Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
2016-07-24ChengLanXiaoNingSunXuChunZhouBoYangBaiLiHuangTaoZhiDengZhouTaoHeXiangYangHanDepartmentofGastroenterologyHainanProvincialGeneralHospitalHaikou5703HainanProvinceChinaDepartmentofGastroenterologystAffiliatedHospitalofCh
Cheng Lan, Xiao-Ning Sun, Xu-Chun Zhou, Bo Yang, Bai-Li Huang, Tao-Zhi Deng, Zhou-Tao He, Xiang-Yang HanDepartment of Gastroenterology, Hainan Provincial General Hospital, Haikou 5703, Hainan Province, ChinaDepartment of Gastroenterology, st Affiliated Hospital of Chongqing Medical University, Chongqing 40006, Chongqing, China
Contents lists available at ScienceDirect
Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
Cheng Lan1*, Xiao-Ning Sun1, Xu-Chun Zhou1, Bo Yang2, Bai-Li Huang1, Tao-Zhi Deng1, Zhou-Tao He1, Xiang-Yang Han1
1Department of Gastroenterology, Hainan Provincial General Hospital, Haikou 570311, Hainan Province, China
2Department of Gastroenterology, 1st Affiliated Hospital of Chongqing Medical University, Chongqing 400016, Chongqing, China
ABSTRACT
Objective: To investigate the impact of the preinduced intestinal heat shock protein 70 (HSP70) on the visceral hypersensitivity and abnormal intestinal motility in a post-infectious irritable bowel syndrome (PI-IBS) mouse model. Methods: Eighty-four female C57BL/6 mice were randomly assigned to four groups: control group (n=21) and induction+PI-IBS group (n=21), PI-IBS group (n=21) and induction group (n=21). The mice in PI-IBS group were infected in vivo with trichinella spiralis by oral administration. The visceral hypersensitivity and intestinal motility were evaluated respectively with abdominal withdrawal refl ex and colon transportation test. The intestinal HSP70 protein¬¬ and mRNA level was measured by Western blot and realtime PCR. Meanwhile, the intestinal proinfl ammatory cytokines IL-10 and TNF-α level was detected by ELISA. Results: Compared with their counterparts in PI-IBS group, the animals in the Induction+PI-IBS group show significantly increased intestinal level of HSP70 and obviously ameliorative clinical fi gures, including abdominal withdrawal refl ex score, intestine transportation time and Bristol scores (P<0.05). Meanwhile, the intestinal post-infl ammatory cytokines remarkably changed, including increased IL-10 level and decreased TNF-αlevel (P<0.05). Conclusions: Intestinal HSP70 may play a potential protective role through improving the imbalance between the intestinal post-infl ammatory and anti-infl ammatory cytokines in PI-IBS.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Post-infectious irritable bowel syndrome
Heat shock protein 70
Proinfl ammatory cytokine
Visceral hypersensitivity
Intestinal motility
1. Introduction
As a kind of clinical syndrome characterized by abdominal pain, discomfort and bloating accompanied with abnormal defecation, the precise pathological mechanism of irritable bowel syndrome (IBS) remains unclear[1-3]. During the last two decades, abundant clinical and experimental research focused on the role of infection and infl ammation in the pathogenesis of IBS, called as post-infectious irritable bowel syndrome (PI-IBS)[5-7]. Recently, it was reported that heat shock protein 70 (HSP70) has an unique capability of regulating the protein misfolding, aggregation and serves critical roles in some diseases[8,9]. Thus the aim of the current study is to investigate the potential role of HSP70 in PI-IBS.
2. Materials and methods
Eighty-four female 57BL/6 mice with (6-8) weeks and (13-15) g were purchased from Kunming Institute of Zoology, Chinese Academy of Science. All animals were housed in sterile, pathogenfree, temperature controlled facility on normal 12-h light/dark cycle, and standard diet and water were provided ad libitum.The experiment was carried out in accordance with the Chinese guidelines for animal welfare. Experimental protocol was approved by the Animal Care and Use Committee of the Hainan Provincial General Hospital. The animals were randomly assigned into four groups: control group, induction+PI-IBS group, PI-IBS group and induction group (n=21 in each group). In each group, 7 mice were sacrificed for the detection of the intestinal HSP70 protein level and 7 mice for that of the intestinal HSP70 mRNA level. The other 7 mice were examined for the visceral hypersensitivity and the intestinal motility. Trichinella spiralis were purchased from Lanzhou Animal Medical Institute.
2.1. Main reagents
Pepsin (Invitrogen Coporation, U.S.A); histochemistry and Western blot agents (Wuhan Boster Coporation, China); antibodies (BDBioscience Co., U.S.A).
2.2. Modeling of PI-IBS
The mice were infected with Trichinella spiralis as described previously[10]. Briefl y, the Trichinella spirali lavace were separated from Sprague-Dawley rats 60 d after infection of Trichinella spirali’s cryst by digestion with 1.5% gastric pepsin. The mice were fed with the lavarce in 0.2 mL saline (300 lavarce per mouse). The animals in the control group were fed with only 0.9% saline.
2.3. Abdominal withdrawal reflex (AWR)
AWR was used to evaluate the visceral hypersensitivity[11]. The anesthetized animals were inserted via their anus with air chamber and catheter. The air chamber was distended at volume of 0.25/0.5/0.75 mL×l5 min×3 times. Between each distending time, the animals were permitted to have a rest for 30 s. The AWR scoring standard: when stimulated, the animals are in stable mood, 0 point; if the animals are in unstable mood, twisting their heads once in a while, 1 point; slightly contracting their abdomen and back muscles, 2 points; intensively contracting their abdomen muscles and uplifting the abdomen from the ground, 3 points; intensively contracting abdomen muscles, bowing abdomen and uplifting the abdomen and perineum, 4 points.
2.4. Colon transportation test
Colon transportation test was used to evaluate the status of the intestinal motility. After filled into stomach with 0.4 mL active carbon, the fi rst black stool time was recorded. The total stool within 8 h was collected and evaluated by Bristol stool grade[12]: normal shaped stool, 1 point; soft or deformed stool, 2 points; water-like stool, 3 points.
2.5. Preinduction of HSP70
Expression of HSP70 in mice was induced by heat treatment according to previous reports[13]. Briefl y, mice were anesthetized with sodium pentobarbital (50 mg/kg). Rectal temperature was monitored with a thermistor inserted into the rectum in a baking oven with constant temperature 50 ℃. After the body temperature was maintained at 41 ℃ for 20 min, the mice were return to their cages at room temperature and allowed water and food ad libitum. Nonheated mice were only anesthetized but received no hyperthermic stress.
2.6. Determination of intestinal HSP70
HSP70 protein level and mRNA expression was measured by Western blot and real-time PCR respectively. The tissue sample was grinded and cracked with RIPA. The homogenate was centrifuged for 30 min. The protein concentration in the supernatants was measured by Bradford Assay. Tissue sample of 40 g was separated by SDS page gel electrophoresis and transferred to the PVDF membrane. The membrane was blotted with TBST for 1 h, then was added with goat-anti-mouse HSP70 multiple clone antibodies (1:1 000) and rabbit-anti-mouse β-actin multiple clone antibodies (1:1 000) at 4 ℃for 12 h. One day later, the membrane was washed in TBST and autographied by ECL chemiluminescent assay. The gray-scale value of HSP70/β-actin represented the relative expression level of HSP70. Total RNA was isolated from the terminal ileum tissue with Trizol liquid and treated with DNAaseI. Primer was designed according to mouse gene sequence. β-actin was used as an internal control.
HSP70 gene primer: F: 5’-GAAGGTGCTGGACAAGTGC-3’[(1 903-1 921) bp), R: 5’-GCCAGCAGAGGCCTCTAATC-3’(2 120-2 139) bp]. β-actin gene primer (470 bp): F: 5’-A G G C T G T G C T G T C C C T G T A T G-3’, 5’-GAGGTCTTTACGGATGTCAACG-3’.
Real-time PCR was operated with following protocol: 1. Predenaturation program (5 min at 94 ℃); 2. Denaturation program (1 min at 94 ℃); 3. Amplifi cation and qualifi cation program, repeated 30 cycles (50 s at 57 ℃, 20 s at 60 ℃); 4. Prolonging program (7 min at 72 ℃). The relative expression was expressed as a ratio of the target gene to the control gene.
2.7. Determination of proinflammatory cytokines
The tissue sample was ultrasonically shivered and centrifuged at 4 ℃. The concentration of cytokines IL-10 and TNF-α in the supernatants was measured by ELISA.
2.8. Statistics analysis
Data were analyzed using Student’s t-test (SPSS 17.0 software). Data were expressed as the mean±SE. Values in the same row with different superscripts are significant (P<0.05), while values with same superscripts are not signifi cantly diff erent (P>0.05).
3. Results
3.1. Expression of intestinal HSP70
Western blot and RT-PCR show that the intestinal HSP70 protein and mRNA level in Induction+PI-IBS mice was far more than that in PI-IBS mice (P<0.05) (Table 1).

Table 1HSP70 protein and mRNA level in heat pretreated PI-IBS mice.
3.2. AWR
At the distending air volume of 0.25 mL and 0.65 mL, there was no signifi cant diff erence between the AWR score of the Induction+PIIBS mice and the PI-IBS mice (P>0.05); but at the distending air volume of 0.35 mL and 0.5 mL, the AWR score of Induction+PI-IBS mice was obviously lower than that of PI-IBS mice (P<0.05) (Table 2).
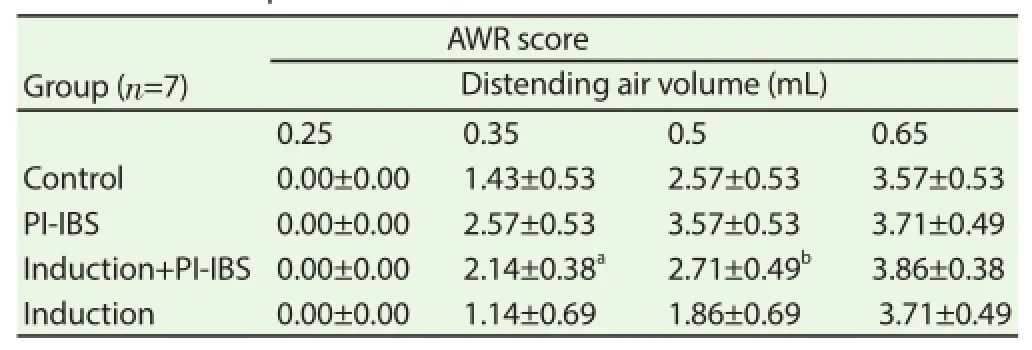
Table 2AWR score in heat pretreated PI-IBS mice.
3.3. Intestinal motility
The colon transport time of the mice in Induction+PI-IBS group was signifi cantly longer than that of the mice in the PI-IBS group (P<0.05); the Bristol score of the eight hours stool from the PI-IBS mice was obviously less than their counterparts in Induction+PI-IBS group (P<0.05) (Table 3).

Table 3Changes of the intestinal mobility in heat pretreated PI-IBS mouse.
3.4. Expression of intestinal proinflammatory cytokines
The intestinal post-infl ammatory cytokines remarkably changed, with decreased IL-10 level and increased TNF-α level (P<0.05) (Figure 1 and Figure 2).

Figure 1. Intestinal IL-10 level in heat-pretreated PI-IBS mouse.

Figure 2. Intestinal TNF-α level in heat-pretreated PI-IBS mouse.
4. Discussion
With high incidence rate and refractory history, IBS seriously impact the patients’ life quality, exerting a huge burden on the patients. There is no eff ective therapy for this kind of disease up to now[14].
It has been proved that IBS results from a synergy of multiple etiological factors includingthe viceroy hypersensitivity, the abnormal intestinal motility, local immune response and socialpsychological factors[15-18]. Recently, researches mainly focus on two aspects: the neuropeptides induced viceroy hypersensitivity and abnormal intestinal motility, the low grade intestinal infl ammation and continuous immune activating process. The concept of PIIBS was considered as concurs[7,19,20]. The current study was to investigate the role of the intestinal mucosa immune system using a Trichinella spiralis infected mice model.
HSP70 is a kind of chaperons whose structures remain unchanged, structurally expressed or induced by heat shock, trauma and other stress stimulation. HSP70 could help the essential proteins to refold their structure, whose folding structure were changed by the stress and inflammatory injure, thus fixed the lesion[18]. Furthermore, the infl ammatory cells expressing HSP70 could be recognized anderadiated by immunocytes[19,20]. PI-IBS is regarded as a disorder induced by the intestinal infection and infl ammation. Our previous study found that the intestinal HSP70 level increased during PIIBS. Thus HSP70 could exert a negative modulating role in PI-IBS. In the current study, heat pre-treatment signifi cantly increased the intestinal HSP70 level in normal mice, but did not induced visceral hypersensitivity and abnormal intestinal motility. Interestingly, heat pre-treatment up-regulated the increasing HSP70 level in PIIBS mice. Simultaneously, viceroy hypersensitivity and abnormal intestinal motility in PI-IBS were improved after heat pretreatment. These results suggest that HSP70 could protect mice against PIIBS. On the other hand, heat pre-treatment could not wholly reverse the clinical symptoms, suggesting that there could be other pathway through which PI-IBS occurred.
Furthermore, the inflammatory cytokines level was detected to explain how HSP70 exerted its impact on the intestinal infl ammation during PI-IBS. IL-10 was a kind of cytokine inhibiting inflammation[21]. TNF-α was considered as an important proinfl ammatory cytokines in some intestinal infl ammatory diseases[22].
We found that during PI-IBS, the IL-10 level decreased and the TNF-α increased significantly. Whereas preinduction of HSP70 obviously improved the changes of these cytokines. These results suggest that HSP70 could exert its protective role via proinfl ammatory cytokines pathway.
In conclusion, our results indicated that preinduction of HSP70 could protect the mice against PI-IBS via improving the imbalance of the intestinal pro-infl ammatory cytokines. Our fi nding could help to optimize the treatment for PI-IBS, although the precise underlying cellular and molecular mechanism of the regulation remains to be explored.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol 2014; 20(34): 12144-12160.
[2] Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313(9): 949-958.
[3] Sperber AD, Drossman DA, Quigley EM. The global perspective on irritable bowel syndrome: a Rome Foundation-World Gastroenterology Organisation symposium. Am J Gastroenterol 2012; 107(11): 1602-1609.
[4] Drossman DA, Dumitrascu DL. Rome Ⅲ: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006; 15: 237-241.
[5] Rajilić-Stojanović M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, et al. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol 2015; 110(2): 278-287.
[6] Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther 2015; 41(2): 177-188.
[7] Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six years follow up study. Gut 2002; 51(3): 410-413.
[8] Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J. The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem 2015; 78: 243-273.
[9] Shiber A, Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules 2014; 4(3): 704-24. PMID: 25036888.
[10] Yang B, Zhou X, Lan C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterol 2015; 15: 43.
[11] Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013; 8(5): e63893.
[12] Caroff DA, Edelstein PH, Hamilton K, Pegues DA. The Bristol stool scale and its relationship to Clostridium difficile infection. J Clin Microbiol 2014; 52(9): 3437-3439.
[13] Itoh YH, Noguchi R. Pre-treatment with mild whole-body heating prevents gastric ulcer induced by restraint and water-immersion stress in rats. Int J Hyperthermia 2000; 16(2): 183-191.
[14] Hungin AP, Molloy-Bland M, Claes R, Heidelbaugh J, Cayley WE Jr, Muris J, et al. Systematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care-a Rome Foundation working team report. Aliment Pharmacol Ther 2014; 40(10): 1133-1145.
[15] Lindfors P, Törnblom H, Sadik R, Björnsson ES, Abrahamsson H, Simrén M. Eff ects on gastrointestinal transit and antroduodenojejunal manometry after gut-directed hypnotherapy in irritable bowel syndrome (IBS). Scand J Gastroenterol 2012; 47(12): 1480-1487.
[16] Bian ZX. Novel insights about the mechanism of visceral hypersensitivity in maternally separated rats. Neurogastroenterol Motil 2012; 24(7): 593-596.
[17] Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, et al. Pathogenesis of irritable bowel syndrome-review regarding associated infection and immune activation. Digestion 2013; 87(3): 204-211.
[18] Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol 2014; 20(39): 14126-14131.
[19] Schwille-Kiuntke J, Frick JS, Zanger P, Enck P. Post-infectious irritable bowel syndrome-a review of the literature. Z Gastroenterol 2011; 49(8): 997-1003.
[20] Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr 2009; 48(Suppl 2): S95-S97.
[21] Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR, et al. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been diffi cult to harness for immunotherapy. Cytokine 2015; 74(1): 27-34.
[22] Bashashati M, Rezaei N, Shafi eyoun A, McKernan DP, Chang L, Öhman L, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 2014; 26(7): 1036-1048.
Document heading 10.1016/j.apjtm.2016.01.022
15 December 2015
*Corresponding author: Cheng Lan, M.D, Ph.D, Professor, Department of Gastroenterology, Hainan Provincial General Hospital, Haikou 570311, Hainan Province, China.
E-mail: lancheng71@163.com
Foundation project: It is supported by Natural Science Foundation of China Grant (No. 81160057); International Science and Technique Corporation Foundation of Hainan Province Grant (No. KJHZ2013-14).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Immune formulation-assisted conventional therapy on anti-infective effectness of multidrug-resistant Mycobacterium tuberculosis infection mice
- Effect of dimethyl fumarate on rats with chronic pancreatitis
- Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
- Effect of miR-467b on atherosclerosis of rats
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
