Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
2016-07-24LeeCraineyllioRomRibeirodaSilvargioLuizBessaLuzEcologiadeDoenasTransmissveisnaAmazniaInstitutoLenidasMariaDeaneFiocruzAmazniaRuaTerezina476AdrianpolisCEP69057070ManausAmazonasBrazil
J. Lee Crainey, Túllio Romão Ribeiro da Silva, Sérgio Luiz Bessa LuzEcologia de Doenças Transmissíveis na Amazônia, Instituto Leônidas e Maria Deane-Fiocruz Amazônia Rua Terezina, 476. Adrianópolis. CEP: 69.057-070 Manaus, Amazonas, Brazil
Contents lists available at ScienceDirect
Historic accounts of Mansonella parasitaemias in the South Pacific and their relevance to lymphatic filariasis elimination efforts today
J. Lee Crainey*, Túllio Romão Ribeiro da Silva, Sérgio Luiz Bessa Luz
Ecologia de Doenças Transmissíveis na Amazônia, Instituto Leônidas e Maria Deane-Fiocruz Amazônia Rua Terezina, 476. Adrianópolis. CEP: 69.057-070 Manaus, Amazonas, Brazil
ABSTRACT
There are two species of fi larial parasites with sheathless microfi lariae known to commonly cause parasitaemias in humans: Mansonella perstans and Mansonella ozzardi. In most contemporary accounts of the distribution of these parasites, neither is usually considered to occur anywhere in the Eastern Hemisphere. However, Sir Patrick Manson, who fi rst described both parasite species, recorded the existence of sheathless sharp-tailed Mansonella ozzardilike parasites occurring in the blood of natives from New Guinea in each and every version of his manual for tropical disease that he wrote before his death in 1922. Manson's reports were based on his own identifi cations and were made from at least two independent blood sample collections that were taken from the island. Pacifi c region Mansonella perstans parasitaemias were also later (in 1923) reported to occur in New Guinea and once before this (in 1905) in Fiji. Although Mansonella-parasitaemias are generally regarded as benign, they are thought to be of public health importance because they can aff ect the epidemiological monitoring of other fi larial diseases. In this article, we reviewed the historic literature concerning Pacifi c-origin Mansonella-parasitaemias in an attempt to explain how, despite repeated reports of Pacifi cregion Mansonella-parasitaemias, by as early as the 1970s, the WHO had arrived at the presentday view that Wuchereria bancrofti is the only cause of fi larial parasitaemias in Papua New Guinea. We have also evaluated the evidence supporting the contemporary existence of Pacifi carea parasitaemia-causing Mansonella parasites and assessed the relevance such parasites could have for present-day lymphatic fi lariasis elimination eff orts in the region.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Mansonella ozzardi
Mansonella perstans
Wuchereria bancrofti
Papua New Guinea
Lymphatic fi lariasis
Global Programme to Eliminate
Lymphatic Filariasis
1. Present perspectives on the global distribution of human Mansonella parasitism
Bain et al recently reviewed the genus of Mansonella placing three species known to parasitize humans in the genus: Mansonella perstans (M. perstans), Mansonella ozzardi (M. ozzardi) and Mansonella streptocerca (M. streptocerca)[1]. Other species of Mansonella parasites, which principally infect animals, are also known to cause zoonanosis in humans, however, such events are rare and have not yet been recorded to cause human parasitaemias, which would require an infecting female parasite to develop to maturity, mate, and then begin releasing microfi lariae into an infected individual’s blood[2,3]. M. streptocerca is also not usually regarded as causing human parasitaemias as M. streptocerca microfi lariae, like those of Onchocerca volvulus, are usually found only in the skin and not in the blood[2]. Both M. perstans and M. ozzardi, however, are thought to commonly cause blood parasitaemias. M. perstans, for example, has been estimated to be infecting around 114 million Africans and is also thought to occur in South America[4-6]. M. ozzardi is thought to be widespread throughout Latin America and the Caribbean but is not usually recorded outside the New World[4-11]. Most contemporary accounts of the distribution of Mansonella parasites exclude the Pacifi c and, in fact, all of the Eastern Hemisphere[4-11].
2. Historic accounts of Mansonella parasitaemias from the Pacific region
In 1897, Sir Patrick Manson reported that he had found ‘a small sharp-tailed, sheathless fi laria closely resembling, if not identical with, Filaria demarquayi’ in the blood from a native living on the ‘north-east coast’ of New Guinea (NG)[12]. Shortly after this first report, which most likely came from material originating in what is present day Northern or Oro Provence of present day Papua New Guinea (PNG), Manson went on to describe further Mansonella parasites from blood slides sent to him by Seligmann and taken from a native residing in what is now the Kairuku-Hiri district of PNG. Manson reportedly described these parasites as ‘identical in appearance to Filaria demarquaii (F. demarquaii)’[12,13]. Today, most contemporary identifi cations of fi larial worms are done using microfilariae and ‘Filaria demarquayi’ and ‘F. demarquaii’ are both regarded as synonyms of M. ozzardi[1,14,15]. Using the modern WHO’s ‘Bench Aids for the Diagnosis of Filarial Infections’, thus, the New Guinean parasitaemias that Manson reported as being caused by ‘minute non-sheathed, sharp-tailed’ microfi lariae can be confi dently classifi ed as M. ozzardi parasites — exactly as Manson tentatively classifi ed them more than 100 years ago[13-16]. Lynch reported fi nding M. perstans (under the synonym F. perstans) occurring in the blood of a native from the Fijian Provence of ‘Lau’and Cilento reported M. perstans from the Dutch side of NG[17-19]. After Cilento’s report, Manson's Tropical Diseases began recording the parasite as probably occurring in NG and the parasite can be seen recorded on the Dutch side of the island up to and including the 18th edition of the book[20]. Sharp also described M. perstans as occurring in NG writing that its presence in the island was 'recorded by Manson himself'[21], which may have been a reference to Manson-Bahr's reporting of the parasite in Manson's Tropical Diseases. Given Manson’s unquestionable interest in M. perstans and the fact that Lynch’s report was published in the highly infl uential British journal the Lancet, it would be surprising if Manson was unaware of Lynch’s report. Manson’s son-in-law (Manson-Bahr) who edited his ‘manual of diseases of warm climates’ was certainly aware of Lynch’s report as he cited the paper in which the report was made[22]. The omission of Fijian M. perstans from Manson’s tropical disease manual and elsewhere cannot therefore be viewed as a simple oversight on the part of the fi larial worm establishment and thus the possibility that there were (presently unclear) well-grounded genuine scientifi c reasons to doubt the legitimacy of Lynch’s report can not be reasonably ignored[5,16,23-31].
3. The disappearance of NG Mansonella parasitaemias from the scientific literature
In contrast to Lynch’s reports of Fijian M. perstans, Manson’s reports of M. ozzardi or a M. ozzardi-like parasite in NG were widely accepted and extensively cited during his life-time[12,16,21-25,32-38]. The reports appeared in the first edition of Manson’s ‘manual of diseases in warm climates’ and in all subsequent editions he published up to and including the last he wrote before his death[16,22-25]. The disappearance from the mainstream scientifi c literature of these NG Mansonella parasites and the M. perstans that Cilento reported was a gradual process that seems to have begun with the publication of the 7th edition of Manson’s Tropical Diseases in which Manson’s son-in-law (Manson-Bhar) effectively declared PNG free of M. ozzardi with the remark: ‘As far as it is known, Filaria ozzardi (F. ozzardi) is confi ned to the West Indies and South America.’[26]. This revised distribution is not accompanied by a retraction of Manson’s original reports and occurred at the same time that the book began formally synonymizing Filaria demarquayi (F. demarquayi) and F. demaraquaii with F. ozzardi (see Table 1) — all of which are today regarded as synonyms of M. ozzardi[26]. Manson-Bahr’s remarks can be seen as a natural progression of the revisions that Manson himself carried-out to the previous editions of this book. In the fi rst edition of this manual it appears Manson is actually naming the PNG microfilariae M. ozzardi (under the synonym F. demarquaii) (see Table 1). As early as the second edition (published in 1900), however, signs of Manson’s reticence to name the PNG microfi lariae as M. ozzardi are evident as Manson introduces the caveat that (before PNG M. ozzardi parasites can be classifi ed as such) adult forms need to be: 'discovered and compared' (to other fi larial parasites)[16]. Manson’s growing reticence is evident from the qualifi cation he added to this caveat in the 5th and 6th editions of his book from when he begins to write that adults need to be 'discovered and carefully compared'[24,25]. By the 11th edition of the book Manson-Bahr is referring to these very same PNG parasites as belonging to a similar or related species and remarking that M. ozzardi is diffi cult to distinguish from the M. perstans, which by this time he is acknowledging probably also occur in the island. Manson’s grandson’s fi larial parasite distribution map also acknowledged the occurrence of M. perstans on the Dutch side of NG, however, he made no mention of any parasitism-causing Mansonella parasite occurring anywhere on the island within the text of the book he edited[20]. In-line with the rest of mainstream scientific literature and Hawking and Denham who asserted in their 1970s WHO report that 'Wuchereria bancrofti' (W. bancrofti) periodic type is the only human fi larial worm present' in the PNG[39], Manson's Tropical Diseases had wiped all Mansonella parasites clean from the Eastern Hemisphere of their maps by the 20th edition of their book[28].
4. A modern perspective on Manson’s descriptions of parasitaemia-causing filariae from PNG
There are presently no parasitaemia-causing microfilariaematching Manson’s description thought to occur in PNG, the Pacifi c region or in fact anywhere in the Eastern Hemisphere[1,5,6]. Just like today, at the time of Manson’s first report of M. ozzardilike parasites, W. bancrofti was the only parasitaemia-causing filaria thought to occur in PNG and then, as now, these two parasites were regarded as easy to discriminate on the basis of microfi lariae alone and thus the possibility that Manson repeatedly misidentifi ed W. bancrofti as M. ozzardi-like parasites seems even less likely than the possibility that M. ozzardi-like parasites are routinely being misidentifi ed as W. bancrofti today. Owing to the diffi culty in obtaining adult specimens, since Manson’s death very few (if any) adult M. ozzardi have been recovered from humans and contemporary accounts of their distribution are based almost entirely on the identifi cation of microfi lariae alone[1,5,6]. Since the advent of molecular systematics, however, it has been possible to look for filarial parasite diversity below the level visible from microfilariae morphology (without obtaining adult specimens) by comparing fast evolving DNA sequences like the CO1 and certain rDNA sequences of the parasites[1,10,40-46]. While this approach to the parasite identifi cation has not been as widely used as it could or should have been, it has been used and to date there is evidence for just two parasitaemia-causing sheathless filarial parasites — named M. ozzardi and M. perstans — which are the same two sheathless parasitaemia-causing fi laria which Manson was aware of at the time of his death[1,4-6,10,40-46]. Today, both M. perstans and M. ozzardi parasitaemias are most often diagnosed using almost the same criteria that Manson used, namely using the morphology of the microfi lariae and their absence of periodicity in the blood[4-6,15]. Morphologically, Mansonella genus microfi lariae that cause parasitaemias can be discriminated from the other known parasitaemia-causing fi larial parasite species and from each other by their size, their absence of a sheath and their tail shape[1,4-6,15,45].
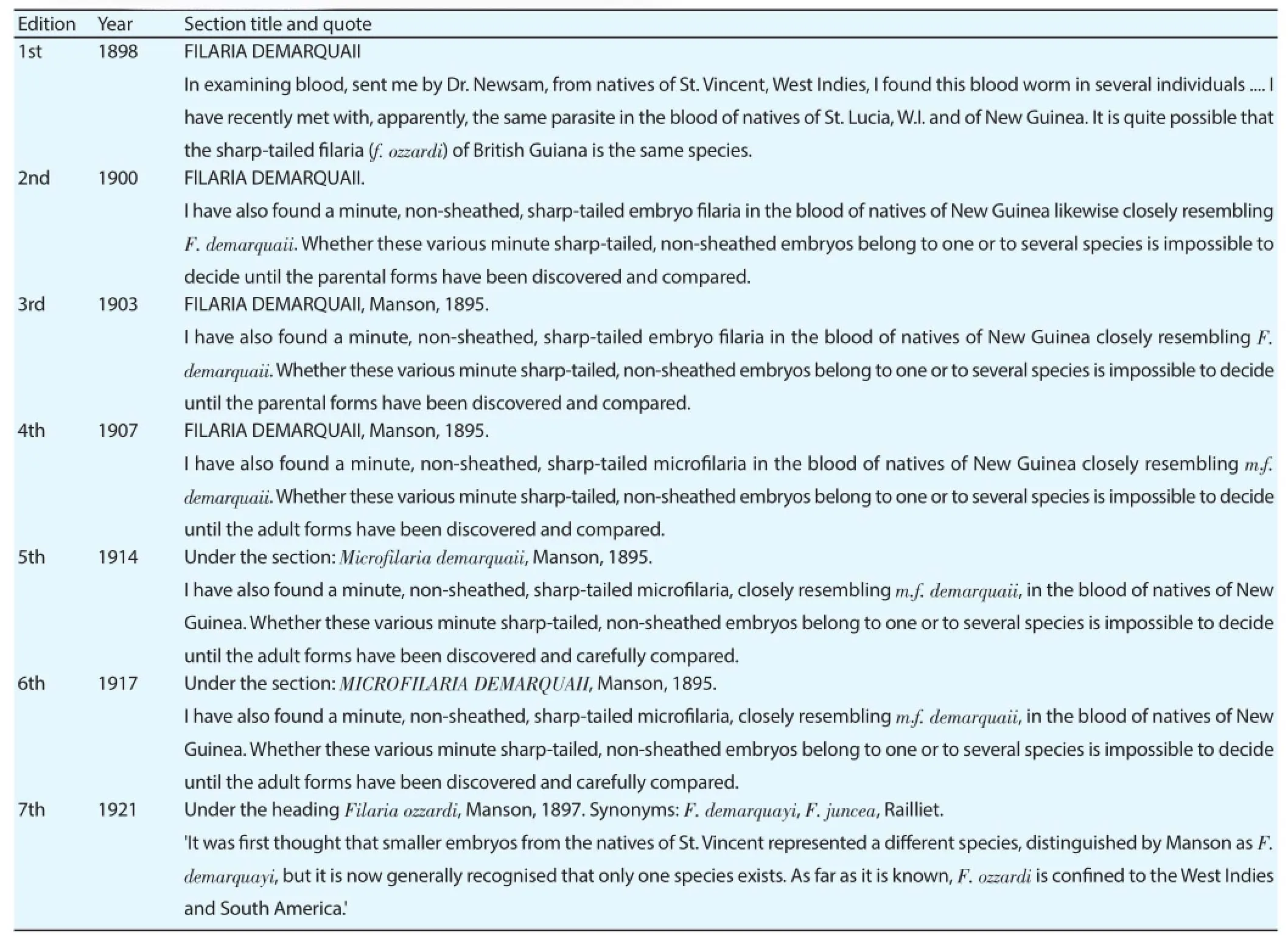
Table 1Manson’s reporting on M. ozzardi-like parasites from Papua New Guinea.
5. The absence of M. ozzardi-like parasites from modern PNG filarial maps
As human Mansonella parasites are not known to be vectored by mosquitoes, W. bancrofti transmission monitoring of wild-caught mosquito vectors is unlikely to detect or be aff ected by the existence of occult M. ozzardi-like filarial populations[2,4-6]. Similarly as Mansonella parasitaemias are mostly or entirely asymptomatic, and certainly have no clearly defi ned symptoms to diagnose them, clinicians are in no position to diagnose infections without bloodtests[2,4-6]. Blood examinations are thus the only method that parasites might be expected to be detected[2,4-6]. Over the last 15 years, fi larial parasite mapping in PNG has been done almost exclusively using immunological assays that test blood samples for circulating W. bancrofti antigens and most recently predominately with ICT cards, which would not reveal the presence of an unexpected M. ozzardi-like parasite[46-49].
Although, intuitively, light-microscopy-based malaria blood surveys might be thought to detect fi larial parasites if any were present and such surveys have detected M. ozzardi in the past, this type of survey cannot reasonably be expected to have found Mansonella parasites in PNG[50]. This is because malaria parasites are found using highmagnifi cation objectives rather than the low magnifi cation objectives that are used to fi nd fi larial parasitaemias[51-53]. Sir Patrick Manson actually advocated the need for schools of tropical medicine (before the London School of Hygiene and Tropical Medicine and the Liverpool School of Tropical Medicine existed) using this counterintuitive fact as an example of the sort of thing that needs to be taught. In his address on 'the necessity for special education in tropical medicine' Manson is reported as saying: 'filariae should be sought with an inch objective otherwise they will be missed' and that although 'in searching the blood for fi laria - a large fi eld is indispensable' this 'self-evident fact' is one that 'He (the student of tropical medicine) seldom arrives at spontaneously'[51-53]. Microscope based filarial surveys – which the WHO presently recommends should use a 10× objective to fi nd W. bancrofti – should, however, quite reasonably, have been expected to also have found Manson’s M. ozzardi-like parasites if any had occurred in the tested blood samples[51-53]. Trained microscopists should have no problem discriminating between the much larger periodic sheathed W. bancrofti and the unsheathed M. ozzardi-like parasites that Manson reported even if they were not expecting to see them[15,53].
In a 2013 review about lymphatic filariasis in PNG, 155 microscopy-based fi larial surveys from PNG were reported to have been conducted between 1980 and 2011, without any new reports of Mansonella or Mansonella-like parasites causing parasitaemias in the area[47]. Similarly, in two other detailed reviews of microfi larial surveying covering the period from Manson’s first report of M. ozzardi in 1897 up to Hawking and Denham’s 1971 WHO report declaring W. bancrofti as the only fi larial parasite to occur in PNG, only Seligmann (1901) and Cilento (1923) have provided fi rst-hand accounts of Mansonella parasites causing parasitaemias[12,13,18,39,54]. The primary source data supporting the contemporary existence of Mansonella-caused parasitaemias occurring in NG cannot be, therefore, regarded as strong. Likewise, however, when this survey data is assessed by geography, it can be seen that the localities that the parasites were fi rst recorded in have not been rigorously surveyed and thus that the evidence that the parasites have been lost from NG is also not strong[39,47,54]. Between 1980 and 2011, there were no microfi larial surveys in the “Central” Provence where Seligmann’s report fi rst originated and there seems to have been just one survey conducted in the Provence (in 1936) after his report[47,55]. Although it is more difficult to be sure where exactly the M. ozzardi-like parasites that Manson recorded as deriving from the 'north-east coast' of NG originated, it is also likely that this locality has also not been rigorously surveyed[12,39,47,54]. At the time of Manson’s report what is now known as the Northern or Oro province was the most northeasterly part of what was then British NG and thus this Provence is likely to be where the parasites he described came from[12,39]. Oro Provence, which is more than 20 000 km2in size, was surveyed at just one coastal locality (Oro Bay) between 1980 and 2011 and prior to this once at the inland town of Popondetta in 1966[39,47,54].
Little is known of the epidemiology of M. ozzardi, however, from what is known it is clear that M. ozzardi can be stably maintained over long periods of time in very localized foci[11,12,36,37,50,56-58]. In at least three of the Caribbean islands where M. ozzardi has been recorded (St. Vincent, St Lucia and Nevis), the parasites seem to have been stably maintained with just a single village focus[12,36,37,49,55]. And in almost all of the islands where the parasites’ distribution has been studied, it appears that their distribution is localized to just one or two areas[11,12,36,37,50,57,58]. While most of the Caribbean islands have not been well-sampled since the 1940s, the evidence that the parasites can be maintained (even with ongoing anti-fi larial mass-drug distribution) has been clearly shown in both Trinidad and Haiti[11,36,37,50,57-58]. In Haiti, where most of the recent Caribbean surveying has occurred it is apparent the parasites have been maintained in the island for over 90 years[11,36,37,56,58]. If the known M. ozzardi epidemiology in the Caribbean is taken as a guide for what could be happening in NG, the possibility of occult M. ozzardi or M. ozzardi-like parasitaemias continuing to occur in localized foci of NG is diffi cult to rule out.
6. The possible impact of occult M. ozzardi-like blood parasitaemias in PNG on W. bancrofti epidemiological monitoring and control
Accurate fi larial parasite distribution maps are key to the success of the Global Programme to Eliminate Lymphatic Filariasis (GPELF)[47-48,59,60]. Historically, lymphatic fi lariasis control eff orts have made use of light-microscopy based microfilariae surveys, circulating antigen and antibody detection assays to plan control programs in PNG[47]. Since 2000, however, most mapping data have derived from circulating antigen assays and most predominately from diagnoses made with the immunochromatographic card test (ICT), which have been performed without the fear of other fi larialparasite species aff ecting their accuracy[47]. Although the specifi city of ICT cards has been tested against M. ozzardi parasites from Bolivia and ICT-based mapping of W. bancrofti has been carried out in co-endemic counties like Haiti, there are no guarantees that W. bancrofti elimination eff orts would be unaff ected by the existence of occult M. ozzardi or M. ozzardi-like parasitaemias in PNG or indeed the existence of such parasites in Haiti or beyond[61-63]. Preliminary testing of the ICT cards suggested that they did not cross-react with Loa loa and/or M. perstans, however, recent fi eld studies in Africa have shown that they can[64]. Other immunological assays for W. bancrofti detection can and have been used for W. bancrofti diagnoses in the Pacific region, but none of the cross-reactivity testing of these tests could be regarded as much more rigorous than the ICT evaluations[65,66]. Cross-reactivity issues are also known to aff ect the security of antibody detecting immunological assays. For example, the ov16 antibody detection assay, which has been used eff ectively for onchocerciasis control and proven especially important for recrudescence monitoring throughout Africa and in many parts of Latin America, cannot be used safely in the Amazonia onchocerciasis focus because it can cross-react with M. ozzardi-positive sera[67,68]. Similarly, alternative anti-O. volvulus antibody detecting assays have also been shown to cross-react with M. ozzardi-positive sera from some geographic regions and not others[68].
Present plans for W. bancrofti elimination in PNG involve focusing resources and control eff orts on the endemic areas. As Kairuku-Hiri district (where M. ozzardi were last recorded to occur) is classifi ed as non-endemic for W. bancrofti, it is unlikely to be surveyed as part of the GPELF's existing plans[47]. Extrapolating from recent surveying eff orts, it would seem, moreover, that even if the site were to be surveyed for the presence of W. bancrofti, it is unlikely that it would be surveyed with the sort of PCR or light-microscopy microfi larial surveys that might be expected to uncover the existence of M. ozzardi parasitaemias[47]. Because more than 1 000 ICT card tests have been conducted in this district without W. bancrofti being detected, it is clear that M. ozzardi parasites have not previously impacted on W. bancrofti monitoring in Kairuku-Hiri district and so an argument could be made that the presence or absence of M. ozzardi or M. ozzardi-like parasites in this area (or indeed all of PNG) is irrelevant for GPELF eff orts[47]. Even if this perspective is adopted, however, surveying the Kairuku-Hiri district for M. ozzardi or M. ozzardilike parasites could still prove very useful to control efforts. This is because uncovering a M. ozzardi or M. ozzardi-like focus could provide a useful resource not only to confi rm the security of ICT card testing in the area, but also as a useful resource for testing the security of other diagnostic tools such as Bm14-based assays which are likely to prove important for the recrudescence monitoring of the GPELF[47,48,59,60,66].
Because M. ozzardi or M. ozzardi-like infections have not been recorded from over 155 microscope surveys in the last 30 years, it is unlikely that they are presently common in PNG. It is, thus, likewise unlikely that they have had a major impact on W. bancrofti mapping eff orts to date whether they still occur in PNG or not. It is, however, important to note that if such parasites do exist and interfere with the accuracy of lymphatic fi lariasis disease and disease-risk mapping eff orts, their importance will only grow as the GPELF gets nearer its target and the number of W. bancrofti parasites in the PNG diminishes[47,48,59,60]. In light of this, whether surveys in Kairuku-Hiri district fi nd the parasite or not, it may prove prudent for the GPELF to shift away from its present focus on immunological-based diagnosis of W. bancrofti blood infections in the PNG and begin to introduce at least some W. bancrofti DNA-detection based diagnostic techniques, with the capacity to detect more than one type of fi larial parasite[44,46,49]. Many PCR tests of this nature have been developed and could be used for this purpose[44,46,49]. Tang et al. reported one such test that is presently being recommended for the detection of Onchocerca volvulus DNA[44] and which has recently been shown to be highly sensitive at detecting M. ozzardi blood samples[40,49]. This test has also been shown to be capable of amplifying W. bancrofti DNA and therefore could be, potentially, adapted to reliably diagnose W. bancrofti infections and distinguish them from any M. ozzardi or M. ozzardi-like blood parasitaemias[40,44,49].
Conflict of interest statement
The authors declare that they have no confl ict of interest.
Acknowledgments
The authors would like to thank Tony Shelley of the Natural history Museum London for helpful comments on an earlier draft of this manuscript and acknowledge FAPEAM for funding J. L. Crainey and Túllio Romão Ribeiro da Silva with research fellowships. This paper is contribution number 26 of the Research Programme on Infectious Disease Ecology in the Amazon of the Instituto Leônidas & Maria Deane – Fiocruz Amazônia.
References
[1] Bain O, Mutafchiev Y, Junker K, Guerrero R, Martin C, Lefoulon E, et al. Review of the genus Mansonella Faust, 1929 sensu lato (Nematoda: Onchocercidae), with descriptions of a new subgenus and a newsubspecies. Zootaxa 2015; 3918: 151–193.
[2] Downes BL Jacobsen KH. A systematic review of the epidemiology of mansonelliasis. Afr J Infect Dis 2010; 4: 7–14.
[3] Orihel TC, Eberhard ML. Zoonotic filariasis. Clin Microbiol Rev 1998; 11: 366–381.
[4] Simonsen PE, Onapa A, Asio S. Mansonella perstans filariasis in Africa. Acta Trop 2011; 120: S109–120.
[5] Simonsen PE, Fischer PU, Hoerauf A, Weil GJ. The filariases. In: Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson's tropical diseases. 23rd ed. Philadelphia: Elsevier Saunders; 2014, p. 737–765.
[6] Muller R. Worms and human disease. 2nd ed. London; CABI publishing; 2002.
[7] Shelley AJ, Coscarón S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem Inst Oswaldo Cruz 2001; 96: 451–458.
[8] Basano SA, Fontes G, Medeiros JF, Aranha Camargo JS, Souza Vera LJ, Parente Araújo MP, et al. Sustained clearance of Mansonella ozzardi infection after treatment with ivermectin in the Brazilian Amazon. Am J Trop Med Hyg 2014; 90: 1170–1175.
[9] Vianna LM, Martins M, Cohen MJ, Cohen JM, Belfort R Jr. Mansonella ozzardi corneal lesions in the Amazon: a cross-sectional study. BMJ Open 2012; 2: pii: e001266.
[10] Marcos LA, Arrospide N, Recuenco S, Cabezas C, Weil GJ, Fischer PU. Genetic characterization of atypical Mansonella (Mansonella) ozzardi microfi lariae in human blood samples from northeastern Peru. Am J Trop Med Hyg 2012; 87: 491–494.
[11] Raccurt CP, Brasseur P, Cicéron M, Boncy J. Epidemiologic survey of Mansonella ozzardi in Corail, Haiti. Am J Trop Med Hyg 2014; 90: 1167–1169.
[12] Manson P. On certain new species of nematode haematozoa occurring in America. Brit Med J 1897; 2: 1837–1838.
[13] Seligmann CG. Filariasis in British New Guinea. J Pathol Bacteriol 1901; 7: 308.
[14] Post RJ, Adams Z, Shelley AJ, Maia-Herzog M, Luna Dias AP, Coscarón S. The morphological discrimination of microfilariae of Onchocerca volvulus from Mansonella ozzardi. Parasitol 2003; 127: 21–27.
[15] World Health Organization. Bench aids for the diagnosis of filarial infections. Geneva: World Health Organization; 1997.
[16] Manson P. Tropical disease: A manual of diseases of warm climates. 2nd ed. London: Cassell and Company; 1900.
[17] Backhouse TC, Heydon GA. Filariasis in Melanesia: observations at Rabaul relating to incidence and vectors. Trans R Soc Trop Med Hyg 1950; 44: 291–306.
[18] Cilento RW. Australia. Department of Health. Service publication (Tropical Division); no. 4. Backhouse and Heydon, editors. Filariasis: with especial reference to Australia and its dependencies : a review and compilation. Commonwealth of Australia and Dependencies. Australian Department of Health Service Publication (Tropical division). 1923.
[19] Lynch GWA. A note on the occurrence of fi laria in Fijians. Lancet 1905; 165: 21–22.
[20] Manson-Bahr PEC, Apted FIC. Manson's tropical diseases. 18th ed. London: Bailliè re Tindall; 1982.
[21] Sharp N. Filaria perstans: its development in Culicoides austeni. Trans R Soc Trop Med Hyg 1928; 21: 371–396.
[22] Manson-Bahr P, Muggleton WJ. Further research on fi lariasis in Fiji (A study of host-parasite relationships, with special reference to the status of the Pacifi c fi laria Wuchereria pacifica). Trans R Soc Trop Med Hyg 1952; 46: 301–326.
[23] Manson P. Tropical disease: A manual of diseases of warm climates. 3rd ed. London: Cassell and Company; 1903.
[24] Manson P. Tropical disease: A manual of diseases of warm climates. 4th ed. London: Cassell and Company; 1907.
[25] Manson P. Tropical disease: A manual of diseases of warm climates. 5th ed. New York: William Wood and Company; 1914.
[26] Manson P. Tropical disease: A manual of diseases of warm climates. 6th ed. London: Cassell and Company Limited; 1917.
[27] Manson P, Manson-Bahr P. Manson’s tropical diseases V2: A manual of the diseases of warm climates. 7th ed. London: Cassell and Company Limited; 1921.
[28] Manson PH. Tropical disease: A manual of diseases of warm climates. 11th ed. London: Cassell and Company; 1940.
[29] Cook G. Manson’s tropical diseases. 20th ed. London: WB Saunders Company Ltd; 1996.
[30] Cook G, Zumla A. Manson’s tropical diseases. 21st ed. London: WB Saunders Company Ltd; 2003.
[31] Cook G. A manson’s tropical diseases. 22nd ed. London, Philadelphia, Toronto, Sydney, Tokyo: WB Saunders Company Ltd.; 2009.
[32] Braun M. Filaria demarquayi, Manson, 1895. The animal parasites of man: A handbook for students and medical men. New York: Wood; 1906, p. 296–297.
[33] Galgey O. Filaria demarquaii in St. Lucia, West Indies. Brit Med J 1899; 1: 145–146.
[34] Johnston TH. Notes on Australian Entozoa No. 1. (Communication from the Government. Bureau of Microbiology, Sydney. Australia). Rec Aust Mus 1909; 7: 329–344.
[35] Johnston TH. Notes on some Australian parasites. Agric gaz NSW 1909; 20: 581–584.
[36] Low GC. The unequal distribution of filariasis in the tropics. Lancet 1908; 171: 279–281.
[37] Low GC. The unequal distribution of fi lariasis in the tropics. Trans R Soc Tropical Med Hyg 1908; 1: 84–95.
[38] Sweet G. The endoparasites of Australian stock and native fauna. Proc RSoc Victoria 1908; 21: 454–527.
[39] Hawking F, Denham DA. The distribution of human filariasis throughout the world. Part I: The Pacific region, including New Guinea. Geneva: WHO; 1971: p. 31.
[40] Medeiros JF, Almeida TA, Silva LB, Rubio JM, Crainey JL, Pessoa FA, et al. A field trial of a PCR-based Mansonella ozzardi diagnosis assay detects high-levels of submicroscopic M. ozzardi infections in both venous blood samples and FTA® card dried blood spots. Parasit Vectors 2015; 20: 1–8.
[41] Morales-Hojas R. Molecular systematics of filarial parasites, with an emphasis on groups of medical and veterinary importance, and its relevance for epidemiology. Inf Gen Evol 2009; 9: 748–759.
[42] Morales-Hojas RM, Post RJ. Regional genetic variation in the major sperm protein genes of Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea). Int J Parasitol 2000; 30: 1459–1465.
[43] Morales-Hojas RM, Post RJ, Shelley AJ, Maia-Herzog M, Coscarón S, Cheke RA. Characterisation of nuclear ribosomal DNA sequences from Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) and development of a PCR-based method for their detection in skin biopsies. Int J Parasitol 2001; 31: 169–177.
[44] Tang TH, López-Vélez R, Lanza M, Shelley AJ, Rubio JM, Luz SBL. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon Region. Mem Inst Oswaldo Cruz 2010; 105: 823–828.
[45] Post RJ, Crainey JL, Bivand A, Renz A. Laser-assisted microdissection for the study of the ecology of parasites in their hosts. Mol Ecol Resour 2009; 9: 480–486.
[46] Alhassan A, Li Z, Poole CB, Carlow CK. Expanding the MDx tooltable for fi larial diagnosis and surveillance. Trends Parasitol 2015; 31: 391–400.
[47] Graves PM, Makita L, Susapu M, Brady MA, Melrose W, Capuano C, et al. Lymphatic fi lariasis in Papua New Guinea: distribution at district level and impact of mass drug administration, 1980 to 2011. Parasit Vectors 2013; 6: 1–18.
[48] Rebollo MP, Bockarie MJ. Shrinking the lymphatic fi lariasis map: update on diagnostic tools for mapping and transmission monitoring. Parasitol 2014; 141: 1912–1917.
[49] Ricciardi A, Ndao M. Diagnosis of parasitic infections: What’s going on? J Biomol Screen 2015; 20: 6–21.
[50] Charles LJ. Malaria in the Leeward and Windward Islands, British West Indies. Am J Trop Med Hyg 1952; 1: 941–961.
[51] Manson P. An introductory address: On the necessity for special education in tropical medicine. Br Med J 1897; 2: 985–989.
[52] Manson P. An Introductory Address: On the necessity for special education in tropical medicine. Lancet 1897; 150: 842–845.
[53] World Health Organization. Monitoring and epidemiological assessment of the programme to eliminate lymphatic filariasis at implementation unit level. Geneva: World Health Organization; 2005.
[54] Iyengar MOT. Distribution of filariasis in the South Pacific Region. Noumea: South Pacifi c Commission; 1954, p. 52.
[55] Clements FW. A medical survey in Papua; Report of the fi rst expedition by the School of Public Health and Tropical Medicine to Papua, 1935. Med J Aust 1936; 1: 451–463.
[56] Low GC. Notes on Filaria demarquaii. Br Med J 1902; 1: 196–197.
[57] Chadee D, Tilluckdharry C, Rawlins S, Doon R, Nathan MB. Mass chemotherapy with diethylcarbamazine for the control of Bancroftian filariasis: a twelve-year follow-up in northern Trinidad, including observations on Mansonella ozzardi. Am J Trop Med Hyg 1995; 52: 174–176.
[58] Raccurt CP, Brasseur P, Boncy J. Mansonelliasis, a neglected parasitic disease in Haiti. Mem Inst Oswaldo Cruz 2014; 109: 709–711.
[59] Rebollo MP, Bockarie MJ. Toward the elimination of lymphatic fi lariasis by 2020: treatment update and impact assessment for the endgame. Expert Rev Anti Infect Ther 2013; 11: 723–731.
[60] Rebollo MP, Bockarie MJ. Rapid diagnostics for the endgame in lymphatic fi lariasis elimination. Am J Trop Med Hyg 2013; 89: 3–4.
[61] Bartoloni A, Cancrini G, Bartalesi F, Roselli M, Gamboa Barahona H. ICT Filariasis test in patients harbouring Mansonella ozzardi. Trop Med Int Health 2000; 5: 833.
[62] Rochars MB, Kanjilal S, Direny AN, Radday J, Lafontant JG, Mathieu E, et al. The Leogane, Haiti demonstration project: decreased microfi laremia and program costs after three years of mass drug administration. Am J Trop Med Hyg 2005; 73: 888–894.
[63] Boyd A, Won KY, McClintock SK, Donovan CV, Laney SJ, Williams SA, et al. A community-based study of factors associated with continuing transmission of lymphatic fi lariasis in Leogane, Haiti. PLOS Negl Trop Dis 2010; 4: 1–10.
[64] Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, et al. Filarial antigenemia and Loa loa night blood microfi laremia in an area without bancroftian fi lariasis in the Democratic Republic of Congo. Am J Trop Med Hyg 2014; 91: 1142–1148.
[65] Joseph H, Maiava F, Taseri T, Silva U, Lammie P, Melrose W. Epidemiological assessment of continuing transmission of lymphatic fi lariasis in Samoa. Ann Trop Med Parasitol 2011; 105: 567–578.
[66] Tisch DJ, Bockarie MJ, Dimber Z, Kiniboro B, Tarongka N, Hazlett FE, et al. Mass drug administration trial to eliminate lymphatic fi lariasis in Papua New Guinea: changes in microfi laremia, fi larial antigen, and Bm14 antibody after cessation. Am J Trop Med Hyg 2008; 78: 289–293.
[67] Luz SL, Crainey JL, Shelley AJ, Rubio M. Outstanding insecurities concerning the use of an Ov16-based ELISA in the Amazonia onchocerciasis focus. Mem Inst Oswaldo Cruz 2014; 109: 506–508.
[68] Shelley AJ, Maia-Herzog M, Calvão-Brito R. The specificity of an ELISA for detection of Onchocerca volvulus in Brazil in an area endemic for Mansonella ozzardi. Trans R Soc Trop Med Hyg 2001; 95: 171–173.
Document heading 10.1016/j.apjtm.2016.01.040
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
*Corresponding author: James Lee Crainey, Ecologia de Doenças Transmissíveis na Amazônia, Instituto Leônidas e Maria Deane-Fiocruz Amazônia Rua Terezina, 476. Adrianópolis. CEP: 69.057-070 Manaus, Amazonas, Brazil.
E-mail: james_lee@amazonia.fi ocruz.br
Foundation project: FIOCRUZ, FAPEAM, CNPq.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of miR-467b on atherosclerosis of rats
- Regulating effect of activated NF-KB on edema induced by traumatic brain injury of rats
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Effect of dimethyl fumarate on rats with chronic pancreatitis
