji Co., Ltd. Nanjing, China) fluorescence apoptosis detection kit following the manufacturer’s instruction. Samples were analyzed using a FACSCalibur fl ow cytometer within 1 h after the staining.
2.6. MTT assay
Glioma U87-MG cells from each group were seeded in a 96-well tissue culture plate at a density of 1×104cells per well. Cell proliferation of each treatment group was determined by MTT assay performed for 72 h. Absorbance was read by using an ELx800 absorbance microplate reader (Bio-Tek Instruments, Winooski, VT) at 570 nm. The values of the treated cells were calculated as percentages of the untreated control.
2.7. Statistical analysis
All data are presented as the mean±SD. SPSS 16.0 software (SPSS, Chicago, IL, USA) was employed to determine the statistical signifi cance between samples by using analysis of variance and Dunnett’s t-tests.
3. Results
3.1. Enhanced TRPV1 gene caused increase of intracellular calcium ion concentration in U87MG glioma cells
After group 1-4 were treated, intracellular calcium ion concentration in U87MG glioma cells was detected in different period of time. There were varying degrees of increasing calcium ion concentration in each group along with the time. In TRPV1 gene up-regulation group, the calcium ion concentration in U87MG glioma cells was signifi cantly higher than those in other groups at the same time point (P<0.05). While in TRPV1 gene silencing group, the calcium ion concentration in U87MG glioma cells was signifi cantly lower than those in other groups at the same time point (P<0.05) ( Figure 1-4.).
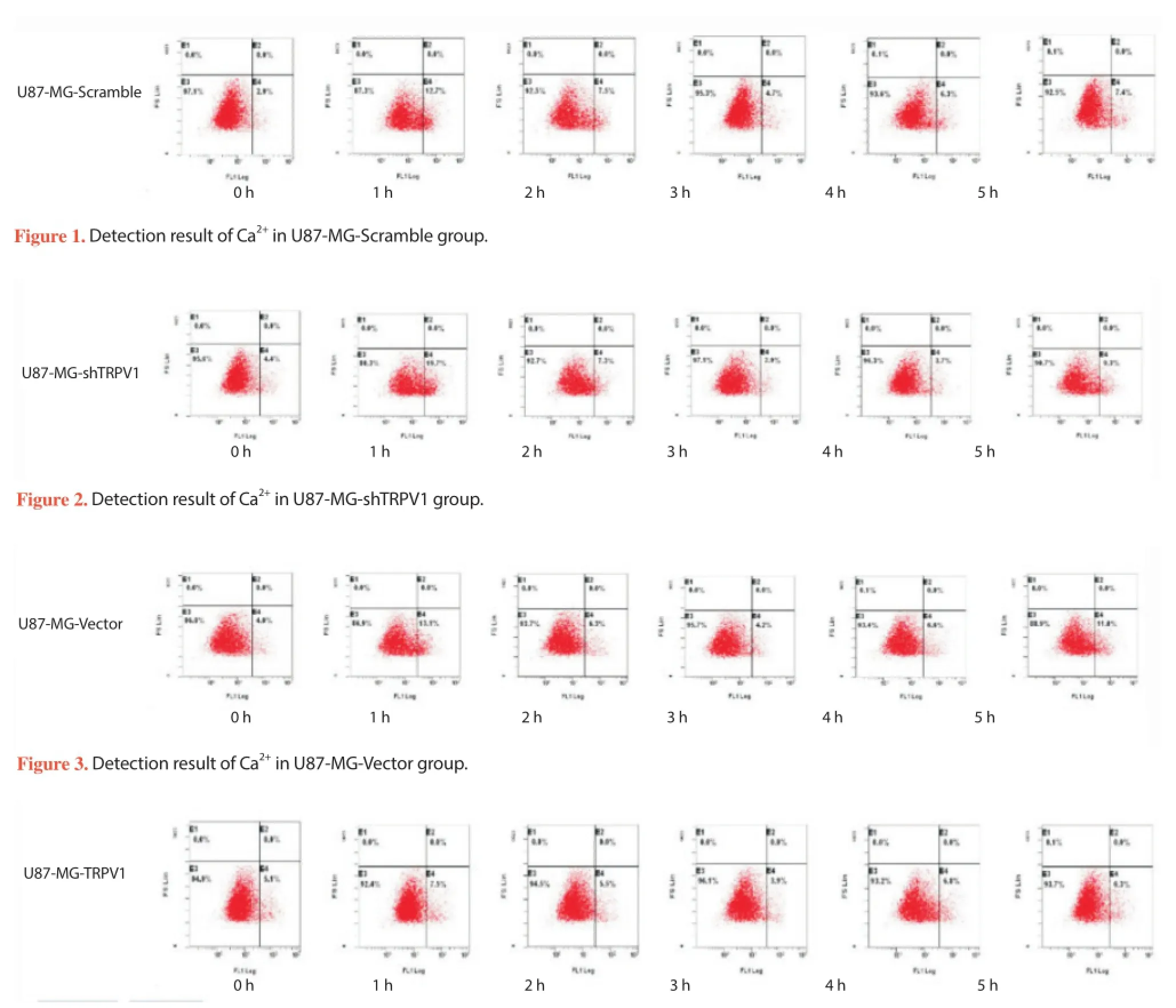
Figure 4. Detection result of Ca2+in U87-MG-TRPV1 group.
3.2. TRPV1 gene up-regulation combined with lidocaine strengthened the mitochondrial membrane potential in U87-MG glioma cells
Besides groups 1-4, groups 5-8 were added to detect the mitochondrial membrane potential in U87-MG glioma cells. The ratio of cells with reduced mitochondrial membrane potential in group 1-8 was respectively 12.4%, 11.5%, 13.9%, 13.0%, 26.0%, 19.6%, 17.8%, and 38.3%. The results showed that the mitochondrial membrane potential in TRPV1 gene up-regulation group was signifi cantly higher lower than in other groups (P<0.05), while in gene silencing group, it was signifi cantly lower higher than that in other groups (P<0.05). Furthermore, after lidocaine was added, the mitochondrial membrane potential signifi cantly increased decreased (P<0.05). The mitochondrial membrane potential TRPV1 gene upregulation combined with lidocaine group was signifi cantly higher lower than that in other groups (P<0.05).
3.3. TRPV1 gene up-regulation combined with lidocaine increased degree of apoptosis in U87-MG glioma cells
Besides groups 1-4, groups 5-8 were added to detect the eff ect of diff erent treatment on cell apoptosis. In fl ow cytometry results, the ratio of apoptotic cell in group 1-8 was respectively 0.5%, 0.3%, 0.3%, 1.4%, 1.5%, 0.9%, 0.5% and 3.1%. Flow cytometry results and MTT results (Figure 5) showed that after the addition of lidocaine, apoptosis signifi cantly increased in each group (P<0.05). This increasing trend in TRPV1 gene up-regulation group was more signifi cant than that in other groups (P<0.05). While it signifi cantly decreased in TRVP1 gene silencing group (P<0.05). Moreover, with the increasing concentration of lidocaine, the apoptosis was more severe (P<0.05).
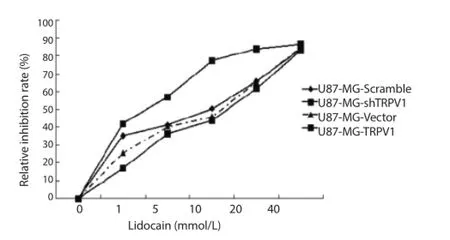
Figure 5. MTT results in each group.
4. Discussion
This experiment demonstrated that lidocaine could dosedependently result in increasing of intracellular calcium concentration, the mitochondrial membrane potential and apoptosis of U87-MG glioma cell line. At the same time, up-regulation of TRPV1 gene increased the cytotoxic effect of lidocaine, which revealed their correlation. Lidocaine probably increased the intracellular calcium concentration and mitochondrial membrane potential by activating TRPV1 gene to cause glioma cell line U87-MG apoptosis.
Studies found that intracellular calcium overload was an important mechanism of LA causing neuronal apoptosis[13,14]. Calcium overload mediates cell damage and apoptosis by the following pathways. Calcium combines phosphate compounds in mitochondria to form insoluble calcium phosphate, interfering oxidative phosphorylation, decreasing production of ATP and causing mitochondrial permeability transition pore open; increasing of calcium ion concentration can activate a variety of phospholipase, promote membrane phospholipids decomposition, thus damaging the cell membrane and organelle membrane structure, increasing membrane phospholipid degradation products, arachidonic acid and lysophosphatidic, increasing cell dysfunction; it can activate calcium-dependent protease, promoting an increase in oxygen free radicals, activating calcium/calmodulin-dependent protein kinase (CaMK Ⅱ), mediated cell apoptosis[15]. LA induced intracellular calcium overload in neurons by calcium-induced calcium release mechanism, that is, after the extracellular calcium ions entering cells, it will induce the release of large amounts of calcium in intracellular calcium stores, suddenly increasing intracellular calcium ion concentration, causing calcium overload[16]. On the other hand, LA can promote the release of a large number of excitatory amino acids in nerve cells, excessively activating NMDA receptors, resulting in large amounts of calcium ions entering the cells through the NMDA receptor. After neuronal excitability increased, extracellular calcium ions can also enter the cells via the membrane voltage-dependent calcium channels, causing intracellular calcium overload[17].
Cell apoptosis is a common physiological phenomenon. Embryonic development, endometrial cyclical shedding, cleaning process immune cells are all accompanied by cell apoptosis. Bacterial toxins, heat shock, radiation and oxidative stress also can affect cell apoptosis by external factors[18-20]. Mitochondria-mediated Caspase-8 precursor activation pathway plays an important role in the induction of cell apoptosis. The main mechanisms are as follows. Cytochrome C released by mitochondria can activate Caspase-8 precursor, generating death stimulus signals including DNA damage and apoptotic precursor protein activation, starting the cascade reaction, inducing cell apoptosis[21-24]. In this study, lidocaine concentration and increased TRPV1 gene plays a synergistic eff ect on mitochondrial membrane potential. With the gradual increase of mitochondrial membrane potential, apoptosis rate of U87-MG glioma cell line was more pronounced, which was consistent with previous studies[25].
In summary, LA drug, lidocaine, could produce neurotoxicity via TRPV1 gene, and promote apoptosis generation. Its mechanismwas associated with the intracellular calcium ion concentration and mitochondrial membrane potential, which provided some references for LA neurotoxicity prevention and application.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Akdere H, Burgazli KM, Aktoz T, Acikgoz A, Mericliler M, Gozen AS. The importance of anatomical region of local anesthesia for prostate biopsy; a randomized clinical trial. Eur Rev Med Pharmacol Sci 2013; 17(21): 2890-2895.
[2] Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier F, et al. Major complications of regional anesthesia in France: The SOS regional anesthesia hotline service. Anesthesiology 2002; 97(5):1274-1280.
[3] Rorarius M, Suominen P, Haanpää M, Puura A, Baer G, Pajunen P, et al. Neurologic sequelae after caesarean section. Acta Anaesthesiol Scand 2001; 45(1):34-41.
[4] Capdevila X, Pirat P, Bringuier S, Gaertner E, Singelyn F, Bernard N, et al. Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1 416 patients. Anesthesiology 2005; 103(5):1035-1045.
[5] Farber SJ, Saheb-Al-Zamani M, Zieske L, Laurido-Soto O, Bery A, Hunter D, et al. Peripheral nerve injury after local anesthetic injection. Anesth Analg 2013; 117(3): 731-739.
[6] Plant TD, Zöllner C, Mousa SA, Oksche A. Endothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptor. Exp Biol Med 2006; 231(6): 1161-1164.
[7] Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fi bres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008; 57(7): 923-929.
[8] Clapham DE, Julius D, Montell C, Schultz G. International union of pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 2005; 57(4): 427-450.
[9] Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. Neurosic 2006; 26(37):9385-9393.
[10] Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-lide substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 2002; 99(12):8400-8405.
[11] Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest 2008; 118(2):763-776.
[12] Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain 2011; 7(1):62.
[13] Johnson ME, Saenz JA, DaSilva AD, Uhl CB, Gores GJ. Eff ects of local anesthetic on neuronal cytoplasmic calcium and plasma membrane lysis (necrosis) in a cell culture model. Anesthesiology 2002; 97(6):1466-1476.
[14] Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med 2008; 33(5): 435-441.
[15] Liu Y, Templeton DM. Cadmium activates CaMK-Ⅱ and initiates CaMK-Ⅱ-dependent apoptosis in mesangial cells. FEBS Lett 2007; 581(7):1481-1486.
[16] Richter TA, Kolaj M, Renaud LP. Low voltage-activated Ca2+channels are coupled to Ca2+-induced Ca2+release in rat thalamic midline neurons. J Neurosci 2005; 25(36):8267-8271.
[17] Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal fi ring. Channels (Austin) 2010; 4(6):44-51.
[18] Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondriadependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 2013; 87(7): 1157-1180.
[19] Lirk P, Haller I, Myers RR, Klimaschewski L, Kau YC, Hung YC, et al. Mitigation of direct neurotoxic eff ects of lidocaine and amitriptyline by inhibition of p38 mitogen-activated protein kinase in vitro and in vivo. Anesthesiology 2006; 104(6):1266-1273.
[20] Lirk P, Haller I, Colvin HP, Frauscher S, Kirchmair L, Gerner P, et al. In vitro, lidocaine-induced axonal injury is prevented by peripheral inhibition of the p38 mitogen-activated protein kinase, but not by inhibiting caspase activity. Anesth Analg 2007; 105(6):1657-1664.
[21] Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011; 21(1): 92-101.
[22] Nguyen A, Chen P, Cai H. Role of CaMKⅡ in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett 2004; 572(1-3):307-313.
[23] Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome coxidase and cytochrome c in ischemia/reperfusion injury and infl ammation. Biochim Biophys Acta 2012; 1817(4): 598-609.
[24] Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 2004; 74(21): 2643-2653.
[25] Jhaveri MD, Elmes SJ, Kendall DA, Chapman V. Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naive, carrageenan-infl amed and neuropathic rats. Eur J Neurosci 2005; 22(2):361-370.
Document heading 10.1016/j.apjtm.2016.01.030
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Jun Lu, Doctorate degree, Department of Anesthesiology, The Second Affi liated Hospital to Nanchang University, Nanchang, Jiangxi 330006, China.
Tel: 13732901831
E-mail: liujunnc1978@163.com
Foundation project: The study was supported by the National Natural Science Foundation (NO. 81260176).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of miR-467b on atherosclerosis of rats
- Effect of dimethyl fumarate on rats with chronic pancreatitis
