Effect of miR-467b on atherosclerosis of rats
2016-07-24XiaoMeiGuanYongXinLiHaiXinJunLiZongGangZhaoYueWeiWangHaoFuWangDepartmentofVascularSurgeryTheAffiliatedHospitalofQingdaoUniversityQiangdaoShandong266005China
Xiao-Mei Guan, Yong-Xin Li, Hai Xin, Jun Li, Zong-Gang Zhao, Yue-Wei Wang, Hao-Fu WangDepartment of Vascular Surgery, The Affiliated Hospital of Qingdao University, Qiangdao, Shandong 266005, China
Contents lists available at ScienceDirect
Effect of miR-467b on atherosclerosis of rats
Xiao-Mei Guan, Yong-Xin Li, Hai Xin, Jun Li, Zong-Gang Zhao, Yue-Wei Wang, Hao-Fu Wang*
Department of Vascular Surgery, The Affiliated Hospital of Qingdao University, Qiangdao, Shandong 266005, China
ABSTRACT
Objective: To observe the effect of miR-467b on the atherosclerosis (AS) of rats with apolipoprotein E (ApoE) gene knockout (ApoE-/-). Methods: ApoE-/-rats were fed with high fat and high cholesterol diet and were randomly divided into group A, group B and group C, with 10 rats in each group. Group A: rats were injected with ApoE agonist through the caudal vein; Group B: rats were injected with ApoE antagonist through the caudal vein; Group C: as negative control group. Enzyme oxidation method was used to detect the blood lipid levels of rats. Western blotting method was used to detect the aortic lipoprotein lipase (LPL) expression levels of rats. HE staining and oil red o staining were performed to observe the AS lesions and lipid accumulation state. Results: Compared with Group C, blood lipid level, aortic intima and aortic sinus lipid accumulation area ratio, aortic sinus lesion area and LPL expression level in Group A signifi cantly reduced; while blood lipid level, aortic intima and aortic sinus lipid accumulation area ratio, aortic sinus lesion area, and LPL expression level in Group B signifi cantly increased, with the statistical diff erence (P<0.05). Conclusions: miR-467b can alleviate the AS lesions of ApoE-/-rats, and its inhibiting eff ect on AS may be related to LPL expression.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
miR-467b
Lipoprotein lipase
Apolipoprotein E
Atherosclerosis
1. Introduction
Atherosclerosis (AS) refers to the complex pathological process induced by many factors. Cardiovascnlar and cerebrovascular disease, which are induced by AS, would bring great harm to health, especially for middle-aged and aged people[1]. The lipid metabolism disorders, excessive cholesterol accumulation and vascular infl ammatory responses for AS patients are the key factors resulting in AS[2,3]. Apolipoprotein E (ApoE) plays an important role during the process of lipid transmission and metabolism[4]. In this study, ApoE gene knockout (ApoE-/-) rats fed with high fat and high cholesterol diet were selected for the building of AS model.
miRNA is a kind of endogenous single mature non-coding RNA, which could induce the miRNA degradation and inhibit its transcription translation process through incomplete pairing with target mRNA. In addition, miRNA is involved in many physiological process, like regulating cell viability, embryonic development, lipid metabolism, infl ammatory response and autoimmunity[5,6]. Recent researches found that miR-467b expression was increased in ApoE-/-mice, which might play an important role in the occurrence and development of AS and its action mechanism maybe achieved through target regulating of lipoprotein lipase (LPL)[7]. LPL in vascular cells could promote the formation of foam cells, increase the infl ammatory factor expression, cause the lipid accumulation and then result in the occurrence of AS[8]. Some researches showed that the AS lesions of rats with high fat and high cholesterol diet feeding and macrophage LPL gene deletion were obviously alleviated[9]. In this study, intravenous injection of miR-467 agonist and antagonist for ApoE-/-rats was performed to observe the LPL, lipid levels and AS lesions, which could provide theoretical basis for the prevention and targeted treatment of AS.
2. Materials and methods
2.1. Animals
A total of 30 healthy male SPF ApoE-/-rats were provided by the Institute for Experimental Animals of Chinese Academy of Medical Sciences, with weight range (250-300) g, and were fed at room temperature range (18-20)℃, with illumination for at least 12 h and humidity (45%-55%).
2.2. Instruments and reagents
Real-time quantification PCR Amplifier Lightcycler 480 was purchased from Roche Life Science; the high speed microcentrifuge G508009 from Sangon; the precise one-way adjustable-Volume Pipettor from Sangon; the geldocument system GOS7500 from UVP; the electronic balance from Sartorius; the ultra cold storage freezer (-86℃ DW-86W100) from Haier; Real-time quantifi cation PCR kit from Roche Group; miRNA agomir/antagomir from RiboBio; Bovine Serum Albumin (A7030) from Sigma; the oil red O staining reagents from Sigma; BCA protein quantification kit from Pulitzer gene technology Co., LTD; the LPL primary antibody from Abcam; Goat Anti-rabbit second antibody from Abcam; and GoScriptTMReverse Transcription System from Promega.
2.3. Experimental methods
2.3.1. Animal grouping
All experimental ApoE-/-rats were fed with high fat and high cholesterol diet. After 1 week, these rats were randomly divided into group A, group B and group C (n=10). Group A: rats were injected with ApoE agonist through the caudal vein; Group B: rats were injected with ApoE antagonist through the caudal vein; Group C: as negative control group. Rats in Group A were given ApoE agonist for once, and rats in Group B were given ApoE antagonist for once respectively, with continuous treatment for 10 d. In addition, rats in A and Group B were fed with high fat and high cholesterol diet, with diet formula (large oil, unprocessed food grains and 0.35% of cholesterol).
2.3.2. Tissues sampling and trimming
Fasting for 12 h before rats were put to death and rats were anesthetized with intraperitoneal injection of 10% of chloral hydrate, with dosage of 300 mg/kg. After adequate anesthesia, blood was collected from eyeballs for high speed centrifugation. Supernate was obtained and enzyme oxidation method was used to detect the venous blood lipid indexes of rats, with indexes including triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low density lipoprotein (LDL). The aorta of rats was obtained and Western blotting method was used to detect the LPL expression levels of aortic tissues of rats. 4% of neutral formalin was used for 30 min perfusion for rats. Heart and partial aorta were obtained from rats: one part was applied to the quick frozen sections for aortic sinus tissue, with section thickness (10 μm), then oil red o staining was performed; the other part was applied to quick slicing for heart tissue, which was fi xed with 10% of paraformaldehyde, embedded with paraffi n, with serial section and section thickness (10 μm), then HE staining was performed.
2.3.3. Method statement
Serum of rats with a certain dilution multiple was added into the purified inflammatory factor antibody coating microplates successively. After washing and coloration, spectrophotometer was used to detect the homologous OD value and homologous inflammatory factor concentration was calculated. Aorta was obtained and fixed on the paraffin plate, with oil red o staining. After sealing piece, observation and statistics were performed under light microscope. Image Analysis Software was used to detect the percentage of fatty streak/total aortic area. Paraffin section was prepared with HE staining for 10 min, 1% of hydrochloric acid differentiation and PBS solution for washing, and then HE staining for 2 min once more. After dehydration, transparency and sealing piece were fi nished, observations were performed under microscope. Aortic tissues of rats were obtained and separated using polyacrylamide gel electrophoresis for extracting the protein. After coloration, gel image analysis system was performed for scanning and the gray area ratio of object of study/β-action was calculated.
2.4. Statistical method
SPSS 20.0 software was used for experimental data treatment. Blood lipid levels and lesion locations were expressed by Mean±SD deviation. One-way ANOVA analysis or t test was used for comparison among groups, where P<0.05 showed the statistical signifi cance.
3. Results
3.1. Blood lipid level comparison of rats
Fully automatic biochemical analyzer was used to detect the TG, TC, HDL levels of experimental rats in three groups. Compared with Group C (control group), blood lipid levels in Group A obviously reduced, while blood lipid levels in Group B obviously increased, where diff erence had statistical signifi cance (P<0.05). It could be proved that the usage of ApoE agonist could reduce the blood lipid levels, while the usage of antagonist could increase the blood lipid levels (Table 1).
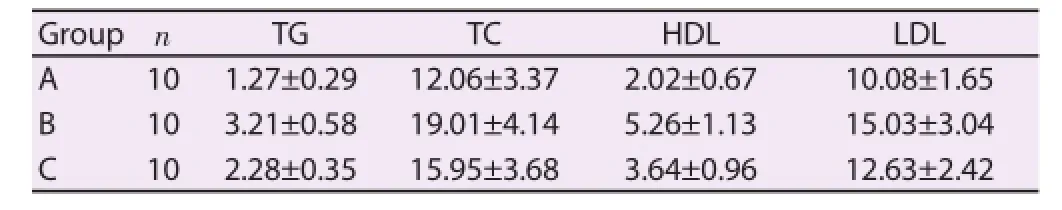
Table 1Blood lipid level comparison of rats (mmol/L).
3.2. Aortic AS lesions of rats
The oil red 0 staining was employed to detect the aortic intima lipid accumulation and aortic sinus lipid accumulation of rats. The proportion of lipid accumulation area, intima area and aortic lumen area were calculated by software. Compared with Group C, the proportion of aortic intima lipid accumulation and aortic sinus lipid accumulation of rats in Group A signifi cantly reduced; while the proportion of aortic intima lipid accumulation and aortic sinus lipid accumulation of rats in Group B signifi cantly increased. Comparative diff erence among groups had statistical signifi cance (P<0.05). HE staining was performed to detect the aortic sinus AS lesion area of rats. Group C: obvious infl ammatory cell infi ltration and the formation of foam cells were visible. Group A: infl ammatory cell infiltration was reduced and foam cells were little and even invisible. Group B: a large number of infl ammatory cell infi ltration and the formation of foam cells were visible. The proportion of AS lesion area accounted for the total lumen area was calculated by software. Compared with Group C, the aortic sinus AS lesion area proportion of rats in Group A signifi cantly reduced; while the aortic sinus AS lesion area proportion of rats in Group B significantly increased. Comparative difference among groups had statistical signifi cance (Table 2) (P<0.05).

Table 2Aortic AS lesions of rats (%).
3.3. Aortic LPL protein expression levels of rats
Western blot method was used to detect the aortic LPL protein expression levels of rats (Figure 1). Compared with Group C, aortic LPL protein expression levels in Group A significantly reduced, while aortic LPL protein expression levels in Group B signifi cantly increased, and comparative diff erence among groups had statistical signifi cance (P<0.05).

Figure 1. Aortic LPL protein expression levels of rats in three groups.
4. Discussion
AS often occurred in the large and medium-sized artery. The appearance of lipid accumulation (cholesterol, glucolipid etc.) in vessel wall intima was the key factor of the occurrence and development of AS. Cholesterol mainly came from plasma lipoprotein. Plasma lipoprotein could increase the cholesterol internal fl ow and deposition within arterial walls, like LDL could promote the AS occurrence. In addition, plasma lipoprotein could promote the cholesterol outward transport from blood vessels, like HDL had inhibition eff ects on AS[10-13]. ApoE was an important component of plasma lipoprotein and played an important role in regulating the cholesterol etc[14]. Related report[15] showed that pathological symptoms of ApoE-/-rats were in accordance with all stages of AS and were similar to human beings. Therefore, in this study, ApoE-/-rats were selected and were fed with high fat and high cholesterol diet for successful building of AS model of rats to discuss the eff ect mechanism of miR-467b on AS.
Not only miRNA could play effects in the non-coding region of targeting miRNA but also could miRNA play effects in coding region of targeting miRNA, which could affect the transcription translation process, increase the degradation speed of mRNA, involve and regulate the endothelial cell growth, infl ammatory response, lipid regulation etc, which were related to AS, and fi nally aff ected the occurrence and development of AS[16,17]. Recent researches indicated that numerous miRNA therapeutic targets involved in the process of AS, in addition, miR-33, miR-122 were closely related to AS[18,19]. Some report[7] showed that miR-467b also involved in the occurrence and development of AS, but its specifi c mechanism was not clear. In this study, we also found that miR-467b could obviously reduce the blood lipid levels of ApoE-/-rats. In addition, LDL was obviously reduced, in the meantime, HDL was also reduced, which indicated that miR-467b acted on the ATP binding cassette transponer 1 A1 and acyl-coenzyme A: Acyl Cholesterol Acyl Transferase 1, which indirectly inhibited the reverse transport process of cholesterol, and then resulted in the blocking of HDL formation.
Through the observations of lipid accumulation area and lesion area, we found that miR-467b could obviously improve the aortic intima and aortic sinus lipid accumulation area, meanwhile, obviously reduce the atherosclerotic lesion area. The worsening of lipid accumulation area and lesion area appeared in miR-467b antagonist group. AS lipid infiltration theory was the common accepted explanation for AS pathogenesis. Lesion limitation began from aortic intima, then lipid, carbohydrate compound accumulation and endothelial injuries resulted in the metamorphosis, calcifi cation and decreased fl exibility of arterial intima; in addition, the deposition of cholesterol, glucolipid materials also accelerated the formation of atheromatous plaque. Monocytes in blood were attracted to the subcutaneous tissues after lipid particles were modifi ed. Monocytes were translated into macrophages, which could phagocytose thelipid particles largely. When the outward transport capacity of which was exceeded, foam cells, which were formed by macrophages, began to die, and the accumulation of dead foam cells formed the lipid pool, which was covered by collagen and elastic fi bers, which were released by smooth muscle cells, finally lesions came into being[20,21]. miR-467b involved in multiple steps of the forming process of lesion, which indicated that miR-467b might alleviate the aortic lipid accumulation and AS lesion range.
There were researches showed that miR-467b could targetedly regulate the LPL expression of RAW 264.7 macrophagocytes of rats and aff ect the lipid accumulation and infl ammatory factor release. This study also indicated that miR-467b could obviously reduce the LPL expression levels of aorta of rats. LPL was a kind of TG degradation rate-limiting enzyme and a kind of glycoprotein which was secreted by adipocytes, myocardial cells and macrophagocytes. LPL in macrophagocytes could mediate the lipid retention and modification, accelerate the formation of foam cells, promote the inflammatory factor expression and then promote the AS occurrence[22]. On the other hand, LPL also had a certain anti-AS eff ect through reducing plasma TG and increasing HDL levels[23]. Therefore, LPL could not explain the inhibiting eff ect of miR-467b on AS. The relationship between miR-467b and LPL in vivo should be analyzed in the further research.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Monneret D, Bonnefont-Rousselot D, Roche F. Letter by Monneret et al. Regarding article, ‘Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: The atherosclerosis risk in the community study’. Circ Heart Fail 2015; 8(5): 1009.
[2] Kali A,Yayar O, Erdogan B, Eser B, Buyukbakkal M, Ercan Z, et al. Is hepcidin-25 a predictor of atherosclerosis in hemodialysis patients? Hemodial Int 2015; doi:10.1111/hdi.12355.
[3] Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, et al. Erratum:G protein-coupled estrogen receptor protects from atherosclerosis. Sci Rep 2015; 5: 13510.
[4] Tao J, Liu CZ, Yang J, Xie ZZ, Ma MM, Li XY, et al. ClC-3 deficiency prevents atherosclerotic lesion development in ApoE(-/-)mice. J Mol Cell Cardiol 2015; 87: 237-247.
[5] Zhang C, Pang QH. Silencing mechanism comparison of siRNA and miRNA in organisms. Chin J Biochem Mol Biol 2012; 28(5): 393-398.
[6] Chen Y, Xu XX, Chen LB. Treatment strategy for tumor stem cells of target miRNA. Tumor 2013; 1: 97-102.
[7] Tian GP. Eff ect of MiR-467b target regulating of macrophagocyte-LPL of rats on atherosclerosis [Thesis]. Hunan: University Of South China: 2013.
[8] Cai W, Zhou Y, Miao L, Sun YP, Yao H. Relationship between lipoprotein lipase gene Hind 栿 and Pvu Ⅱ locus polymorphism and blood liquid in Xinjiang Uygur aged people. Zhongguo Manxingbing Yufang Yu Kongzhi 2013; 4: 402-405.
[9] Gu B, Zhao YC. Research progress of lipoprotein lipase and atherosclerosis and cerebral infarction. J Int Neurol Neurosur 2012; 6: 534-537.
[10] Son KY, Son HY, Chae J, Hwang J, Jang S, Yun JM, et al. Genetic association of APOA5 and APOE with metabolic syndrome and their interaction with health-related behavior in Korean men. Lipids Health Dis 2015; 14(1): 105.
[11] Pleva L, Kusnierova P, Plevova P, Zapletalova J, Karpisek M, Faldynova L, et al. Increased levels of MMP-3, MMP-9 and MPO represent predictors of in-stent restenosis, while increased levels of ADMA, LCAT, ApoE and ApoD predict bare metal stent patency. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015; doi: 10.5507/bp.2015.037.
[12] Cheng C, Jiang L, Yang Y, Wu H, Huang Z, Sun X. Eff ect of APOE gene polymorphism on early cerebral perfusion afteraneurysmal subarachnoid hemorrhage. Transl Stroke Res 2015; 6(6): 446-450.
[13] González-Castro TB, Tovilla-Zárate CA, Hernández-Díaz Y, Fresán A, Juárez-Rojop IE, Ble-Castillo JL, et al. No association between ApoE and schizophrenia: Evidence of systematic review and updated metaanalysis. Schizophr Res 2015; doi:10.1016/j.schres.2015.08.031.
[14] Zhong YJ, Zhang ZW, Xu Y, Hua XM, Hu Y. Effect of atorvastatin on the aortic atherosclerosis and calcification of ApoE~ (-/-)rats. Chin J Arteriosclerosis 2013; 4: 305-310.
[15] Jiang JJ, Wang HF, Tang JN, Guo L, Liang HS. Eff ect of zinc sulfate on the aortic matrix metalloproteinase (MMP)-9 and CD40 expression of apolipoprotein E knockout mouse with high fat diet. Zhongguo Yaowu Yu Linchuang 2013; 12: 1548-1550.
[16] Zhang HX, Gong H. Research progress of miRNA and atherosclerosis. J Chin Prac Diagn Ther 2014; 1: 1-3.
[17] Zhang H, Zhang K, Wang Z. The regulation network of DNA methylation and miRNA in atherosclerosis. Chin J Biochem Mol Biol 2014; 30(10): 985-992.
[18] He XL, Lin XL, Wang Z. miRNA-transcription factor-target gene feedforward loop: regulating and controlling new mechanism of tumor and atherosclerosis. Chem Life 2013; 6: 621-626.
[19] Song CZ, Guo R, Zhang BK. Regulating eff ect and clinical application of MicroRNA to atherosclerosis occurrence. Chin J Biochem Mol Biol 2013; 29(1): 13-18.
[20] Breitling C, Gross A, Büttner P, Weise S, Schleinitz D, Kiess W, et al. Genetic contribution of variants near SORT1 and APOE on LDL cholesterol independent of obesity in children. PLoS One 2015; 10(9): e0138064.
[21] Liao YH, Yang Y. New exploration of infl ammation and atherosclerosis. J Clin Cardiol 2014; 6: 461-463.
[22] Zhao YF, Pan SJ, Gao ZZ, Zhang HB. Research progress of the infl ammatory signaling pathways of atherosclerosis. Chin J Geriatr Heart Brain Vessel Dis 2014; 12: 1332-1334.
[23] Guo YQ, Li YF, Wang ZH. Eff ect of excited β_3 adrenergic receptor on the atherosclerotic plaque of apolipoproteinE knockout mouse. Chin J Geriatr Heart Brain Vessel Dis 2013; 11: 1192-1195.
Document heading 10.1016/j.apjtm.2016.01.026
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*
Hao-Fu Wang, M.D., Attending Physician, Physician’s Offi ce, Department of Vascular Surgery, Medical Technology Building, No.16, Jiangsu Rd., Shinan District, Qiangdao, Shandong 266005, China.
Tel: 18661803315
E-mail: guanxiaomei002@163.com
Foundation project: It was supported by Science and Technology Support Program Project of Qingdao Public Domain [No.: 2012-1-3-2- (16)-nsh].
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Effect of dimethyl fumarate on rats with chronic pancreatitis
