Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
2016-07-24YanJiangLinZhangDepartmentofOphthalmologyRenJiHospitalSchoolofMedicineShanghaiJiaoTongUniversityShanghai200127China
Yan Jiang, Lin ZhangDepartment of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127,China
Contents lists available at ScienceDirect
Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
Yan Jiang, Lin Zhang*
Department of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127,China
ABSTRACT
Objective: To explore the mechanism of all-transretinoic acid (ATRA) increasing retinoblastoma (RB) sensitivity to vincristine, and the inhibiting eff ect of vincristine combined with ATRA treatment on the SO-RB50 cell proliferation. Methods: SO-RB50 cells were cultivated by routine culture method. Diff erent concentrations of vincristine or ATRA were added into culture solution. After 48 h, cell counting kit-8 was used to detect the median inhibitory concentration (IC50) of vincristine combined with ATRT treatment to SO-RB50 cells. SO-RB50 cells were divided into drug combination group, vincristine group, ATRA group and control group. Diff erent drugs were added into the culture solution respectively for cell culture based on the IC50value. Cell counting kit-8 was used to detect the cell proliferation every 24-h cultivation. After continuous determination for 6 d, data was processed to draw the cell growth curve. After drug use for 72 h, fl ow cytometry was used to detect the proportion of diff erent cell cycles of SO-RB50 cells in each group. After drug use for 48 h, annexin V/ propidium iodide method was used to detect the SO-RB50 cell apoptosis in each group. Results: The IC50value of vincristine treatment on the SO-RB50 cells was 0.11 μmol/L, and ATRT was 12.84 μmol/L. The cell growth curve in control group rose gradually along with the extended culture time, but after vincristine and ATRA treatment, the cell growth curve was smooth and steady. The cell increment was the least in drug combination group and its cell growth curve was the smoothest. There was signifi cant diff erence in A45048 h and 72 h after treatment (Fgrouping=77.316, P<0.001; Ftime=86.985, P<0.001). Compared with control group, A450value in drug combination group, vincristine group, ATRA group was significantly lower (P<0.001). Compared with control group, the G2/M phase cell proportion in vincristine group was signifi cantly increased, while the G0/G1phase cell proportion was signifi cantly decreased; the G0/G1phase cell proportion in ATRA group was significantly increased, while the S phase cell proportion was signifi cantly decreased (FG0/G1=85.878, Fs=56.455, FG2/M=85.878, P<0.001). After 48 h, there was signifi cant diff erence in SO-RB50 cell apoptosis rate among groups (F=11.312, P<0.05). The apoptosis rate in drug combination group was signifi cantly higher than that of other groups (P<0.001). Conclusions: ATRA can increase the sensitivity of SO-RB50 cells to vincristine. Vincristine combined with ATRA treatment can signifi cantly increase the inhibiting eff ect on SO-RB50 cells, which may be related with promoting cell apoptosis and involving in cell cycle control.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 Narch 2016
All-transretinoic acid
Retinoblastoma
Vincristine
Cell cycle
Apoptosis
1. Introduction
Retinoblastoma (RB) is a kind of intraocular malignant tumor. Most infants are vulnerable to RB, accounting for 3%-4% of children's malignant tumors. RB has brought a great threat to the eyesight and life of children[1]. Survival rate of children with RB in developed countries is up to 95%, while it is only 50% worldwide[2,3]. Clinical treatments for RB mainly include chemotherapy, radiotherapy, surgery and other local treatments[4]. Among them, chemotherapy plays a very important therapeutic eff ect. However, the drug resistance of tumour cells easily leads to the chemotherapy failure[5,6]. Therefore, the research hotspotin this world is focused on looking for a kind of more sensitive chemotherapy[7]. All-transretinoic acid (ATRA) can effectively promote the cell differentiation and obviously inhibit the cell proliferation[8]. There are researches showing that ATRA can eff ectively increase the sensitivity of multiple tumour cells like breast cancer and liver cancer to chemotherapies. However, researches on increasing the sensitivity of RB chemotherapies are relatively less[9,10]. In this study, the mechanism that ATRA could increase the sensitivity of RB to vincristine and the inhibiting eff ect of vincristine combined with ATRA treatment on the SO-RB50 cell proliferation have been discussed.
2. Meterials and methods
2.1. Materials
2.1.1. Cell sources
SO-RB50 cell lines were provided by Pathology Lab of Zhongshan Eye Center, Zhongshan University. RPMI/1640 culture solution (containing 1% mass fraction of mycillin antibody and 10% volume fraction of fetal calf serum) was used to culture these cells. Culture condition: temperature (37℃), saturation humidity and volume fraction (5% CO2). Cells showed a suspended growth and cells in logarithmic phase were selected for detection.
2.1.2. Key instruments and reagents
CO2incubator was from Japan SANYO Co.; ELIASA was from Beijing Perlong New counting Co., Ltd.; fl ow cytometry (FCM) was from America BD Co. RPMI/1640 culture medium and fetal calf serum were from America Hyclone Co.; ATRA was from America Sigma Co.; vincristine was from Zhejiang Hisun Pharmaceutical Company Ltd.; cell apoptosis detection kit was from Beijing 4A Biotech Co., Ltd.; cell counting kit-8 (CCK-8) was from Japan Dojindo Co.; cell cycle detection kit was from Hangzhou MultiSciences Biotech Co., Ltd.
2.2. Methods
2.2.1. IC50value of SO-RB50 cells after vincristine and ATRA treatment detected by CCK-8
SO-RB50 cells in logarithmic phase were inoculated into 96-well culture plates evenly, with 5 000/hole. Diff erent mass concentrations of 0.005, 0.010, 0.050, 0.100, 0.500 and 1.000 μmol/L of vincristine and 2.50, 5.00, 10.00, 20.00 and 40.00 μmol/L of ATRA were added respectively. A total of 5 holes were set up for each concentration. After being cultured for 48 h, 10 mL of CCK-8 reagent was added into each hole for 2 h incubation at 37℃. ELIASA method was used to detect the A450value at 450 nm[11].
2.2.2. Cell proliferation detected by CCK-8
The cultured cells were divided into drug combination group, vincristine group, ATRA group and control group. SO-RB50 cells in logarithmic phase were inoculated into 96-well culture plates, with 2.5伊104/mL of density. A total of 200 μL was added into each hole. IC450of vincristine (0.11 μg/mL) or ATRA (12.84 μmol/L) was added respectively according to the grouping. Five holes were set up in each group. CCK-8 was used to detect the A450value of cells in each group at 450 nm every 24 h for 6 continuous days. Then the average value was calculated and the cell growth curve was drew[12].
2.2.3. Cell cycle detected by FCM
SO-RB50 cells in logarithmic phase were inoculated into sixwell culture plates, with 2.5伊104/mL of density. Each hole was inoculated with 2 mL. IC450of vincristine or ATRA was added respectively. A total of 72 h after drug treatment, PBS was used for washing for 2 times, with centrifugation for 5 min (1 000 r/min and 12 cm of centrifugal radius). A total of 1伊106cells were collected in each group and were fi xed with ethyl alcohol with 70% of volume fraction. Temperature was controlled at 4℃ and treatment was performed after overnight. After PBS washing for once, 100 μL of buff er solution (containing 100 μg/mL of RNA enzyme and 0.2% volume fraction of Triton X-100) was added for 30 min incubation at 37℃; then 400 μL of propidium iodide (PI) staining solution was added for 30 min incubation at 4℃, with keeping out of the sun. FCM was performed to detect the cells of diff erent cell cycles. Cell proportion was calculated[13].
2.2.4. Apoptosis rate of cells detected by Annexin V/PI
SO-RB50 cells in logarithmic phase were inoculated into six-well culture plates, with 2.5伊104/mL of density. Each hole was inoculated with 2 mL. IC450of vincristine or ATRA was added respectively. A total of 72 h after drug treatment, PBS was used for washing for 2 times, with centrifugation for 5 min (1 000r/min). A total of 1伊106cells were collected in each group. A total of 100 μL of binding buffer solution was added for cell resuspension. Then 10 μL of annexin V-FITC and 5 μL of PI were added into culture solution for mixing, with incubation for 30 min at 4℃. Afterwards, incubation for 15 min was performed with keeping out of the sun and 400 μL of binding buff er solution was added once more. FCM was used to make a comparison of the ratio of apoptotic cells[14].
2.3. Statistical method
SPSS 13.0 statistical package was used for experimental data analysis. The data information in this study showed a normal distribution by Shapiro-Wilk test. Data was expressed by mean±SD and was detected by Levene homogeneity test of variances. Grouping was based on the balanced grouping and multilevel experimental design method. Block design two-way analysis of variance (ANOVA) was adopted to compare the overall diff erence of A450value changes in diff erent time in drug combination group, vincristine group, ATRA group and control group. SNK-q test was used for multiple comparisons among groups. One-factor ANOVA was used to compare the cell percentage and apoptosis rate in diff erent cell cycles in each group. When P<0.05, the diff erence hadstatistical signifi cance.
3. Results
3.1. IC50value of vincristine and ATRA treatment for SORB50 cells
The SO-RB50 cells were treated with diff erent concentrations of vincristine working solutions, respectively, and the cell proliferation inhibition rate was measured and IC50value of vincristine calculated. The SO-RB50 cells were treated with different concentrations of ATRA, respectively, and the cell proliferation inhibition rate was measured and IC50value of ATRA calculated. The results were shown in Table 1.
3.2. Cell proliferation in each group
Vincristine and ATRA treatment was performed respectively for SO-RB50 cells for 6 d. The change in cell growth rate with time in control group was significantly larger than that in vincristine group, ATRA group and drug combination group, and that in drug combination group was the minimum. There were significant diff erence in cell proliferation rates among the 4 groups 48 h and 72 h after drug treatment (P<0.001). A total of 48 h after drug treatment, compared with vincristine group, ATRA group and control group, A450 value in drug combination group was significantly lower (P<0.001) (Table 2).
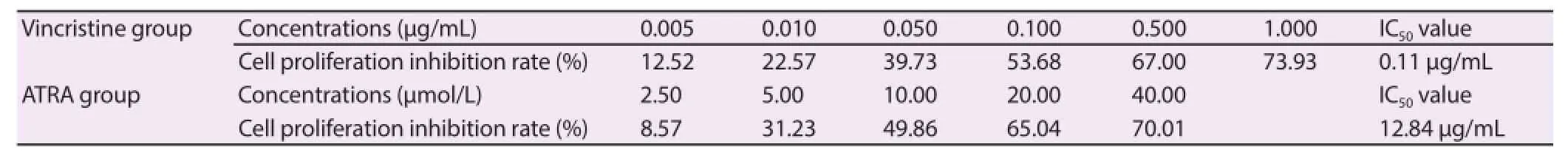
Table 1IC50 values of vincristine and ATRA for SO-RB50 cells.
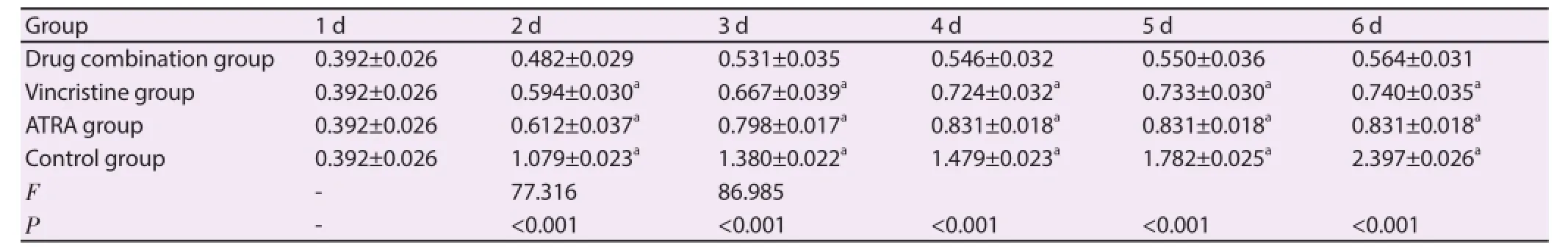
Table 2Cell proliferation in each group.
3.3. Proportion of SO-RB50 cells in different cell cycles 72 h after drug treatment
There were signifi cant diff erences in proportion changes of SORB50 cells in G0/G1phase, G2/M phase, S phase 72 h after treatment of diff erent drugs (F=130.565, F=114.290, F=57.435; P<0.001). The G0/G1phase cell proportion in vincristine group was signifi cantly reduced; G2/M cell proportion was signifi cantly increased (P<0.001). The G0/G1phase cell proportion in ATRA group was significantly higher than that in control group; S phase cell proportion was signifi cantly lower than that in control group (P<0.001) (Table 3).
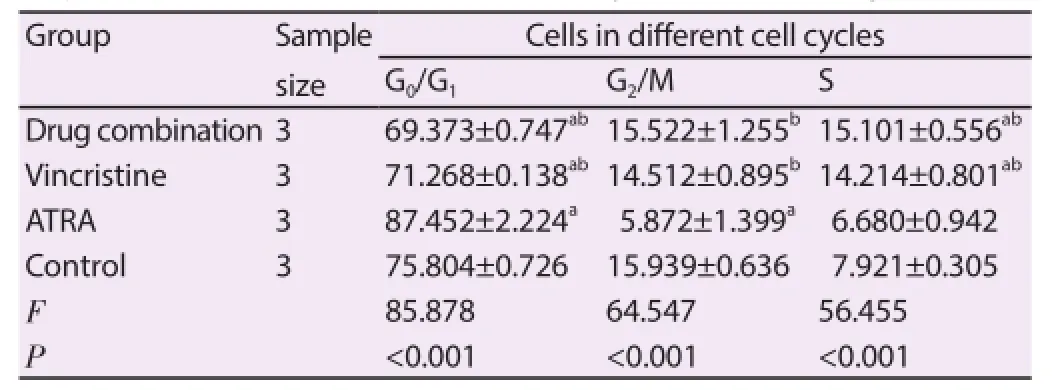
Table 3Proportion of SO-RB50 cells in diff erent cell cycles 72 h after drug treatment.
3.4. Apoptosis rate of SO-RB50 cells in each group 48 h after treatment of different drugs
A total of 48 h after diff erent drug treatment, there was signifi cant difference in apoptosisrate of SO-RB50 cells among four groups (P<0.05). Compared with control group, the apoptosis rate in drug combination group, vincristine group and ATRA group were significantly higher (P<0.001). Compared with vincristine group and ATRA group, the apoptosis rate in drug combination group was signifi cantly higher (P<0.001). There was no signifi cant diff erence in the apoptosis rate between vincristine group and ATRA group (P=0.919) (Table 4).

Table 4Apoptosis rate of SO-RB50 cells in each group 48 h after drug treatment.
4. Discussion
In recent years, the therapeutic goal for RB have changed from increasing the survival rate of children to the living quality of children. The treatment mode has also changed gradually from eyeball extraction to conservative treatment[15,16]. Chemotherapies in clinic could increase the retention rate of eyeballs in a certain degree, but the drug resistance of tumour cells to the traditional drugs of chemotherapies would easily lead to the treatment failure[17]. At present, research hotspots are focused on looking for effective chemosensitizer.
There were researches showing that ATRA could effectively increase the chemical sensibility of multiple tumors[18,19]. Through experiments in vitro and vivo, reserachers found that ATRA could effectively induce the liver cancer stem cell differentiation and increase the sensitivity of tumour cells to cis-platinum[20]. Through phase Ⅱ clinic trial, it was found that ATRA combined with paclitaxel or cis-platinum could increase the effi cacy of patients with advanced non-small cell lung cancer[21]. There were reports showing that ATRA combined with paclitaxel could relieve the metastatic or recurrent breast cancer in a certain degree and bring better tolerance for patients[22,23]. In this study, the eff ect of the sensitivity of ATRA to vincristine, the typical chemotherapy drug for RB, was mainly discussed. Research results showed that ATRA combined with vincristine treatment had obvious inhibiting eff ects and apoptosispromoting effects on the SO-RB50 cell proliferation, and the effi cacy of the combined drugs was obviously better than single drug use of vincristine. That was to say, ATRA could obviously increase the sensitivity of SO-RB50 cells to vinblastine.
Vitamin A metabolism in vivo could produce ATRA and ATRA combined with cell receptor could regulate and control the gene expression, and then promote the cell diff erentiation and inhibit the cell proliferation[24]. There were researches showing that ATRA could eff ectively inhibit the proliferation of multiple tumors, like RB, glioma, leukemia, and neuroblastoma[25]. In this research, CCK-8 detection method was adopted to prove the fact that ATRA could inhibit the cell growth, after playing eff ects, G0/G1phase SO-RB50 cells were obviously increased; while S phase SO-RB50 cells were obviously decreased. ATRA could make cell stasis at G0/G1phase and then slower the cell growth speed, which was in accordance with the previous reports[26,27]. Vincristine was a kind of cell mitosis inhibitor, action mechanism of which was that vincristine combined with microtubulin specifi cally could block the microtubule formation and result in the stasis of cell division at the metaphase[28]. This research indicated that after vincristine treatment, SO-RB50 cells at G0/G1phase were obviously reduced; while SO-RB50 cells at G2/ M phase were obviously increased, which was in accordance with the previous reports that vincristine could produce periodic blocking to neuroblastoma[29,30]. Its mechanism was that vincristine was a key factor resulting in the cell cycle changes. Vincristine and ATRA could effectively play inhibiting effects on cell proliferation for diff erent phase of cells. In addition, in this study, also it was also found that ATRA could increase the apoptosis rate of cells, which indicated that ATRA might induce the cell apoptosis and then play inhibiting eff ects on cell proliferation.
In conclusion, through experiments in vitro in this study, it was proved that ATRA can effectively increase the sensitivity of SORB50 cells to vincristine, which provide theoretical basis for exploring new drug combination scheme for neuroblastoma. The possible mechanism of that inducing the cell apoptosis, and regulating and controlling the cell cycle. In addition, further research is necessary to discuss other possible action mechanism.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Steinmetz B, Hackl H, Slabáková E, Schwarzinger I, Smějová M, Spittler A, et al. The oncogene EVI1 enhances transcriptional and biological responses of human myeloid cells to all-trans retinoic acid. Cell Cycle 2014, 13(18): 2931-2943.
[2] Yang F, He Y, Liu HX, Tsuei J, Jiang X, Yang L, et al. All-trans retinoic acid regulates hepatic bile acid homeostasis. Biochem Pharmacol 2014; 91(4): 483–489.
[3] Komori R, Kobayashi T, Matsuo H, Kino K, Miyazawa H. Csn3 gene is regulated by all-trans retinoic acid during neural differentiation in mouse P19 cells. PloS One 2013; 8(4): e61938. doi: 10.1371/journal. pone.0061938
[4] Zhan XX, Liu Y, Yang JF, Wang GY, Mu L, Zhang TS, et al. All-transretinoic acid ameliorates experimental allergic encephalomyelitis by affecting dendritic cell and monocyte development. Immunol 2013; 138(4): 333–345.
[5] Lou Y, Qian W, Meng H, Mai W, Tong H, Tong Y, et al. High efficacy of arsenic trioxide plus all-trans retinoic acid based induction and maintenance therapy in newly diagnosed acute promyelocytic leukemia. Leuk Res 2013; 37(1): 37-42.
[6] Masetti R, Vendemini F, Zama D, Biagi C, Gasperini P, Pession A. All-trans retinoic acid in the treatment of pediatric acute promyelocytic leukemia. Expert Rev Anticancer Ther 2012; 12(9): 1191-1204.
[7] Lei GS, Zhang C, Shao S, Jung HW, Durant PJ, Lee CH. All-trans retinoic acid in combination with primaquine clears Pneumocystis infection. PloS One 2013; 8(1): e53479. doi: 10.1371/journal.pone.0053479
[8] Rubin SM. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci 2013; 38(1):12–19.
[9] Ajioka, I. Coordination of proliferation and neuronal diff erentiation by the retinoblastoma protein family. Dev Growth Differ 2014; 56(5): 324-334.
[10] Houston SK, Murray TG. Microarray gene-expression analysis in ocular oncology: uveal melanoma and retinoblastoma. Expert Rev Ophthalmol 2014; 6(4): 477-485.
[11] Zhu SJ, Pearson BJ. The retinoblastoma pathway regulates stem cell proliferation in freshwater planarians. Dev Biol 2013; 373(2): 442–452.
[12] Kapatai G, Brundler MA, Jenkinson H, Kearns P, Parulekar M, Peet AC. Gene expression profi ling identifi es diff erent sub-types of retinoblastoma. Brit J Cancer 2013; 109(2): 512-525.
[13] Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic diff erentiation of myeloid cells in cancer. Nat Immunol 2013; 14(3): 211-220.
[14] Witkiewicz AK, Cox DW, Rivadeneira D, Ertel AE, Fortina P, Schwartz GF, et al. The retinoblastoma tumor suppressor pathway modulates the invasiveness of ErbB2-positive breast cancer. Oncogene 2014; 33(30): 3980-3991.
[15] Philippeit C, Busch M, Dünker N. Epigenetic control of trefoil factor family (TFF) peptide expression in human retinoblastoma cell lines. Cell Physiol Biochem 2014; 34(3): 1001-1014.
[16] Chawla B, Jain A, Azad R. Conservative treatment modalities in retinoblastoma. Indian J Ophthalmol 2013; 61(9): 479-485.
[17] Silverman JA, Deitcher SR. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemoth Pharm 2013; 71(3): 555-564.
[18] Dun L, Li Y, Xu Y, Zhou R, Ma L, Jin S, et al. Antinociceptive effect of matrine on vincristine-induced neuropathic pain model in mice. Neurol Sci 2014; 35(6): 815-821.
[19] Lewin J, Wieringa S, Collins M, Desai J, Orme L, Lingaratnam S, et al. Intra-patient dose escalation in Ewing's sarcoma treated with vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide and etoposide: a retrospective review. Clin Sarcoma Res 2013; 3(1): 15.
[20] LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Eff ects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 2013; 37: 231–239.
[21] Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014; 32(8): 783-790.
[22] Cunningham D, Hawkes A, Jack A, Qian W, Smith P, Mouncey P, et al. . Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diff use large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensifi cation with 14-day versus 21-day cycles. Lancet 2013; 381(9880): 1817-1826.
[23] Kapinas K, Grandy R, Ghule P, Medina R, Becker K, Pardee A, et al. The abbreviated pluripotent cell cycle. J Cell Physiol 2013; 228(1): 9-20.
[24] Patrício P, Mateus-Pinheiro A, Sousa N, Pinto L. Re-cycling paradigms: cell cycle regulation in adult hippocampal neurogenesis and implications for depression. Mol Neurobiol 2013; 48(1): 84-96.
[25] Noatynska A, Tavernier N, Gotta M, Pintard L. Coordinating cell polarity and cell cycle progression: what can we learn from fl ies and worms? Open Biol 2013; 3(8): 130083. doi: 10.1098/rsob.130083
[26] Weinstein N, Ortiz-Gutiérrez E, Muñoz S, Rosenblueth DA, Álvarez-Buylla ER, Mendoza L. A model of the regulatory network involved in the control of the cell cycle and cell diff erentiation in the Caenorhabditis elegans vulva. BMC Bioinformatics 2015; 16: 81. doi: 10.1186/s12859-015-0498-z
[27] Arlt A, Müerköster SS, Schäfer H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett 2013; 332(2): 346–358.
[28] Zeestraten ECM, Benard A, Reimers MS, Schouten PC, Liefers GJ, Cornelis JH, et al. The prognostic value of the apoptosis pathway in colorectal cancer: a review of the literature on biomarkers identifi ed by immunohistochemistry. Biomark in Cancer 2013; 5: 13-29.
[29] Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol 2011; 5(2): 201-212.
[30] Lee CH, Wu SB, Hong CH, Yu HS, Wei YH. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci 2013; 14(3): 6414-6435.
Document heading 10.1016/j.apjtm.2016.01.028
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Lin Zhang, Professor, Department of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
Tel: 13611794943
E-mail: linlinrj172@hotmail.com
Foundation project: It is supported by Projects of Science and Technology Commission of Shanghai Municipality (No. 11ZR1421300).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Effect of miR-467b on atherosclerosis of rats
- Effect of dimethyl fumarate on rats with chronic pancreatitis
