CART在不同发育阶段牛卵泡颗粒细胞中的表达和定位
2016-07-18李鹏飞毕锡麟景炅婕吕丽华
李鹏飞,毕锡麟,王 锴,景炅婕,吕丽华
(1山西农业大学生命科学学院,山西太谷 030801;2山西农业大学动物科技学院,山西太谷 030801)
CART在不同发育阶段牛卵泡颗粒细胞中的表达和定位
李鹏飞1,毕锡麟2,王锴2,景炅婕2,吕丽华2
(1山西农业大学生命科学学院,山西太谷 030801;2山西农业大学动物科技学院,山西太谷 030801)
摘要:【目的】探讨牛卵泡颗粒细胞中可卡因-苯丙胺调节转录肽(cocaine-and amphetamine-regulated transcript, CART)的表达对牛不同阶段卵泡发育的影响。【方法】通过对牛卵泡转录组ODF1(the largest onset of deviation follicle)和ODF2(the second largest onset of deviation follicle)颗粒细胞(granulesa cells, GCs)构建RNA文库,Illumina HiSeq 2000平台测序;采集屠宰牛双侧卵巢,通过黄体形态特征筛选处于第一卵泡波阶段的最大卵泡和第二大卵泡;分别抽取卵泡液,竞争性ELISA法测定卵泡液雌激素(estrodiol, E2)和孕酮(progestin, P)浓度,确定优势卵泡(dominant follicles,DF)和从属卵泡(subordinate follicles,SF);分别分离DF和SF颗粒细胞(granulosa cells, GCs),提取总RNA;设定3个重复样本,以RPLP0作为内参基因,设计引物在牛DF和SF进行qRT-PCR表达量检测,GraphPad Prism 5.0作图并进行显著性分析;兔抗CART一抗检测CART在牛卵泡的表达。【结果】转录组测序结果显示,CART在ODF1的表达量是ODF2的38.2倍;通过黄体形态特征及激素测定共筛选出3头牛的卵泡发育处于第一卵泡波,并获得DF和SF;qRT-PCR结果显示CART 在DF与SF的表达量差异极显著(P<0.01),高达2 310倍,与转录组测序结果的表达差异趋势一致;免疫组化结果表明,CART在牛DF和SF中均有表达,且DF表达量明显高于SF,同时,CART主要在卵泡颗粒细胞层表达。【结论】在牛卵泡发育过程中,卵泡发育选择期有其它抑制因子抑制了大多数卵泡的发育,阻止其成为DF;当出现偏差后,CART的高表达抑制了DF进入排卵期,推测CART可能是通过促进卵泡颗粒细胞的凋亡,进一步抑制了颗粒细胞层E2的分泌,最终导致DF闭锁。
关键词:牛;卵泡;发育;CART;表达
联系方式:李鹏飞,E-mail:adamlpf@126.com。通信作者吕丽华,E-mail:lihualvsxau@126.com
0 引言
【研究意义】单胎动物在一个发情周期均会出现2—3个卵泡发育波,一般只有最后一个卵泡波中的优势卵泡最终排卵,排卵率低一直是影响单胎家畜繁殖环节的关键因素。可卡因-苯丙胺调节转录肽(cocaine-and amphetamine-regulated transcript, CART)属于动物下丘脑分泌的神经肽,课题组前期研究表明 CART对牛[1-2]、绵羊[3]、猪[4]卵泡颗粒细胞(granulesa cells, GCs)的增殖及雌激素(estrodiol, E2)的分泌具有抑制作用。本研究从分子和蛋白水平探究CART在牛不同发育阶段卵泡的表达变化,为后期进一步研究 CART对牛卵泡发育的影响奠定基础。【前人研究进展】 对牛卵泡发育和排卵相关的研究一直以来集中在人工调控发情持续时间及排卵时间和数量上[5]。近年来,研究人员通过促性腺激素与卵泡内生长因子互作[5-7]、GCs凋亡与卵泡闭锁的关系[8-11]来探讨牛优势卵泡(dominant follicles,DF)生长发育和闭锁规律。BABITHA等[12]在对水牛卵泡液VEGF研究证实卵巢毛细血管组织增生有助于牛卵泡波启动及卵泡发育。GUPTA等[13]研究发现卵泡液中的活性氧浓度对牛卵泡波发生和卵子发育具有调控作用。此外,高浓度的 E2和孕酮(progestin, P)以及 IGF-Ⅰ可显著促进末期卵母细胞的成熟[14-16]。【本研究切入点】在牛发情周期第一卵泡波中,ODF1最终必将发育为DF,ODF2无疑为从属卵泡(subordinate follicles,SF),而第一卵泡波中的DF和SF随着第一卵泡波发育的结束最终都将闭锁,本研究选择第一卵泡波中的卵泡作为研究对象,旨在探究CART在卵泡发育的不同阶段是否为促进卵泡颗粒细胞凋亡的主要因子。【拟解决的关键问题】本试验通过高通量测序、qRT-PCR和免疫组织化学定位技术探讨CART在不同发育阶段卵泡的表达,从而探明CART在卵泡发育各阶段的调控作用及其信号传导途径。
1 材料与方法
1.1 试验动物及材料
高通量测序用试验牛选取及ODF1和ODF2卵泡分离方法[17]。qRT-PCR和免疫组化试验用海福特牛选自山西文水肉牛屠宰场(采样时间为2015年9月24日),采集双侧卵巢标记并投入灭菌DPBS中,带回山西农业大学动物繁殖实验室处理。
1.2 试验方法
1.2.1 Illumina平台测序 卵泡 GCs的分离、总RNA的提取以及 Illumina平台对转录组 ODF1和ODF2进行测序[17]。
1.2.2 通过黄体特征确定第一卵泡波 确定牛发情周期所采集的卵泡属于第一卵泡波依据IRELAND等的研究[18],可通过卵巢黄体表观变化特征准确判断母牛发情阶段。处于第一卵泡波阶段的黄体要求处于牛发情周期的第5—6天,这时的黄体外观呈红色,表明近日排卵;裂点未被上皮细胞覆盖;切开黄体内部呈红色,偶尔充满血液,细胞松弛且有规则;黄体直径0.5—1.5 cm;黄体表面的脉管系统外围固有;此时卵巢上最大卵泡直径 6—10 mm(图1)。
1.2.3 采集卵泡液 用于 qRT-PCR的卵泡需确定DF和SF,将采集到的卵泡分别置于无菌培养皿上,超净台中用1 mL注射器及针头注入卵泡,缓慢抽取各组卵巢中最大和第二大卵泡液,并分别分离GCs,-20℃保存待激素测定和总RNA提取。
1.2.4 E2和P的测定 卵泡液E2和P的测定选用竞争性ELISA法,使用牛E2ELISA Kit和PROG ELISA Kit试剂盒,具体方法参照试剂盒说明书进行。
1.2.5 qRT-PCR反应 依据 NCBI上Bos taurus的CART mRNA序列,Primer 5.0在线设计qRT-PCR引物;牛RPLP0作为内参基因,上海生工合成引物。引物序列见表1。
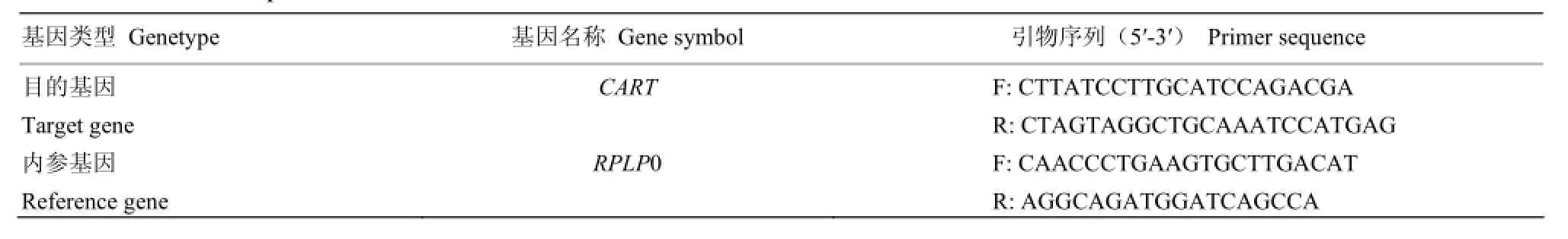
表1 qRT-PCR引物序列Table 1 Primers of quantitative PCR
qRT-PCR反应体系如下:SYBR® Premix Ex TaqⅡ 10 μL,Primer 各0.8 μL,ROX Reference Dye Ⅱ0.4 μL,cDNA 2 μL,灭菌H2O至总体系20 μL。配置好反应体系按如下条件进行反应,95℃ 30 s,95 ℃ 5 s,60℃ 30 s,72℃ 15 s,45个循环;95℃ 15 s,60℃ 1 min,95℃ 15 s。目的基因和内参基因在同一条件反应。
1.2.6 免疫组织化学分析 卵泡分离后放入 4%的多聚甲醛溶液中固定24 h后,70% 酒精洗脱黄色,脱水包埋,制成5 μm厚的切片;二甲苯脱蜡后梯度酒精浸泡,蒸馏水冲洗;3% H2O2阻断后消化酶抗原修复;5% BSA室温封闭30 min,滴加500倍稀释的山羊抗兔CART一抗(Abcam, America),湿盒4℃孵育10—12 h;滴加二抗,DAB显色20 min;苏木精复染25 s后,梯度酒精脱水,二甲苯透明;中性树胶封片。
1.3 数据处理与分析
结果采用平均值±标准差表示,GraphPad Prism 5.0作图并进行显著性分析。
2 结果
2.1 高通量测序后CART差异表达
通过 AUDIC[19]等的研究算法从两个文库中获得差异表达基因,设定条件RPKM>0.5,FDR校正后的P<0.05,通过设定ODF1-RPKM/ODF2-RPKM >5,在ODF1和ODF2文库中共发现147个高差异表达基因,其中 CART的差异表达倍数最高,为38.1519倍(表2)。

表2 Illumina平台测序后CART的高差异表达Table 2 Highly differentially expressed CART in ODF1 and ODF2 by sequencing platform of Illumina
2.2 发情期第一卵泡波卵巢的筛选
在采样过程中,共获得11对卵巢的黄体形态符合第一卵泡波黄体形态的要求(图 1-A为对照组)。确定第一卵泡波通过黄体形态特征观察,该黄体为上一发情周期排卵后形成,主要从黄体的外部和内部特点、黄体大小以及此时卵巢上最大卵泡的直径来确定,图 1为编号为 3、5、7的牛卵巢及其黄体图(1-B、C、D)。
2.3 激素测定
本试验确定牛DF和SF卵泡的方法参考XU等[20]的研究结果,测定每一对卵巢中最大卵泡和第二大卵泡卵泡液中E2和P的浓度平均值,通过比值确定DF和SF,4次重复测定数值是同一卵泡液的激素浓度,因此,不进行统计分析。利用E2Elisa Kit和PROG Elisa Kit试剂盒、酶标仪分别对采集的 11对牛卵巢各组最大和第二大卵泡的卵泡液进行E2、P测定(表3),结果表明:第3、5、7组E2和P测定值中,最大卵泡的E2∶P值>1,第二大卵泡的E2∶P值<1,确定第3、5、7组卵巢采集的最大和第二大卵泡分别为第一卵泡波中的DF和SF。
2.4 CART基因在第一卵泡波DF和SF中表达差异分析
通过样本重复校正、物理校正和RPLP0内参基因校正,利用△△CT算法对CART在牛发情周期第一卵泡波DF和SF的表达量进行分析,结果见图2。结果显示:CART在DF中的表达量极显著高于SF(P<0.01),表达倍数高达2 310倍。
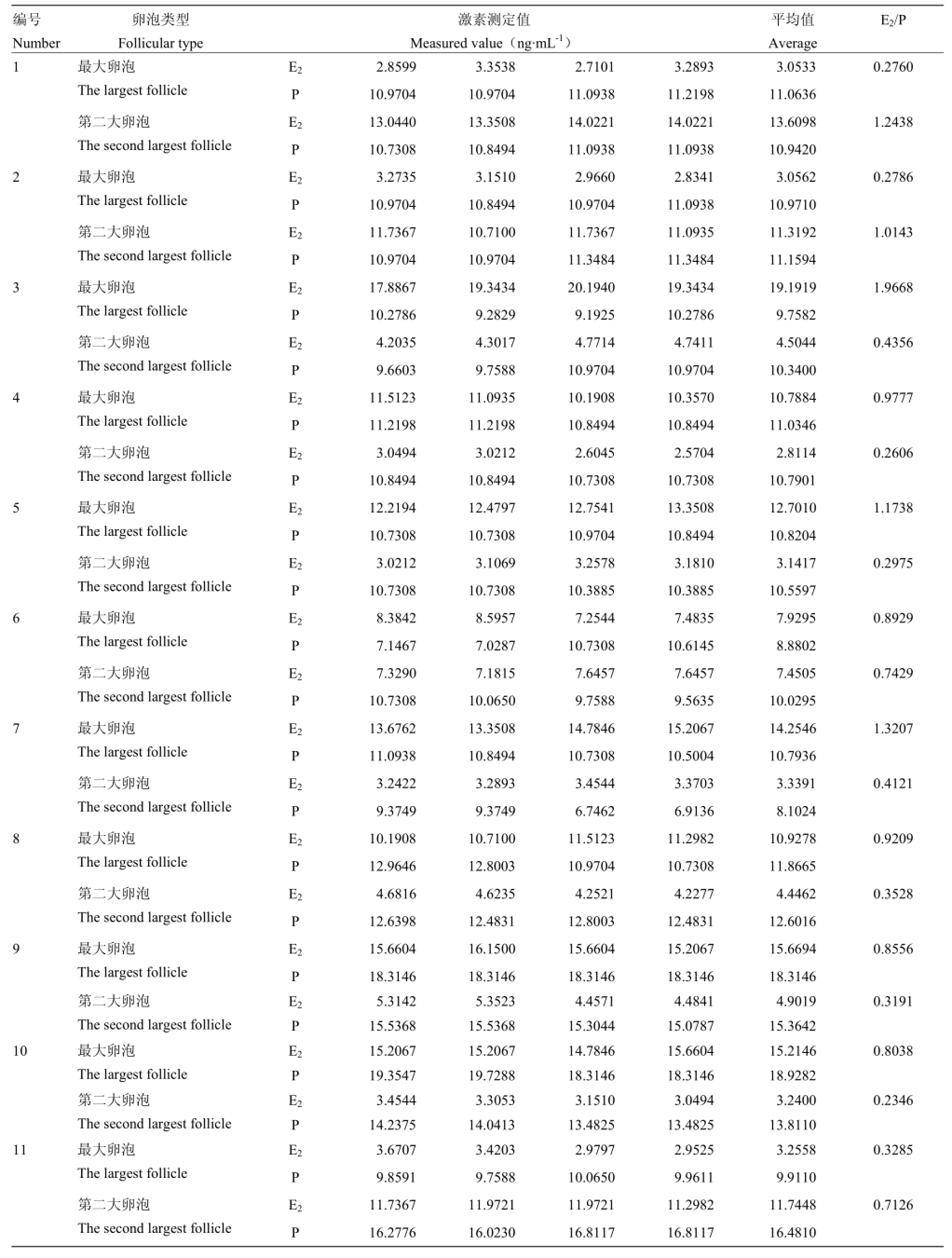
表3 各组卵巢卵泡液E2和P的测定Table 3 The E2and P determination results from groups of ovarian follicle fluid
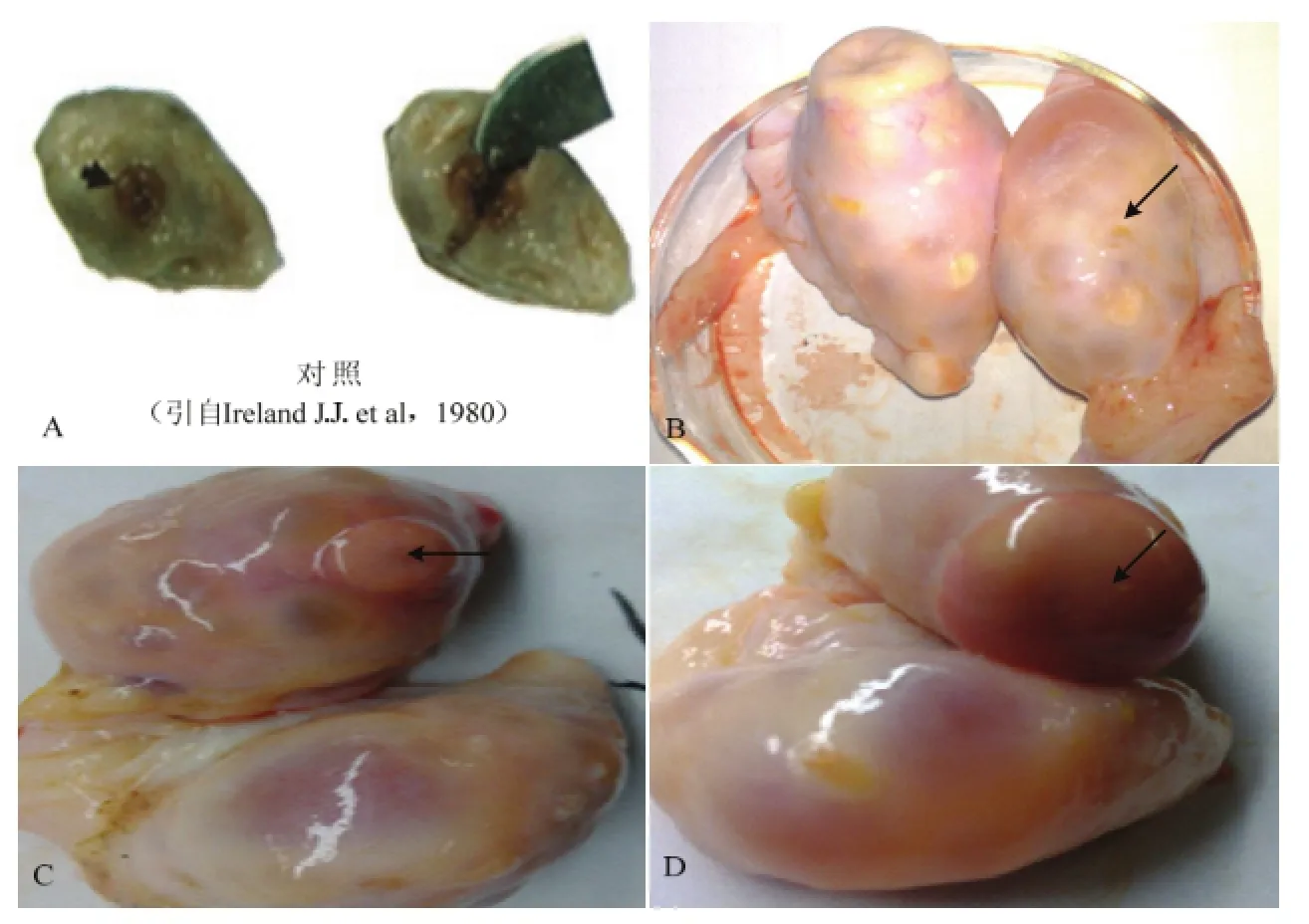
图1 牛发情周期第一卵泡波黄体形态Fig. 1 Corpus luteum morphological feature of the first follicular wave during the estrous cycle of cattle.
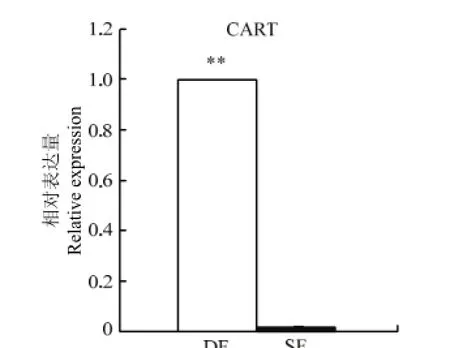
**表示在0.01水平上的比较结果** indicate significantly different at the level of 0.01图2 CART在优势卵泡和从属卵泡中的表达Fig. 2 Real time PCR analysis of CART expression in DF vs SF
2.5 CART免疫组织化学定位分析
免疫组化分析表明,CART在牛卵巢DF和SF均有表达(图3-B, D),对照组无特异性显色(图3-A, C);DF的GCs层和TCs层CART表达量明显高于SF。
3 讨论
卵泡发生闭锁的典型特征就是 E2的分泌能力丧失[21-22]和卵泡内E2与P比值的降低。E2分泌活跃的卵泡具有健康生长卵泡的生物化学特性和高的E2:P值;相对于闭锁卵泡或向闭锁趋势发育的卵泡,其E2分泌能力低下,E2:P值较低,其比值通常以1为分界[22-24]。因此,在本试验中采用第一卵泡波中最大卵泡卵泡液E2:P>1和第二大卵泡卵泡液 E2:P<1来确定 DF和SF。在一个卵泡发育波中,卵巢内部和表面分布有大量卵泡。在实际采样过程中,所采集的最大卵泡和第二大卵泡是从母牛双侧卵巢中挑选,只能根据突出卵巢表面部分的卵泡大小情况,肉眼来估测最大卵泡和第二大卵泡,这会造成所采集的最大卵泡可能与卵泡发育的实际情况不符。因此,筛选的处于第一卵泡发育波阶段的11对卵巢,只有第3、5、7组分离的卵泡为DF和SF。同时,也进一步表明对卵泡液E2和P测定的必要性。
牛发情周期内通常出现2个[25]或3个[26]卵泡波,但排卵卵泡仅出现在最后一个卵泡波的DF中。在牛卵泡发育的卵泡期和黄体期,E2分泌量较高的卵泡的生长与GCs增殖与卵泡内E2的浓度、GCs和膜细胞(theca cells, TCs)中促黄体素(luteinizing hormone,LH)结合位点数量有关;反之,E2分泌量较低的卵泡GCs中促卵泡素(follicle-stimulating hormone, FSH)结合位点数量减少[22-23,27]。ILHA等研究表明,FSH浓度降低导致SF颗粒细胞LIF-STAT3信号活性降低,最终造成卵泡闭锁,当 FSH浓度提高可恢复LIF-STAT3信号活性[28]。GARCIA等通过试验发现卵泡发育波启动前FSH和LH的过量刺激可增强较小的有腔卵泡发育能力,但不会使原始卵泡持续募集,延长刺激时间可促进大卵泡的成熟和排卵[29]。ATANASOV等通过在海福特牛和荷斯坦奶牛阴道内放置P缓释装置,发现海福特牛排卵时血液P浓度较高且排卵提前,获得的DF数量增加,排卵卵泡直径较大[30]。哺乳期奶牛通过生物刺激和暂时性断奶协同作用也可显著提高卵泡发育、同期排卵,进而提高母牛的受孕率[31]。
课题组通过体外细胞培养技术,对CART与卵泡GCs激素分泌和调控研究发现,牛卵泡GCs雌激素的分泌需在FSH诱导下产生,CART对卵泡发育有负调控作用,能够引起牛卵泡闭锁[1,32-33]。本研究通过采集牛发情期第一卵泡波ODF1/DF和ODF2/SF,通过高通量测序、qRT-PCR和免疫组化试验,从mRNA和蛋白水平证明CART在牛DF和SF中高差异表达,而第一卵泡波中DF和SF注定均会发生闭锁,这表明在第一卵泡波中当卵泡发育出现偏差前,卵泡能否优势化取决于卵泡内因子和相关激素浓度;当发育波出现偏差后,DF能否最终排卵,可能是由于GCs高表达的CART决定的。同时,免疫组化结果表明,GCs 层CART的表达量明显高于TCs层,而牛卵泡E2主要由GCs分泌,高表达的CART可能通过促进GCs凋亡,抑制颗粒细胞E2的分泌,进而对卵泡的发育产生调控作用。
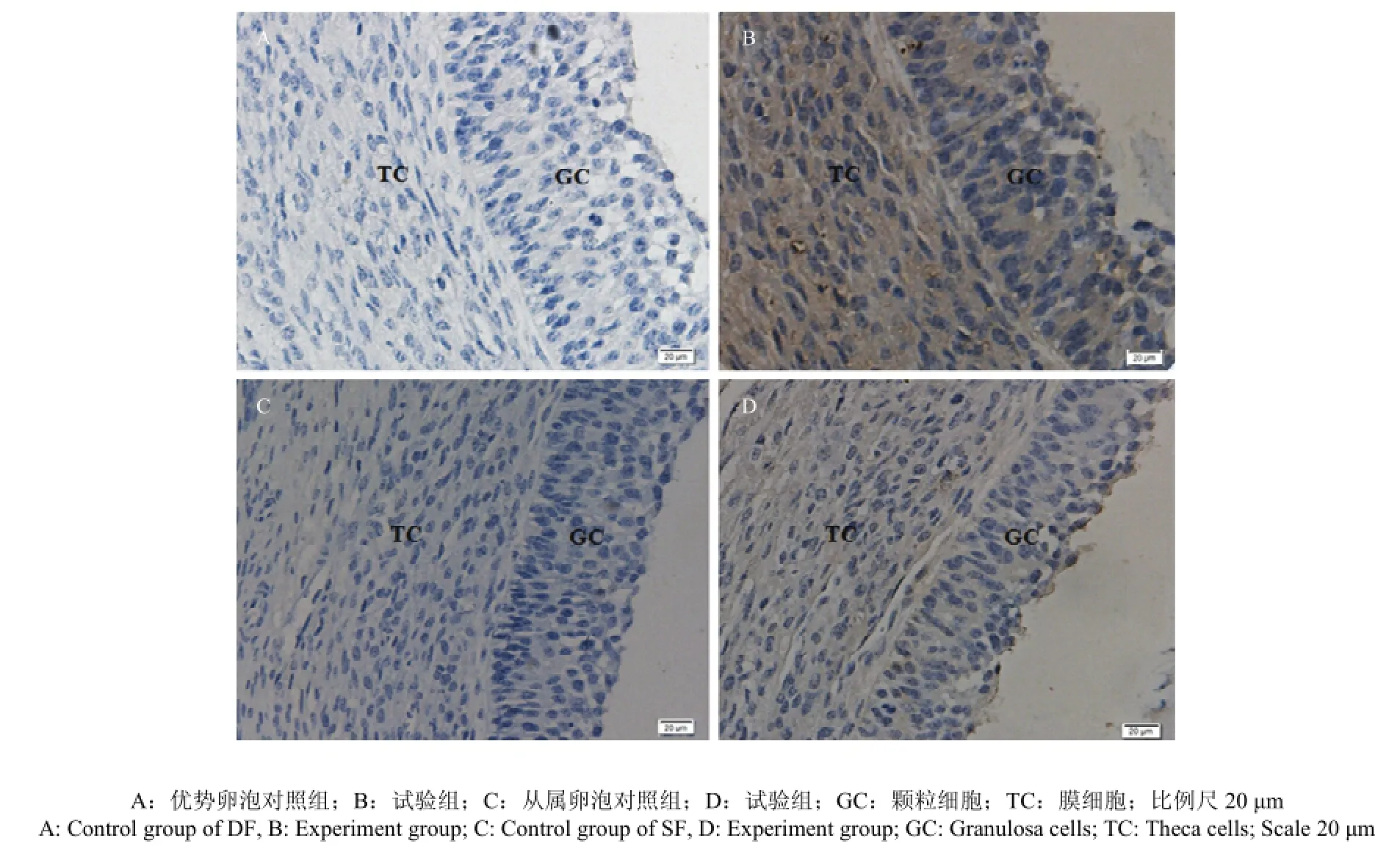
图3 卵巢组织 CART 的表达(免疫组化 400×)Fig. 3 Expressions of CART in the ovary (immunohistochemistry 400×)
4 结论
牛卵泡发育过程中,第一卵泡波卵泡发育选择期卵泡内因子和相关激素抑制了大多数卵泡的发育,阻止其成为DF;当出现偏差后,CART的高表达抑制了DF进入排卵期,推测CART可能是通过促进卵泡颗粒细胞的凋亡,最终导致DF闭锁。
References
[1] SEN A, LÜ L, BELLO N, IRELAND J J, SMITH G W. Cocaine-and amphetamine-regulated transcript (CART) accelerates the temination of FSH induced ERK1/2 and AKT activation by regulating the expression and degradation of specific map kinase phosphatases. Molecular Endocrinology, 2008, 22(12): 2655-2676.
[2] LÜ L, JIMENEZ-KRASSEL F, SEN A, BETTEGOWDA A,MONDAL M, FOLGER J K, LEE K B, IRELAND J J, SMITH G W. Evidence supporting a role for cocaine and amphetamine regulated transcript (CART) in control of granulosa cell estradiol productionassociated with dominant follicle selection in cattle. Biology of Reproduction, 2009, 81(3):580-586.
[3] 李鹏飞, 岳文斌, 黄洋, 孙晋艳, 李晓明, 庞钰莹, 于学静, 贺俊平,孟金柱, 任有蛇, 吕丽华. 可卡因-苯丙胺调控转录肽对绵羊卵巢卵泡颗粒细胞雌激素产生的影响. 畜牧兽医学报, 2013, 44(6):853-857. LI P F, YUE W B, HUANG Y, SUN J Y, LI X M, PANG Y Y, YU X J, HE J P, MENG J Z, REN Y S, LÜ L H. Effects of CART on estradiol production of sheep ovarian follicular granulosa cells. Acta Veterinaria et Zootechnica Sinica, 2013, 44(6):853-857. (in Chinese)[4] 李鹏飞, 岳文斌, 李富禄, 黄洋, 孙晋艳, 朱芷薇, 于秀菊, 贺俊平,范瑞文, 任有蛇, 吕丽华. 可卡因-苯丙胺调控转录肽(CART)对猪卵巢卵泡颗粒细胞雌激素产生的影响. 畜牧兽医学报, 2012,43(12):1879-1886. LI P F, YUE W B, LI F L, HUANG Y, SUN J Y, ZHU Z W, YU X J, HE J P, FAN R W, REN Y S, LV L H. Effects of CART on estradiol production of pig ovarian follicular granulosa cells in vitro culture. Acta Veterinaria et Zootechnica Sinica, 2012, 43(12): 1879-1886. (in Chinese)
[5] ROCHE J F, AUSTIN E J, RYAN M, OROURKE M, MIHM M,DISKIN M G. Regulation of follicle waves to maximize fertility in cattle. Journal of Reproduction and Fertility, 1999, 54(Suppl):61-71.
[6] GINTHER O J, WILTBANK M C, FRICKE P M, GIBBONS J R,KOT K. Selection of the dominant follicle in cattle. Biology of Reproduction, 1996, 55(6):1187-1194.
[7] WEBB R, ARMSTRONG D G. Control of ovarian function; effect of local interactions and environmental influences on follicular turnover in cattle: a review. Livestock Production Science, 1998, 53(2):95-112.
[8] 卫晓红, 祁丽花, 迟晓春, 董静霞, 杨京京, 徐健. Smad2/Smad3蛋白在大鼠卵巢颗粒细胞中的表达及FSH对其活化的影响. 解剖学报, 2007, 38 (2): 205-208. WEI X H, QI L H, CHI X C, DONG J X, YANG J J, XU J. The effects of FSH on the phosphorylation of smad2/smad3 protein in rat ovarian granulosa cells. Acta Anatomica Sinica, 2007, 38 (2): 205-208. (in Chinese)
[9] PEDERSON H G, WATSON E D, TELFER E E. Analysis of atresia in equine follicles using histology, fresh granulosa cell morphology and detection of DNA fragmentation. Reproduction, 2003, 125(3):417-423.
[10] ZEUNER A, MÜLLER K, REGUSZYNSKI K, JEWGENOW K. Apoptosis within bovine follicular cells and its effect on oocyte development during in vitro maturation. Theriogenology, 2003,59(5-6): 1421-1433.
[11] WONGSRIKEAO P, KANESHIGE Y, OOKI R, TANIGUCHI M,AGUNG B, NII M, OTOI T. Effect of the removal of cumulus cells on the nuclear maturation,fertilization and development of porcine oocytes. Reproduction in Domestic Animals, 2005, 40(2): 166-170.
[12] BABITHA V, PANDA R P, YADAV V P, CHOUHAN V S, DANGI S S, KHAN F A, SINGH G, BAG S, TARU SHARMA G, SILVIA W J, SARKAR M. Amount of mRNA and localization of vascular endothelial growth factor and its receptors in the ovarian follicle during estrous cycle of water buffalo(Bubalus bubalis). Animal Reproduction Science, 2013, 137(3-4):163-176.
[13] GUPTA S, CHOI A, YU H Y, CZERNIAK S M, HOLICK E A,PAOLELLA L J, AGARWAL A, COMBELLES C M. Fluctuations in total antioxidant capacity, catalase activity and hydrogen peroxide levels of follicular fluid during bovine folliculogenesis. Reproduction Fertility and Development, 2011, 23(5):673-680.
[14] KAFI M, MESBAH S F, DAVOODIAN N, KADIVAR A. Fine structures of the oocyte in relation to serum, follicular fluid steroid hormones and IGF-I in the ovulatory-sized follicles in one-humped camel (Camelus dromedarius). Avicenna Journal of Medical Biotechnology, 2014, 6(1):57-61.
[15] AAD P Y, ECHTERNKAMP S E, SPICER L J. Possible role of IGF2 receptors in regulating selection of 2 dominant follicles in cattle selected for twin ovulations and births. Domestic Animal Endocrinology,2013, 45(4):187-195.
[16] DIAS F C, KHAN M I, ADAMS G P, SIRARD M A, SINGH J. Granulosa cell function and oocyte competence: Super-follicles,super-moms and super-stimulation in cattle. Animal Reproduction Science, 2014, 149(1-2):80-89.
[17] 李鹏飞, 孟金柱, 谢建山, 朱芷葳, 刘岩, 姜晓龙, 陈建伟, 姚晓磊, 赵妙妙, 吕丽华. 牛卵泡ODF1与ODF2转录组发育相关基因筛选及表达差异分析. 畜牧兽医学报,2015, 46(11):1961-1966. LI P F, MENG J Z, XIE J S, ZHU Z W, LIU Y, JIANG X L, CHEN J W, YAO X L, ZHAO M M, LÜ L H. Screening and analyse study of genes associated with follicular development in bovine ODF1 and ODF2 transcript. Acta Veterinaria et Zootechnica Sinica, 2015, 46(11):1961-1966. (in Chinese)
[18] IRELAND J J, MURPHEE R L, COULSON P B. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. Journal of Dairy Science, 1980, 63(1):155-160.
[19] AUDIC S, CLAVERIE J M. The significance of digital gene expression profiles. Genome Research, 1997, 7(10):986-995.
[20] XU Z, GARVERICK H A, SMITH G W, SMITH M F, HAMILTON S A, YOUNGQUIST R S. Expression of messenger ribonucleic acidencoding cytochrome P450 side-chain cleavage, cytochrome p450 17 alpha-hydroxylase, and cytochrome P450 aromatase in bovine follicles during the first follicular wave. Endocrinology, 1995, 136(3):981-989.
[21] KOBAYASHI Y, JIMENEZ-KRASSEL F, IRELAND J J, SMITH G W. Evidence of a local negative role for cocaine and amphetamine regulated transcript (CART), inhibins and low molecular weight insulin like growth factor binding proteins in regulation of granulose cell estradiol production during follicular waves in cattle. Reproductive Biology and Endocrinology, 2006, 4(1):22-33.
[22] IRELAND J J, ROCHE J F. Development of nonovulatory antral follicles in heifers: changes in steroids in follicular fluid and receptors for gonadotropins. Endocrinology, 1983, 112(1):150-156.
[23] IRELAND J J, ROCHE J F. Development of antral follicles in cattle after prostaglandin-induced luteolysis: changes in serum hormones,steroids in follicular fluid, and gonadotropin receptors. Endocrinology,1982, 111(6):2077-2086.
[24] SUNDERLAND S J, CROWE M A, BOLAND M P, ROCHE J F,IRELAND J J. Selection, dominance and atresia of follicles during the oestrous cycle of heifers. Journal of Reproduction and Fertility, 1994,101(3):547-555.
[25] GINTHER O J, BERGFELT D R, KULICK L J, KOT K. Selection of the dominant follicle in cattle: establishment of follicle deviation in less than 8 hours through depression of FSH concentrations. Theriogenology, 1999, 52(6):1079-1093.
[26] GOODMAN A L, NIXON W E, JOHNSON D K, HODGEN G D. Regulation of folliculogenesis in the cycling rhesus monkey: selection of the dominant follicle. Endocrinology, 1977, 100(1):155-161.
[27] IRELAND J J, ROCHE J F. Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers: changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles. Journal of Animal Science, 1983, 57(1): 157-167.
[28] ILHA G F, ROVANI M T, GASPERIN B G, ANTONIAZZI A Q,GONÇALVES P B, BORDIGNON V, DUGGAVATHI R. Lack of FSH support enhances LIF-STAT3 signaling in granulosa cells of atretic follicles in cattle. Reproduction, 2015, 150(4):395-403.
[29] GARCÍA GUERRA A, TRIBULO A, YAPURA J, ADAMS G P,SINGH J, MAPLETOFT R J. Lengthened superstimulatory treatment in cattle: Evidence for rescue of follicles within a wave rather than continuous recruitment of new follicles. Theriogenology, 2015,84(3):467-476.
[30] ATANASOV B, DE KOSTER J, BOMMELÉ L, DOVENSKI T,OPSOMER G. Pathways of the dominant follicle after exposure to sub-luteal circulating progesterone concentrations are different in lactating dairy cows versus non-lactating heifers. Animal Reproduction Science, 2015, 154(3):8-15.
[31] SILVA FILHO M L, BEZERRA L R, FERREIRA-SILVA J C, SOUTO F M, OLIVEIRA N R, DE LIMA P F, BARTHOLOMEW C C, DE OLIVEIRA M A. Influence of biostimulation and temporary weaning on follicular dynamics and pregnancy rates in Nelore cows (Bos taurus indicus). Tropical Animal Health and Production, 2015, 47(7):1285-1291.
[32] KOBAYASHI Y, JIMENEZ-KRASSEL F, LI Q, YAO J, HUANG R,IRELAND J J, COUSSENS P M, SMITH G W. Evidence that cocaine-and amphetamine-regulated transcript is a novel intraovarian regulator of follicular atresia. Endocrinology, 2004, 145(11): 5373-5383.
[33] SEN A, BETTEGOWDA A, JIMENEZ-KRASSEL F, IRELAND J J,SMITH G W. Cocaine-and amphetamine-regulated transcript (CART)regulation of follicle stimulating hormone signal transduction in bovine granulosa cells. Endocrinology, 2007, 148(9):4400-4410.
(责任编辑 林鉴非)
Research on the Expression and Localization of CART in Bovine Granulosa Cells at Different Developmental Stages
LI Peng-fei1, BI Xi-lin2, WANG Kai2, JING Jiong-jie2, LÜ Li-hua2
(1College of Life Science, Shanxi Agricultural University, Taigu 030801, Shanxi;2College of Animal Science and Technology,Shanxi Agricultural University, Taigu 030801, Shanxi)
Abstract:【Objective】The objective of this paper is to investigate the effect of CART (cocaine-and amphetamineregulated transcript) expression in granulosa cells on bovine follicular development at different stages.【Method】 Two RNA libraries from ODF1 (the largest onset of deviation follicle) and ODF2 (the second largest onset of deviation follicle)granulosa cells were prepared and sequenced using Illumina HiSeq 2000 platform. Cows were slaughtered, and both ovaries were collected, then the largest and the second largest follicles were screened through the morphology of corpus luteum in the first follicular wave. Follicular fluid was extracted for the concentration of E2and P measurement using competitive ELISA method to determine DF (dominant follicles) and SF (subordinate follicles). Granulesa cells were isolated from DF and SF,and total RNA were extracted, respectively. Using RPLP0 as a reference gene, primers were designed. qRT-PCR wasperformed to detect the expression of CART in DF and SF, GraphPad Prism 5.0 was used for construction and significant analysis. Rabbit anti-CART antibody was used to detect the CART expression in cow follicles.【Result】Deep sequencing results showed that CART amounts in ODF1 were 38.2 times more than that in ODF2. Ovaries of 3 cows were screened out which were in the first follicular wave according to the observation of the morphology of corpus luteum, and the DF and SF were obtained. Real-time PCR results showed that CART expressed in DF was extremely significantly higher than that in SF,up to 2310-fold, which is consistent with the deep sequencing results. Immunohistochemistry results showed that CART expressed both in DF and SF, and the amounts in DF was significantly greater than that in SF, at the same time, CART was mainly expressed in granulosa cell layer.【Conclusion】During the process of follicular development in cattle, other inhibitors have suppressed most of the follicles, and prevented them to develop into DF; when the deviation appears, CART inhibited DF to further develop into ovulatory follicle, so it is considered that CART possibly by promoting granulosa cells apoptosis, and suppresses the E2secretion in granulosa cell layer, eventually leads to DF atresia.
Key words:bovine; follicle; development; CART; expression
收稿日期:2015-12-01;接受日期:2016-04-30
基金项目:国家自然基金(31172211)、农业部“948”项目(2010-Z43)、山西省横向协作与委托项目(2010HX54)、山西省回国留学人员科研资助项目(2014-重点5)、山西省科技攻关项目(20130311027-2)、山西省人事厅人才引进项目、山西农业大学引进人才博士科研启动费(2014ZZ04) 和科研管理费资助重大项目和标志性成果培育项目(71060003)
