Hepatoprotective effects of Nigella sativa seed extract against acetaminophen-induced oxidative stress
2016-07-24GareeballaOsmanAdamMdMahbuburRahmanSeiJinLeeGiBeumKimHyungSubKangJinShangKimShangJinKimDepartmentofVeterinaryMedicineandSurgeryCollegeofVeterinaryMedicineSudanUniversityofScienceandTechnologyBoxNo04HilatKukuKharto
Gareeballa Osman Adam, Md. Mahbubur Rahman, Sei-Jin Lee, Gi-Beum Kim, Hyung-Sub Kang, Jin-Shang Kim, Shang-Jin Kim*Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, Sudan University of Science and Technology, P.O. Box No. 04, Hilat Kuku, Khartoum North, SudanDepartment of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Chonbuk National University, Iksan Campus, 79 Gobongro, Iksan-si, Jeollabuk-do 570-75, Republic of KoreaKorea Basic Science Institute Jeonju Center, 567 Baekje-daero, Deokjin-gu, Jeonju-si, Jeollabuk-do 56-756, Republic of Korea
Contents lists available at ScienceDirect
Hepatoprotective effects of Nigella sativa seed extract against acetaminophen-induced oxidative stress
Gareeballa Osman Adam1,2#, Md. Mahbubur Rahman2#, Sei-Jin Lee3, Gi-Beum Kim2, Hyung-Sub Kang2, Jin-Shang Kim2, Shang-Jin Kim2*
1Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, Sudan University of Science and Technology, P.O. Box No. 204, Hilat Kuku, Khartoum North, Sudan
2Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Chonbuk National University, Iksan Campus, 79 Gobongro, Iksan-si, Jeollabuk-do 570-752, Republic of Korea
3Korea Basic Science Institute Jeonju Center, 567 Baekje-daero, Deokjin-gu, Jeonju-si, Jeollabuk-do 561-756, Republic of Korea
ABSTRACT
Objective: To investigate the protective effects of Nigella sativa seed extract (NSSE) against acetaminophen (APAP)-induced hepatotoxicity in TIB-73 cells and rats. Methods: Toxicity in TIB-73 cells was induced with 10 μmol/L APAP and the protective eff ects of NSSE were evaluated at 25, 50, 75, 100 μg/mL. For in vivo examination, a total of 30 rats were equally divided into fi ve experimental groups; normal control (vehicle), APAP (800 mg/kg body weight single IP injection) as a hepatotoxic control, and three APAP and NS pretreated (2 weeks) groups (APAP+NSSE 100 mg; APAP+NSSE 300 mg and APAP+NSSE 900 mg/kg). Results: TIB-73 cell viability was drastically decreased by (49.0±1.9)% after the 10 μmol/L APAP treatment, which also increased reactive oxygen species production. Co-treatment with NSSE at 25, 50, 75, and 100 μg/mL signifi cantly improved cell viability and suppressed reactive oxygen species generation. In vivo, the APAP induced alterations in blood lactate levels, pH, anionic gap, and ion levels (HCO3-, Mg2+and K+), which tended to normalize with the NSSE pretreatment. The NSSE also signifi cantly decreased elevated serum levels of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and alkaline phosphatase induced by APAP, which correlated with decreased levels of hepatic lipid peroxidation (malondialdehyde), increased superoxide dismutase levels, and reduced glutathione concentrations. Improved hepatic histology was also found in the treatment groups other than APAP group. Conclusions: The in vitro and in vivo fi ndings of this study demonstrated that the NSSE has protective eff ects against APAP-induced hepatotoxicity and metabolic disturbances by improving antioxidant activities and suppressing both lipid peroxidation and ROS generation.
ARTICLE INFO
Article history:
Received in revised form 20 January 2016
Accepted 15 February 2016
Available online 20 March 2016
Nigella sativa
Acetaminophen
Antioxidants
Oxidative stress
TIB-73 cells
Rat
1. Introduction
Acetaminophen (APAP) is an antipyretic and analgesic drug that is widely used to treat fevers, headaches, and various forms of pain. APAP is readily available without a prescription, and is considered safe and eff ective when taken at therapeutic doses[1,2]. However, acute or cumulative overdose can result in toxicity in the form of hepatic and renal tubular necrosis, which is lethal in both humans and experimental animals[2-4]. In the United States, APAP overdoses account for more than 56 000 emergency room visits, 2 600 hospitalizations, and an estimated 458 deaths due to acute liver failure each year[5]. APAP-induced hepatotoxicity has been demonstrated in experimental animal models as well as clinical cases[1,3,4]. Specifically, excessive consumption of APAP causes elevated reactive oxygen species (ROS) production[6] and disturbances in the body’s natural defense systems. ROS generation, lipid-peroxidation, oxidative stress, and mitochondrial dysfunction are all thought to contribute to the initiation or progression ofAPAP-induced hepatotoxicity[1,6]. Moreover, APAP may cause lactic acidosis often with coma, occurring prior to the onset of hepatotoxicity or later in the course of poisoning in patients with established acetaminophen related hepatotoxicity. Indeed, elevated blood lactate concentrations have been shown to be a strong predictor of death[7].
Natural traditional remedies for the prevention and treatment of liver diseases have become popular in the last decade worldwide; however, there is limited information on the biological activities and mechanisms of these remedies. Nigella sativa (NS) seed (commonly known as black cumin) is a plant belonging to the Ranunculaceae family and is available in Asian, Mediterranean, Middle Eastern, and African countries[8]. NS is traditionally used for the treatment of many diseases owing to its reported anti-viral, anti-infl ammatory, anti-diabetic, immunomodulatory, anti-cancer, and hepatoprotective activities[9,10]. In addition, NS seed oil attenuates the toxic side eff ects of several chemotherapeutic agents[11].
The liver is a primary metabolic organ and is responsible for many critical functions within the body. Indeed, if the liver becomes diseased or injured, the ensuing threat to the body’s metabolic system can be life threatening. Many researchers have previously demonstrated that antioxidants prevent hepatotoxicity by inhibiting lipid peroxidation, ROS generation, and also by suppressing the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP)[2,12,13]. NS seed is a well-known antioxidant-containing seed. Therefore, the aim of the present study was to investigate the effi cacy of NS seeds extract (NSSE) against APAP-induced hepatotoxicity in TIB-73 cells and male Sprague Dawley rats as well as attenuating the eff ects of metabolic disturbances in APAP intoxicated rats.
2. Materials and methods
2.1. NS seed extracts
NS seeds were purchased from a local herb-store in Republic of Sudan and authenticated by a Taxonomist at Sudan University of Sciences & Technology and further confi rmed by a Taxonomist at Chonbuk National University, Republic of Korea. The NS seeds were washed with running tap water, air dried, and homogenized to a fine powder and stored in air-tight containers at 40 °C. The dried (250 g) powder of the plants was extracted with 2 500 mL of aqueous methanol (methanol: distilled water, 4:1 v/v) for 48 h with occasional shaking. The extract was then fi ltered through Whatman fi lter paper. The resulting residue was extracted twice with the same fresh solvent, and the fi ltrate was concentrated using a rotary vacuum evaporator at low temperature (30–40 °C) and pressure. Finally, a brown grease material was obtained, which was suspended in water prior to use in treatments.
2.2. Cell culture
TIB-73 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Sigma–Aldrich, St. Louis, MO, USA), 5 mmol/L L-glutamine, 50 U/mL penicillin, and 50 g/mL streptomycin in a humidified 5% CO2–95% air environment at 37 °C. 2,7 dichlorodihydro-fl uorescein diacetate was purchased from Molecular Probes (Eugene, OR, USA). 4,6-diamidino-2-phenylindole and 5,5,6,6-tetrachloro-1,1,3,3 tetraethyl- benzimidazolyl-carbocyanine iodide were purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA) and used as described previously[13].
2.3. Cell viability assay
We have previously shown that the TIB-73 cell line is sensitive to 10 μmol/L APAP. After treatment with/without APAP (10 μmol/ L) and NS (25, 50, 75, 100 μg/mL) for 24 h, cell viability of TIB-73 cells was detected by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described previously[13].
2.4. Measurement of intracellular ROS generation
After subjecting TIB-73 cells to the same treatments described above, cells were treated with 10 mol/L dichloro-dihydro-fl uorescein diacetate for 30 min. DCFH fl uorescence was determined using a spectrophotometer at excitation and emission wavelengths of 488 and 515 nm, respectively.
2.5. Animals
A total of thirty male Sprague-Dawley rats (250–300 g, Samtako Biokorea, Daejeon, Korea) were used for this study. The rats were housed in an environment with controlled temperature of (23±2)°C and humidity of (50±5)% with a 12 to 12 h light-dark cycle. Food and water were available ad libitum before acute hepatotoxicity.
All experimental protocols employed herein were approved by the Committee on the Care of Laboratory Animal Resources, Chonbuk National University, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996).
2.6. Experimental design
Animals were divided into fi ve groups containing six animals for each group. Group 1 served as a normal control, which received distilled water only. Group 2 served as the APAP control, and received APAP at a dose of 800 mg/kg (bw) dissolved in DMSO. The selected dose of APAP was relatively high, and was chosen in order to demonstrate the protective eff ect of the NSSE. Groups 3 to 5 received NSSE at 100, 300, and 900 mg/kg bw 2 weeks before induction of liver stress.
Animals were anesthetized by intraperitoneal injection of tiletamine zolazepam (Zoletile, 40 mg/kg). Blood was collected in tubes containing the anticoagulant sodium heparin (5 IU/mL). Serum was separated by centrifugation at 3 000 r/min for 20 min.
2.7. Measurement of blood ions, metabolites, and enzymes
Blood was collected from the caudal vena cava immediately following sacrifice. Blood collection, storage, and measurement of ionized magnesium levels in blood were performed according to established guidelines[14]. We used the Nova Stat Profile 8 CRT (NOVA Biomedical Corp, Waltham, MA, USA) to measure pH and concentrations of hematocrit (Hct), hemoglobin (Hb), ionized sodium ion (Na+), potassium ion (K+), calcium ion (Ca2+), magnesium ion (Mg2+), chloride ion (Cl-), and the anionic gap as described previously[15].
Immediately following sacrifice, serum was separated by centrifugation at 3 000 r/min for 10 min and stored at –20 °C until analysis. AST, ALT, lactate dehydrogenase (LDH), ALP and total magnesium (tMg) levels were determined with a Hitachi 7020 instrument (Hitachi, Tokyo, Japan).
2.8. Measurement of MDA and antioxidant activities
Liver concentrations of malondialdehyde (MDA) were measured with an OXI-TEK TBARS kit (Enzo Life Sciences Incorporated). Reaction products were quantifi ed by measuring the absorbance at 532 nm according to the manufacturer’s suggested protocol. Serum levels of superoxide dismutase (SOD) were quantifi ed using a SOD activity kit (Enzo Life Sciences Incorporated) by measuring the absorbance of the reaction products at 450 nm. Glutathione (GSH) levels were measured using a glutathione (total) detection kit from Enzo Life Sciences according to the suggested protocol, for which the absorbance of the reaction products at 405 nm was recorded.
2.9. Histopathology
Livers were excised for histopathological examination. Portions of the same lobe of liver from each animal were immediately fi xed in 10% (v/v) formaldehyde, embedded in paraffi n, cut into 5–6 μm sections, stained with hematoxylin-eosin (H&E), and observed under a light microscope (100×).
2.10. Statistical analysis
Data were analyzed using t-tests and repeated measures of analysis of variance (ANOVA) followed by a Bonferroni correction. All results are expressed as the mean±SEM. P-values < 0.05 were considered signifi cant.
3. Results
3.1. Effect of APAP with/without NSSE on the viability of TIB-73 cells
As shown in Figure 1, APAP induced a dramatic decrease in the viability of TIB-73 cells to (49.0±1.9)% of control cells (P < 0.001). Co-treatment with APAP and NSSE at 25 μg/mL attenuated the APAP-induced decrease in cell viability to (108.0±0.6)% of that of APAP-treated cells. Likewise, co-treatment with APAP and NSSE at 50 μg/mL attenuated the APAP induced decrease in cell viability to (117.0±0.5)% of that of APAP treated cells. Finally, pretreatment with APAP and NSSE at 75 and 100 μg/mL attenuated the APAP induced decrease in cell viability to (123.0±0.8)% and (131.0±0.5)% of that of APAP-treated cells, respectively.

Figure 1. Eff ects of APAP with/without NSSE on cell viability and reactive oxygen species generation of TIB-73 cell lines.
3.2. Effect of APAP with/without NSSE on the generation of ROS concentrations in TIB-73 cells
As shown in Figure 1, APAP increased the level of ROS production to (141±8)% of the level of control cells. Co-treatment with APAP and NSSE attenuated the APAP-induced increase in ROS [NSSE 25, 50, 75, and 100 μg/mL: (99±5)%, (89±10)%, (77±6)%; and (81±2)% of the levels of APAP treated cells, respectively].
3.3. Effect of APAP with/without NSSE on serum cytosolic enzymes
Figure 2 shows that the activities of serum cytosolic enzymes ALT (8.32 folds), AST (8.79 folds), LDH (4.34 folds), and ALP (2.36 folds) were increased significantly (P < 0.001) after the administration of APAP as compared with the control group. Pretreatment with 100, 300 or 900 mg/kg of NSSE significantly reduced both ALT and LDH (P < 0.01, P < 0.001, P < 0.001 respectively). AST was also signifi cantly (P < 0.001) decreased in all treatment groups, while the decrease in ALP was only signifi cant in the APAP+900 mg/kg NSSE group.
3.4. Effect of APAP with/without NSSE on MDA and antioxidant activities
APAP administration caused a signifi cant (P < 0.001) increase in MDA concentration (2.18 folds) compared with the normal control group. However, pretreatment with 100, 300, 900 mg/kg NSSE signifi cantly reduced the MDA level in liver homogenates by 26% (P < 0.05), 75% (P < 0.001) and 77% (P < 0.001), respectively, as compared with the APAP-treated group (Figure 3A). In addition, the amounts of SOD and GSH were signifi cantly diminished in theAPAP-intoxicated group as compared with the normal control group (P < 0.01) (Figure 3B & 3C). Pretreatment with 100, 300 and 900 mg/kg of NSSE significantly increased the levels of antioxidant enzymes compared with the rats treated with APAP. On the other hand, the increase in GSH level upon treatment with 100 mg/kg NSSE was not signifi cant (Figure 3).

Figure 2. Eff ects of APAP with/without NSSE on the serum levels of cytosolic enzymes.

Figure 3. Effects of APAP with/without NSSE on the serum lipid peroxidation and antioxidant activities.
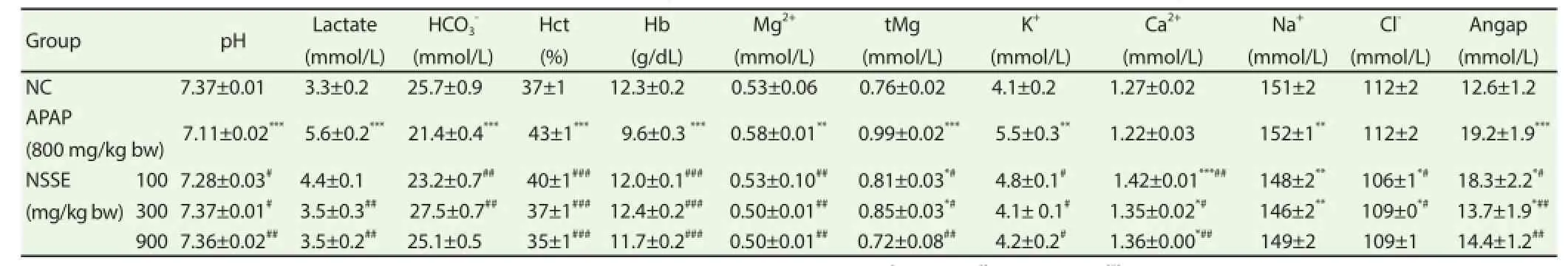
Table 1Ef fects of NSSE on blood pH, lactate, hematocrit, hemoglobin, and electrolyte levels following acetaminophen-induced hepatotoxicity in rats.
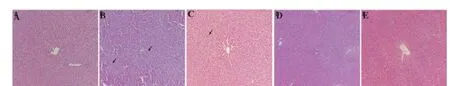
Figure 4. Eff ect of NSSE on APAP-induced hepatotoxicity in rats.
3.5. Effect of APAP with/without NSSE on blood pH, hematocrit, hemoglobin, ions, and metabolites
As shown in Table 1, blood hemoglobin, pH,and hemoglobin decreased while blood lactate, hematocrit, Mg, tMg, K+, and anionic gap were decreased upon treatment with APAP. On the other hand, levels of Ca2+, Na+, and Cl-were not changed by APAP. Pretreatment with 100, 300, and 900 mg/kg of methanolic extracts of NSSE signifi cantly rescued APAP-induced changes.
3.6. Histological analysis
Microscopic examination of the liver sections of the control animals showed normal architecture of hepatic lobules in the form of hepatocytes arranged from the portal vein (Figure 4A). Examination of rat livers from the group treated with APAP showed necrosis and loss of architecture of hepatocytes swelling, slight hydropic degeneration, apoptotic nuclei, occasional binucleation, cellular infi ltration, hemorrhage, and congestion of central veins (Figure 4B). Histopathological changes induced by APAP were remarkably improved by pre-administration with 100 mg/kg (Figure 4C), 300 mg/kg (Figure 4D), and 900 mg/kg (Figure 4E) NSSE.
4. Discussion
The non-steroidal analgesic-antipyretic drug APAP is one of the safest over-the counter drugs when used in recommended doses, but is capable of producing massive hepatic necrosis following acute overdose or chronic low dose use[3,4]. In this study, we focused on determining the protective eff ects of NSSE against APAP-induced toxicity. Importantly, we found that 100 and 900 mg/kg were the most eff ective concentrations of NSSE in attenuating the eff ects of APAP in TIB-73 cells and rats, respectively.
Cell viability was decreased and ROS generation was increased in TIB-73 cells treated with APAP. During the metabolism of APAP in the body, a state of oxidative stress is created as a result of excessive ROS generation, which plays a vital role in the development of hepatotoxicity[6,12]. The viability of NSSE treated cells was signifi cantly increased compared with APAP treated cells, indicating that NSSE might have a protective effect against APAP-induced hepatotoxicity. ROS mediates hepatic tissue damage induced by APAP[6,12], which usually causes a pro-oxidative state in the cellular environment and results in oxidative damage. ROS are generated in many stressful situations and induce cell death by either apoptosis or necrosis and can induce mitogen activated protein kinase activation by which hepatocytes react to oxidative stress[13]. Prevention of overproduction of intra-cellular ROS levels by pretreatment with NSSE indicated that its protective eff ect against APAP-induced liver damage were mainly due to the suppression of an increase in intracellular ROS. This result is consistent with previous observations that administration of nutritional regimens can inhibit excessive levels of intra-cellular ROS[12,13]. Moreover, the anti-cytotoxic activity of NSSE on the human hepatoma HepG2cell line was reported previously[10].
In the present study, APAP-induced liver damage was confi rmed by elevated levels of bio-chemical parameters, namely, ALT, AST, LDH, and ALP. Specifically, elevation of these enzymes in serum is indicative of hepatocytic damage[15]. Specifically, ROS generation and lipid peroxidation of cell membranes leads to loss of membrane integrity, changes in membrane potential, and an increase in membrane permeability, which in turn results in leakage of the enzymes from liver cells into circulation[14,15] resulting in increased serum levels. Oral administration of various doses of NSSE in this study in rats treated with a toxic dose of APAP tended to normalize the serum levels of liver enzymes, confirming that it effectively protected rat livers against severe damage caused by APAP.
Excessive ROS generated during APAP metabolism rapidly reacts with lipid membranes, which initiates a lipid peroxidation chain reaction that produces lipid peroxyl radicals[3,4]. Enhanced levels of hepatic MDA, a major reactive aldehyde that results from the peroxidation of polyunsaturated fatty acids in the cell membrane, is indicative of a causal role of lipid peroxidation in APAP-induced liver damage. Similar to the results of our study, the elevation of lipid peroxidation caused by APAP has been previously reported in a rat model[2]. Our results revealed that pretreatment with NSSE had a signifi cant inhibitory role against lipid peroxidation in rats, resulting in diminished APAP-induced hepatic membrane destruction and hepatic damage. Consistently, NSSE-mediated protection and amelioration of elevated lipid peroxidation caused by APAP has been previously reported to be benefi cial in a rat model[16].
The antioxidant defense system in the body plays an important role in protection against oxidative stress. Antioxidant activities including GSH and SOD were partially inactivated by APAP-induced hepatotoxicity, consistent with the results of a previous study[2]. This might have been largely due to an overwhelming oxidative modifi cation of enzymatic proteins by increased ROS production and lipid peroxidation. Indeed, excess levels of ROS can attack biological molecules such as DNA, protein, and phospholipids, leading to lipid peroxidation and depletion of antioxidant activity, resulting in further oxidative stress[2,3]. Our observations revealed that NSSE supplementation improved the altered antioxidative defense system (SOD and GSH) in rat livers challenged with APAP. From the experimental and clinical studies performed on NSSE, it seems that most of its pharmacological actions were due to its antioxidant activity, as indicated by its ability to scavenge free radicals and/ or inhibit lipid peroxidation[16]. Hepatic GSH is an important nonenzymatic antioxidant that plays a crucial role in scavenging ROS and maintaining enzymatic antioxidants[1]. Depletion of GSH obtained by APAP exposure may be attributed to its rapid utilization by the overproduction of ROS and subsequent oxidative stress[1,4]. In this study, the level of hepatic GSH was the same regardless of pretreatment of NSSE in APAP administered rat, which indicated that ROS detoxification and subsequent prevention of protein oxidation was the primary mechanism by which NS protects against APAP-induced hepatotoxicity. Consistent with this possibility, many studies have showed that NS has antioxidant activity[10].
In the present study, lowered blood pH, reduced HCO3-levels, and increased lactate levels were all observed in the APAP group. In previous reports, severe metabolic acidosis (pH 7.14–7.27) and hyperlactacemia were also shown to be accompanied by hypotension, peripheral vasoconstriction, dehydration, ketoacidosis, and hepatic failure in patients presenting with acetaminophen ingestion[7]. Such increased lactate levels further reduce pH, which results in various biochemical and physiological side effects including glycolysis and phosphofructokinase and ion release via muscular contraction[15]. It is important to note that Hct was increased in the APAP group, indicating hemoconcentration and dehydration[15]. The APAP-induced alterations were suppressed in the NSSE treated group, indicating the protective eff ects of NSSE against metabolic disturbances and hepatotoxicity. The anionic gap was also signifi cantly elevated in APAP intoxicated rats in our study, which may have been due to high levels of lactate[17] and were prevented by NSSE, correlating with improved levels of lactateand HCO3[15,17]. Hypomagnesemia is common in chronic liver disease[17]. In addition, blood levels of magnesium and potassium were increased after 24 h of APAP administration (acute stress) in this study. In general, hypermagnesemia is associated with acute stress while hypomagnesemia occurs during chronic stress[15]. Moreover, dehydration, metabolic acidosis, and muscular damage (hepatic muscle) may be responsible for the acute elevation of blood magnesium and potassium levels[15].
The histopathological results of the current study support the previous observations that the toxic eff ects of APAP on the liver result in necrosis and loss of architecture of hepatocytes, apoptotic nuclei, occasional binucleation, cellular infi ltration, hemorrhage, and congestion of central veins[2]. In this study, we found that NSSE pretreatment was protective against APAP-induced histopathological alterations. Similar protection of hepatic and renal histopathological changes by NSSE was also reported in a previous study[16].
The in vitro and in vivo fi ndings in this present study demonstrated that NSSE has protective effects against APAP-induced hepatotoxicity and metabolic disturbances in rats, which might be related to improved antioxidant activity and attenuation of oxidative stress, lipid peroxidation, and ROS generation.
Conflicts of interest statement
We declare that we have no confl icts of interest.
Acknowledgments
This paper was supported in part by the Brain Korea 21 Plus program of the National Research Foundation of Korea Grant. The authors are very grateful to the proofreading service center of Chonbuk National University and e-World Editing Ltd. for proofreading this manuscript.
References
[1] McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 2012; 264(3): 387–394.
[2] Yen FL, Wu TH, Lin LT, Lin CC. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol 2007; 111(1): 123–128.
[3] Hinson JA, Reid AB, McCullough SS, James LP. Acetaminopheninduced hepatotoxicity: role of metabolic activation, reactive oxygen/ nitrogen species, and mitochondrial permeability transition. Drug Metab Rev 2004; 36(3–4): 805–822.
[4] Hinson JA, Roberts DW, James LP. Mechanisms of acetaminopheninduced liver necrosis. (Volume 196). Handbook of experimental pharmacology. Berlin: Springer; 2010; p. 369–405.
[5] Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 2004; 40(1): 6–9.
[6] Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 2005; 312(2): 509–516.
[7] Shah AD, Wood DM, Dargan PI. Understanding lactic acidosis in paracetamol (acetaminophen) poisoning. British J Clin Pharmacol 2011; 71(1): 20–28.
[8] Abdel-Fattah AM, Matsumoto K, Watanabe H. Antinociceptive eff ects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol 2000; 400(1): 89–97.
[9] Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed 2013; 3(5): 337–352.
[10] Khan MA, Chen HC, Tania M, Zhang DZ. Anticancer activities of Nigella sativa (black cumin). Afr J Tradit Complement Altern Med 2011; 8(5 Suppl): 226–232.
[11] Uz E, Bayrak O, Uz E, Kaya A, Bayrak R, Uz B, et al. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: an experimental model. Am J Nephrol 2008; 28(3): 517–522.
[12] El-Shafey MM, Abd-Allah GM, Mohamadin AM, Harisa GI, Mariee AD. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology2015; 22(1): 49–55.
[13] Park HM, Kim SJ, Mun AR, Go HK, Kim GB, Kim SZ, et al. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J Ethnopharmacol 2012; 141(3): 1071–1076.
[14] Rahman MM, Lee SJ, Mun AR, Adam GO, Park RM, Kim GB, et al. Relationships between blood Mg2+and energy metabolites/enzymes after acute exhaustive swimming exercise in rats. Biol Trace Elem Res 2014; 161(1): 85–90.
[15] Ben Rayana MC, Burnett RW, Covington AK, D'Orazio P, Fogh-Andersen N, Jacobs E, et al. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC); IFCC Scientific Division Committee on Point of Care Testing. IFCC guideline for sampling, measuring and reporting ionized magnesium in plasma. Clin Chem Lab Med 2008: 46: 21-26.
[16] Elkhateeb A, Khishin IE, Megahed O, Mazen F. Eff ect of Nigella sativa Linn oil on tramadol-induced hepato-and nephrotoxicity in adult male albino rats. Toxicol Rep 2015; 2: 512–519.
[17] Zein JG, Wallace DJ, Kinasewitz G, Toubia N, Kakoulas C. Early anion gap metabolic acidosis in acetaminophen overdose. Am J Emerg Med 2010; 28(7): 798–802.
Document heading 10.1016/j.apjtm.2016.01.039
IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
15 December 2015
#These authors contributed equally to this work.
*Corresponding author: Shang-Jin Kim, Associate Professor, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Chonbuk National University, Iksan Campus, 79 Gobong-ro, Iksan-si, Jeollabuk-do 570-752, Republic of Korea.
E-mail: abbasj@jbnu.ac.kr
Tel: +82-63-850-0963
Fax: +82-63-850-0910
Foundation project: This paper was supported in part by the Brain Korea 21 Plus program of the National Research Foundation of Korea Grant.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model
- Mechanism of all-transretinoic acid increasing retinoblastoma sensitivity to vincristine
- Protective effect of apoptosis signal-regulating kinase1 inhibitor against mice liver injury
- Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines
- Effect of miR-467b on atherosclerosis of rats
- Effect of dimethyl fumarate on rats with chronic pancreatitis
