甘蓝型油菜温敏细胞核雄性不育系TE5A花药发育的细胞学研究
2016-07-18李可琪曾新华闫晓红
李可琪,曾新华,袁 荣,闫晓红,吴 刚
(1中国农业科学院油料作物研究所/农业部油料作物生物学与遗传育种重点开放实验室,武汉 430062;2中国农业科学院研究生院,北京 100081)
甘蓝型油菜温敏细胞核雄性不育系TE5A花药发育的细胞学研究
李可琪1,2,曾新华1,袁荣1,闫晓红1,吴刚1
(1中国农业科学院油料作物研究所/农业部油料作物生物学与遗传育种重点开放实验室,武汉 430062;2中国农业科学院研究生院,北京 100081)
摘要:【目的】明确甘蓝型油菜温敏细胞核雄性不育系TE5A花药败育的时期及细胞学特征,为进一步研究其雄性不育的机理奠定理论基础。【方法】用不同温度(16℃和22℃)处理试验材料不育系TE5A,分别观察植株的育性及花器形态;并采用常规半薄切片技术,通过甲苯胺蓝、苯胺蓝和苏丹黑B等染料进行染色,观察并比较该不育系TE5A不育植株和可育植株花药发育的显微结构、胼胝质以及脂质体等脂类物质的异同;进一步通过透射电镜对其花药发育的超微结构进行比较观察。【结果】当把可育株油菜从16℃光照培养箱移到22℃光照培养箱后7 d,观察到花朵的育性转变为雄性不育。把22℃光照培养箱的不育株油菜移到16℃光照培养箱后,观察到先开放的花朵表现为雄性不育,而随后开放的花朵表现为雄性可育。在不育环境下的不育株TE5A花朵的花瓣大小和形态与可育株花朵没有明显差异,但不育株花朵的花丝显著短于可育株的花丝,并且花药萎缩、干瘪,没有花粉粒附着在上面。在不育环境下,不育系TE5A花药败育发生在花粉母细胞减数分裂时期,花粉母细胞减数分裂发生异常,没有二分体及四分体的形成,形成“拟小孢子”。胼胝质在花粉母细胞时期可以正常合成,但后续的降解滞后,在四分体时期没有降解,直至花粉粒成熟期才开始降解。绒毡层没有观察到明显异常,并能分泌脂质体等脂类物质。“拟小孢子”的花粉粒外壁发育异常,无法形成具有特殊柱状体和顶盖结构的花粉粒外壁,因此,不能结合孢粉素和脂质体等物质。随着花药的继续发育,最后“拟小孢子”逐渐降解,只剩下花粉空壳。【结论】甘蓝型油菜温敏细胞核雄性不育系TE5A花朵的花瓣大小和形态与可育株花朵没有明显差异,但花丝显著缩短,花药萎缩、干瘪,没有花粉粒附着在上面。不育系TE5A花药败育发生在花粉母细胞减数分裂时期,减数分裂异常导致无法形成二分体及四分体,并且胼胝质的异常降解及花粉粒外壁的异常对花药败育也有重要影响。甘蓝型油菜温敏细胞核雄性不育系TE5A可以作为一新型甘蓝型油菜温敏细胞核雄性不育系材料。
关键词:甘蓝型油菜;温敏细胞核雄性不育;花药败育;减数分裂
联系方式:李可琪,Tel:18271412601;E-mail:likeqi1218@sina.com。通信作者吴刚,Tel:027-86711501;E-mail:wugang@caas.cn
0 引言
【研究意义】明确甘蓝型油菜温敏细胞核雄性不育系TE5A的花药败育时期及败育的细胞学特征,不仅为彻底解析该不育材料的不育机理奠定基础,而且为甘蓝型油菜细胞核雄性不育系的研究提供依据。【前人研究进展】杂种优势是自然界普遍存在的一种现象,而油菜是杂种优势利用最成功的作物之一[1]。在油菜中杂种优势的利用途径主要有:细胞质雄性不育三系杂交途径,细胞核雄性不育两系、三系杂交途径,化学杀雄杂交途径和光、温敏雄性不育两系杂交途径[2]。自从1973年石明松[3]发现光敏核不育系农垦58S,成功创建了水稻两系杂交途径以来,人们逐渐在小麦[4]、玉米[5]、大豆[6]、水稻[7]、油菜[8]等作物中发现光、温敏雄性不育材料,并得到广泛的推广应用。国内已经报道了大量甘蓝型油菜光、温敏细胞核雄性不育材料:Huiyou50S[9]、SP2S[10]、TE5A[8]、373S[11]、533S[12]和H50S[13]等。已经利用光温敏雄性不育两用系 501-8S培育出国内第一个应用于生产上的生态型雄性不育两系杂交油菜组合两优586[14]。利用湘91S选育的两系杂交种湘杂油5号[15]和利用光温敏不育系26S选育的赣两优二号等品种[16]已通过品种审定。进一步说明光、温敏雄性不育两系杂交油菜在实际生产应用中具有广阔前景。甘蓝型油菜雄性不育材料花药败育的细胞学研究将为其在生产中的推广应用提供理论依据。随着新型甘蓝型油菜细胞核雄性不育材料的不断发现,花药败育的细胞学研究也逐渐发展起来。杨莉芳等[17]根据前人对雄性不育材料花药发育过程及细胞形态变化的研究,将导致油菜雄性不育的原因分为:花粉囊发育异常、减数分裂异常、胼胝质代谢异常、绒毡层发育异常、花粉壁发育异常和花药开裂异常等。董军刚等[12]发现甘蓝型油菜生态雄性不育系 533S不能形成花粉囊,属于花粉囊发育异常导致雄性不育。杨光圣等[18]研究发现细胞核不育系宜3A是因为花粉母细胞减数分裂异常导致雄性不育。YU等[10]发现温敏核不育系 SP2S胼胝质没有降解,从而导致花药败育。葛娟等[9]、孙晓敏等[13,19]发现反型温敏核不育系Huiyou50S、生态雄性不育系 373S和光敏型核不育H50S绒毡层提前或推迟降解导致雄性不育。【本研究切入点】前人对光、温敏雄性不育材料的细胞学研究只是通过一种技术来观察花药发育的过程,本研究综合应用多重技术观察花药发育过程中各部分的变化。甘蓝型油菜温敏细胞核雄性不育系TE5A的育性受温度影响,低温育性正常,当温度高于20℃时则表现为完全不育[8],与之前发现的光、温敏核不育材料相比,以其败育彻底的优势,可以更好地应用于两系杂交育种中。【拟解决的关键问题】本研究采用常规半薄切片技术,通过甲苯胺蓝、苯胺蓝和苏丹黑 B等染料进行染色,来观察和比较该不育系TE5A不育植株和可育植株花药发育的显微结构、胼胝质以及脂质体等脂类物质的异同,并通过透射电镜对其花药发育的超微结构进行比较观察,明确不育系TE5A败育的时期及细胞学特征,为彻底解析其不育机理和应用奠定基础。
1 材料与方法
1.1 材料
甘蓝型油菜温敏细胞核雄性不育系 TE5A,是本课题组从甘蓝型油菜品系TE5中发现的一种新型自然突变不育类型。通过研究发现TE5A育性受温度影响,当温度低于20℃时,表现为完全可育;当温度高于20℃时,则表现为完全不育[8]。
1.2 不育系TE5A的育性转换观察
2015年2月,在中国农业科学院油料作物研究所22℃(16 h光照/8 h黑暗)的温室中播种不育系TE5A的种子,共25盆直径为18 cm的花盆,在五叶期进行春化处理。在植株即将现蕾时,将不育系TE5A分别移到16℃(16 h光照/8 h黑暗)和22℃(16 h光照/8 h黑暗)的光照培养箱(SANYO)中,各10盆。把5盆已经开花的可育油菜从 16℃光照培养箱移到 22℃光照培养箱(SANYO)中生长;把5盆刚现蕾一段时间的 22℃光照培养箱中的油菜移到 16℃光照培养箱中生长。
1.3 不育系TE5A花器特征观察
在甘蓝型油菜温敏细胞核雄性不育系TE5A盛花期,分别观察在22℃光照培养箱中生长的10盆可育油菜和在16℃光照培养箱中生长的10盆不育株的花瓣、花药、花丝等形态。
1.4 不育系TE5A半薄切片观察
当油菜进入初花期时,在上午9:00分别取该不育系TE5A可育环境下的可育植株和不育环境下的不育植株不同大小的花蕾(0.5—4 mm)立即放入FAA固定液(乙醇∶冰乙酸∶甲醛∶水=10∶1∶2∶7)中,抽真空30 min,固定12—24 h后,用浓度依次为50%、70%和95%的乙醇进行梯度脱水,然后渗透、包埋、切片、粘片,最后用 2%甲苯胺蓝、0.1%脱色苯胺蓝和苏丹黑B染液染色,并用Feica DM 2500显微镜进行镜检照相。
1.5 透射电镜观察
选取不同发育阶段的花蕾(0.5—4 mm),用4%戊二醛固定24 h之后,再用1%四氧化锇固定2 h,用丙酮进行梯度脱水,然后用Epon812树脂包埋,进行切片、粘片,最后用乙酸双氧铀和柠檬酸铅染色,并用TEM-1230透射电镜观察并照相。
2 结果
2.1 TE5A花朵形态学观察
当把已开花的可育株油菜从16℃(16 h光照/8 h黑暗)光照培养箱移到22℃(16 h光照/8 h黑暗)后,观察到刚开始开放的花朵仍然表现为可育,但一周之后在花序上开放的花朵转变为雄性不育(图1-a)。把刚现蕾一段时间的22℃(16 h光照/8 h黑暗)光照培养箱的油菜移到16℃(16 h光照/8 h黑暗),则植株先开放的花朵表现为雄性不育,而随后开放的花朵的育性发生了转换,表现为雄性可育(图 1-b)。这一现象说明该温敏材料对高温更敏感。
通过对在不同温度处理(16℃和22℃)下的甘蓝型油菜温敏细胞核雄性不育系TE5A花朵的形态学观察,表明该不育系TE5A不育株的花朵的花瓣大小和形态与可育株的花朵没有明显区别,但不育株花朵的花丝显著短于可育株的花丝,并且不育株的花药萎缩、干瘪,没有花粉粒附着在花药上;而可育株的花药饱满并且明显可以看到花粉粒(图1-c和图1-d)。
2.2 TE5A花药半薄切片观察

图1 不育系TE5A不育花和可育花的花器形态比较Fig. 1 Comparison of morphological characters between the sterile and fertile flowers of the sterile line TE5A at flowering stage
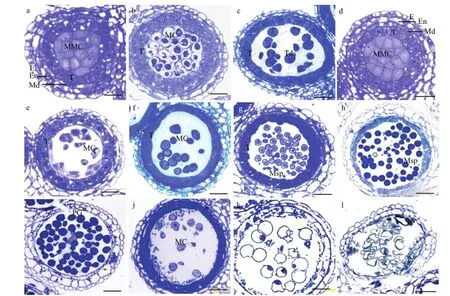
a:可育、花粉母细胞时期;b:可育、减数分裂时期;c:可育、四分体时期;g:可育、单核早期;h:可育、单核靠边期;i:可育、成熟花粉期;d:不育、花粉母细胞时期;e:不育、减数分裂时期f:不育、四分体时期;j:不育、单核早期;k:不育、单核靠边期;l:不育、成熟花粉期;E:外表皮;En:药室内壁;MMC:花粉母细胞;MC:减数分裂细胞;T:绒毡层;Tds:四分体;Msp:小孢子;PG:成熟花粉粒a: Fertile, pollen mother cell stage; b: Fertile, meiotic stage; c: Fertile, tetrad stage; g: Fertile, early uninucleate stage; h: Fertile, late uninucleate stage; i: Fertile,mature pollen stage; d: Sterile, pollen mother cell stage; e: Sterile, meiotic stage; f: Sterile, tetrad stage; j: Sterile, early mononuclear cell; k: Sterile, late uninucleate stage; l: Sterile, mature pollen stage; E: Epidermis; En: endothecium; MMC: Pollen mother cells; MC: Meiotic cell; T: Tapetum; Tds: Tetrads; Msp:Microspore; PG: Mature pollen grain图2 不育系TE5A花药发育的显微结构Fig. 2 The anther's microscopical structure of anthers of the sterile line TE5A
如图2所示,通过2%甲苯胺蓝染色可以看出,甘蓝型油菜温敏细胞核雄性不育系TE5A在不育环境下的不育植株和在可育环境下的正常可育花药都可以正常分化形成花粉母细胞,并且在结构上没有明显区别(图2-a和图2-d)。减数分裂早期,其花粉母细胞仍没有明显的差别,绒毡层细胞都显示轻度的液泡化并且含有2个细胞核(图2-b和图2-e)。减数分裂后期至四分体时期,可育花药可以正常形成四分体,绒毡层细胞明显皱缩,且细胞质染色比较深,表明绒毡层细胞已转化为分泌型(图2-c);不育花药仍然停留在花粉母细胞减数分裂早期的状态,没有二分体及四分体的形成,绒毡层细胞与可育花药没有明显区别(图2-f),说明花粉母细胞减数分裂出现了异常。进入单核期,可育花药随着包裹四分体的胼胝质的降解,释放出单核小孢子(图 2-g),小孢子继续发育直至形成成熟花粉粒(图2-h和图2-i)。在单核早期不育花药和可育花药的绒毡层细胞质进一步浓缩,染色加深,逐渐开始降解(图2-j和图2-k),不育花药花粉母细胞仍然停留在花粉母细胞时期的状态,形成“拟小孢子”,并且随着花药的继续发育而逐渐解体,到成熟花粉粒时期只剩下空瘪的花粉外壳(图2-j、图2-k和图2-l)。
进一步通过苯胺蓝染色观察花药发育过程中胼胝质的变化(图 3),在花粉母细胞时期不育花药和可育花药的胼胝质都分布在花粉母细胞周围,并且在结构上没有明显区别(图3-a和图3-e)。四分体时期,可育花药的胼胝质均匀地包裹在四分体周围,由于胼胝质逐渐降解,荧光强度比花粉母细胞时期弱(图3-f);不育花药的胼胝质将花粉母细胞紧紧地挤在一起,并且胼胝质壁较厚,荧光强度比花粉母细胞时期更亮(图 3-b)。随后可育花药的胼胝质完全降解,释放出小孢子,没有荧光信号(图 3-g),但不育花药的花粉囊中仍然存在非常强烈的胼胝质荧光信号,并且挤压得更加紧密(图3-c),到成熟花粉粒时期,随着“拟小孢子”的逐渐降解,胼胝质荧光信号才完全消失(图3-d)。

图3 不育系TE5A胼胝质观察Fig. 3 The callose staining of the sterile line TE5A
2.3 TE5A花药透射电镜观察
通过透射电镜观察进一步证实该不育系TE5A在不育的环境下,没有形成二分体及四分体(图4-a和图4-e)。在四分体释放出小孢子后,在可育环境下的正常可育花蕾的小孢子形成具有特殊的柱状体和顶盖结构的花粉外壁,这一柱状体和顶盖结构用来结合绒毡层释放的脂类和酚类物质以及孢粉素等,最终形成一层花粉包被(图4-f和图4-g)。而不育环境下的不育花蕾的花粉母细胞不能进行减数分裂,花粉母细胞形成“拟小孢子”(图 4-b),“拟小孢子”周围没有这一特殊的柱状体和顶盖结构,说明其花粉外壁发育异常,并且“拟小孢子”周围充满染色很深的颗粒状物质(图4-c)。成熟花粉粒时期,正常花粉粒形成三边加厚的花粉壁(图 4-h);不育花蕾的“拟小孢子”逐渐降解,只剩下空壳,颗粒状物质分布在“拟小孢子”及花粉囊四周(图4-d)。
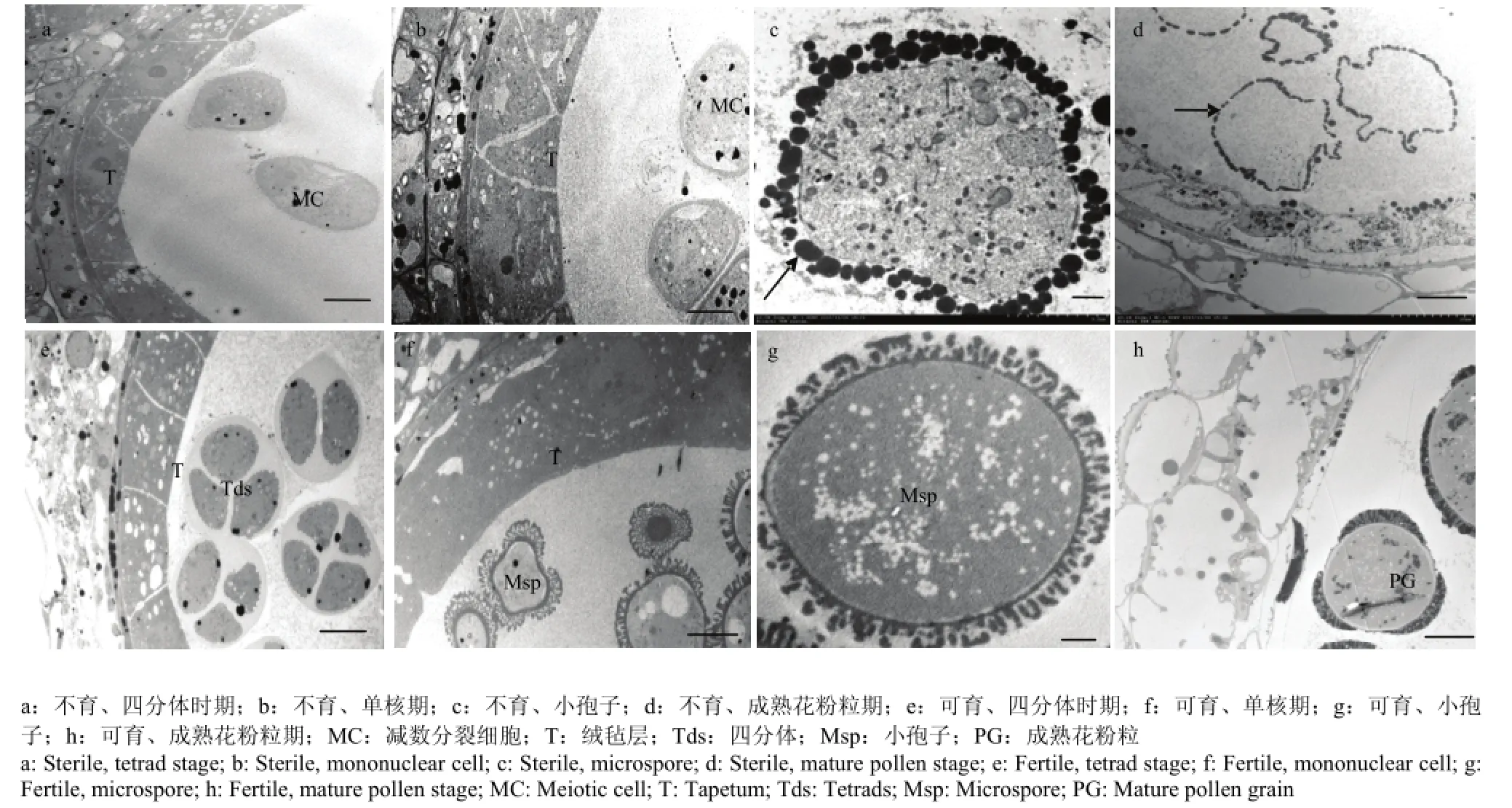
图4 不育系TE5A超薄切片显微解剖结构Fig. 4 The ultrastructure of the sterile line TE5A
2.4 TE5A花药脂类染色观察
根据透射电镜观察到TE5A在不育环境下的不育花蕾“拟小孢子”周围分布着染色很深的颗粒状物质,推测该物质是绒毡层分泌的脂质体等脂类物质。进一步通过脂特异性染料苏丹黑B对不育系TE5A花粉囊切片进行染色,观察到在“拟小孢子”早期(对应可育单核期),绒毡层开始释放出脂类物质,并结合在“拟小孢子”周围(图5-a)。随着花粉囊的继续发育,在“拟小孢子”中期(对应可育三核期),因为“拟小孢子”没有形成正常的外壁结构,所以这些脂类物质不能结合到小孢子外壁上,只是简单堆积在其周围而不能形成真正的花粉外壁(图5-b),最后随着“拟小孢子”的不断降解,这些脂类物质分散到花粉囊周围(图5-d)。

图5 不育系TE5A脂类染色图Fig. 5 Lipids staining of the sterile line TE5A
3 讨论
本研究表明甘蓝型温敏细胞核雄性不育系 TE5A的育性受温度影响,临界温度为20℃,低于该温度表现为完全不育,高于该温度表现为可育。这一育性表现与甘蓝型油菜温敏核不育系 SP2S[10]和甘蓝型油菜细胞质雄性不育系 392A[20]等大多数材料相同,都表现为低温可育、高温不育。但葛娟等[9]发现的发型温敏不育系Hui you50S表现为低温不育、高温可育的现象。
随着甘蓝型油菜光、温敏细胞核雄性不育系的不断发现,其花药发育的细胞学研究也逐渐发展起来。本研究发现温敏核不育系TE5A败育发生在花粉母细胞减数分裂时期,没有二分体及四分体的形成,败育特征与之前的研究材料有相似之处,但具体从减数分裂、胼胝质、绒毡层、花粉外壁发育等来看,不育系TE5A与现有的核不育材料均有不同。不育系TE5A减数分裂异常导致无法形成二分体及四分体,这一现象与宜 3A[18]和 Shaan-GMS[21]的败育特征非常相似,但TE5A的胼胝质推迟降解、绒毡层发育正常,而宜 3A的绒毡层发育异常、胼胝质发育正常,Shaan-GMS的绒毡层细胞在减数第一次分裂中期就开始降解。不育系SP2S[10]、9012A及7365A[22-25]也存在胼胝质推迟降解导致小孢子无法释放的现象,但这些材料可以形成正常的四分体并且绒毡层发育异常,这些特征都与TE5A不同。并且这些材料都没有低温可育的特性。目前报道的光温敏细胞核雄性不育材料H50S、373S和Huiyou50S[9,13,19]败育都发生在小孢子发育时期,小孢子可以正常释放出来但后期发育异常,并且都存在绒毡层降解异常的现象。这些光温敏材料与TE5A败育的特征存在巨大差异。总的来说目前发现的甘蓝型油菜核不育系材料几乎都存在绒毡层发育异常的现象,但不育系TE5A的绒毡层发育正常,并且有研究表明在水稻不育系中也存在花粉母细胞和小孢子发育异常而绒毡层发育正常的现象[26]。综合分析表明不育系TE5A为一新型温敏细胞核雄性不育系材料。
减数分裂是有性生殖生物性母细胞特殊的有丝分裂,减数分裂过程对雌雄配子的育性至关重要。已经发现大量雄性不育材料是因为减数分裂异常造成的,目前报道的有水稻[27]、小麦[28]、高粱[29]、大豆[30]、油菜[18,21]等农作物。在甘蓝型油菜中也存在许多这样的材料,如:杨光圣等[18]发现甘蓝型油菜核不育系宜3A、樊云芳等[31]发现双隐性细胞核雄性不育两用系479A、聂明建等[20]发现隐性两用核不育系86A和显性核不育系629A和XIAO等[21]发现显性核不育系Shaan-GMS均因为减数分裂发生异常,从而导致雄性不育。本研究材料也是因为减数分裂过程出现问题,从而导致花粉母细胞不能形成二分体及四分体。但尚不能确定减数分裂中哪一过程发生异常,接下来将通过DAPI染色进一步观察染色体的行为,研究减数分裂过程中染色体的变化,初步得出减数分裂发生异常的时期及特征。通过免疫荧光、荧光定量PCR 等方法研究减数分裂相关基因 DMC1[32]、RAD51[33]和ASY1[34]等在该不育材料TE5A中的表达情况,为进一步解析该材料的不育机理提供理论依据。
胼胝质代谢在花药发育过程中也起着极其重要的作用,胼胝质围绕在花粉母细胞周围使其不相互粘连、融合,并且使其免受外界渗透压力和有害物质的影响[35-36]。新型甘蓝型油菜温敏核不育系SP2S和甘蓝型隐性核不育9012A和7365A都是因为胼胝质没有降解导致小孢子无法从四分体中释放出来,从而导致花粉败育。HUANG等[37]通过干涉基因CDKG1(该基因调控胼胝质合成酶的基因CalS5),从而干涉的拟南芥植株不能形成正常的花粉外壁表现为雄性不育。SHI等[38]将CalS5在水稻中的CalS5基因敲除后,阳性转基因水稻在花粉母细胞时期胼胝质合成异常,从而导致花粉败育。因此,这些研究充分表明胼胝质在花药发育过程中起着巨大作用。所以该不育系 TE5A胼胝质的异常代谢对其花药败育也有重要影响。
4 结论
甘蓝型温敏细胞核雄性不育系TE5A的育性受温度影响,表现为高温不育、低温可育的特性。不育系TE5A在不育环境和可育环境下,花瓣大小没有明显区别,但不育花的花丝明显变短,花药干瘪、萎缩,没有花粉粒附着在上面。不育系TE5A在不育环境下,因减数分裂异常没有形成二分体及四分体,花粉母细胞形成“拟小孢子”;围绕在其四周的胼胝质推迟降解,直到花粉粒成熟期才降解;“拟小孢子”的外壁结构异常不能结合孢粉素和脂质体等物质,不育系TE5A可以作为新型甘蓝型油菜温敏细胞核雄性不育系材料。
References
[1] FU D, XIAO M, HAYWARDA, FU Y, LIU G, JIANG G, ZHANG H. Utilization of crop heterosis: A review. Euphytica, 2014, 197:161-173.
[2] ZHAO L, JING X, CHEN L, LIU Y J, SU Y A, LIU T T, GAO C B, YI B, WEN J, MA C Z, TU J X, FU T D, SHEN J X. Tribenuron-Methyl induces male sterility through anther-specific inhibition of acetolactate synthase leading to autophagic cell death. Molecular Plant, 2015, 8(12): 1710-1724.
[3] 石明松. 对光照长度敏感的隐性雄性不育水稻的发现与初步研究.中国农业科学, 1985(2): 44-48. SHI M S. The discovery and study of the photo-sensitive recessive male-sterile rice. Scientia Agricultura Sinica, 1985(2): 44-48. (in Chinese)
[4] GUO R X, SUN D F, TAN Z B, RONG D F, LI C D. Two recessive genes controlling thermophotoperiod-sensitive male sterility in wheat. Theoretical and Applied Genetics, 2006, 112(7): 1271-1276.
[5] TANG J H, FU Z Y, HU Y M, LI J S, SUN L L, JI H Q. Genetic analyses and mapping of a new thermo-sensitive genic male sterile gene in maize. Theoretical and Applied Genetics, 2006, 113: 11-15.
[6] FRASCH R M, WEIGAND C, PEREZ P T, PALMER R G,SANDHU D. Molecular mapping of 2 environmentally sensitive male sterile mutants in soybean. Journal of Heredity, 2010, 102: 11-16.
[7] DING J H, LU Q, OUYANG Y D, MAO H L, ZHANG P B, YAO J L, XU C G, LI X H, XIAO J H, ZHANG Q F. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proceedings of the National Academy of Sciences of the USA, 2012, 109(7): 2654-2659.
[8] ZENG X H, LI W P, WU Y H, LIU F, LUO J L, CAO Y L, ZHU L, LI Y J, LI J, YOU Q B, WU G. Fine mapping of a dominant thermo-sensitive genic male sterility gene (BntsMs) in rapeseed (Brassica napus) with AFLP-and Brassica rapa-derived PCR markers. Theoretical and Applied Genetics, 2014, 127: 1733-1740.
[9] 葛娟, 郭英芬, 于澄宇, 张国云, 董军刚, 董振生. 甘蓝型油菜光、温敏雄性不育系 Huiyou50S花粉败育的细胞学观察. 作物学报,2012, 38(3): 541-548. GE J, GUO Y F, YU C Y, ZHANG G Y, DONG J G, DONG Z S. Cytological observation of anther development of photoperiod/thermo sensitive male sterile line Huiyou50S in Brassica napus. Acta Agronomica Sinica, 2012, 38(3): 541-548. (in Chinese)
[10] YU C, GUO Y, GE J, HU Y M, DONG J G, DONG Z S. Characterization of a new temperature-sensitive male sterile line SP2S in rapeseed (Brassica napus L.). Euphytica, 2015, 206(2): 473-485.
[11] 于澄宇, 李玮, 常建军, 胡胜武. 油菜温敏雄性不育系 373S的选育. 中国农学通报, 2007, 23(7): 245-248. YU C Y, LI W, CHANG J J, HU S W. Development of A Thermo-sensitive Male-Sterile Line 373S in Brassica napus L.. Chinese Agricultural Science Bulletin, 2007, 23(7): 245-248. (in Chinese)
[12] 董军刚, 董振生, 刘绚霞, 刘创社, 李红兵. 甘蓝型油菜生态雄性不育系 533S花药发育的细胞学研究. 西北农林科技大学学报,2004, 32(7): 61-66. DONG J G, DONG Z S, LIU X X, LIU C S, LI H B. Cytological studies on anther development of ecological male sterile line 533S in Brassica napus L.. Journal of Northwest Sci-Tech University of Agriculture and Forestry, 2004, 32(7): 61-66. (in Chinese)
[13] 孙晓敏, 胡胜武, 于澄宇. 油菜生态不育系H50S花药败育的细胞学观察. 西北农业学报, 2009, 18(5): 153-158. SUN X M, HU S W, YU C Y. Cytological observation of anther development of an ecological male sterile line H50S in Brassica napus L.. Acta Agricultura Boreali-Occidentalis Sinica, 2009, 18(5):153-158. (in Chinese)
[14] 刘尊文, 袁卫红, 李文信. 甘蓝型两系杂交油菜两优 586的选育.中国油料作物学报, 2000, 22(2): 5-7. LIU Z W, YUAN W H, LI W X. Breeding of two-line system hybrid Liang you 586 in Brassica napus. Chinese Journal of Oil Crop Sciences, 2000, 22(2): 5-7. (in Chinese)
[15] 席代汶, 邬贤梦, 宁祖良, 邓锡兴, 陈卫江, 易冬莲, 李莓, 黄虎兰, 丁登杰. 优质两系杂交油菜湘杂油5号的选育. 中国油料作物学报, 2005, 27(1): 23-25. XI D W, WU X M, NING Z L, DENG X X, CHEN W H, YI D L, LI M, HUANG H L, DING D J. Breeding of a two-line hybrid variety Xiang za you 5 with double-low quality in Brassica napus L.. Chinese Journal of Oil Crop Sciences, 2005, 27(1): 23-25. (in Chinese)
[16] 刘尊文, 吴平, 张迁西, 周贱根, 袁卫红, 周小萍. 优质两系杂交油菜“赣两优二号”的选育. 江西农业学报, 2007, 19(11): 10-12. LIU Z W, WU P, ZHANG Q X, ZHOU J G, YUAN W H, ZHOU X P. Breeding of high quality two-line hybrid rape variety ganliang you 2. Acta Agriculturae Jiangxi, 2007, 19(11): 10-12. (in Chinese)
[17] 杨莉芳, 刁现民. 植物细胞核雄性不育基因研究进展. 植物遗传资源学报, 2013, 14(6): 1108-1117. YANG L F, DIAO X M. Progress in identification of plant male sterility related nuclear genes. Journal of Plant Genetic Resources,2013, 14(6): 1108-1117. (in Chinese)
[18] 杨光圣, 瞿波, 傅廷栋. 甘蓝型油菜显性细胞核雄性不育系宜 3A花药发育的解剖学研究. 华中农业大学学报, 1999, 18(5): 405-408.YANG G S, QU B, FU T D. Anatomical studies on the anther's development of the dominant genic male sterile line Yi-3A in Brassica napus L.. Journal of Huazhong Agricultural University, 1998, 18(5):405-408. (in Chinese)
[19] 孙晓敏, 李玮, 李英, 冯志峰, 李艳明, 习广清, 谌国鹏, 胡胜武.油菜生态型不育系 373S小孢子败育的电镜观察. 中国农学通报,2011, 27(7): 123-132. SUN X M, LI W, LI Y, FENG Z F, LI Y M, XI G Q, ZHAN G P, HU S W. Electronic microscope observations on microsporogenesis of ecol-sensitive male sterile line 373S in Brassica nupas. Chinese Agricultural Science Bulletin, 2011, 27(7): 123-132. (in Chinese)
[20] 聂明建, 王国槐, 朱卫平. 甘蓝型油菜3种类型雄性不育系花药败育的细胞学研究. 中国农业科学, 2007, 40(7): 1543-1549. NIE M J, WANG G H, ZHU W P. Cytology research on the anther abortion of three male sterility lines in rapeseed (Brassica napus L.). Scientia Agricultura Sinica, 2007, 40(7): 1543-1549. (in Chinese)
[21] XIAO Z D, XIN X Y, CHEN H Y, HU S W. Cytological investigation of anther development in DGMS line Shaan-GMS in Brassica napus L.. Czech Journal of Genetics and Plant Breeding, 2013, 49: 16-23.
[22] WAN L L, XIA X Y, HONG D F, LI J, YANG G S. Abnormal vacuolization of the tapetum during the tetrad stage is associated with male sterility in the recessive genic male sterile Brassica napus L. line 9012A. Journal of Plant Biology, 2010, 53: 121-133.
[23] ZHU Y, DUN X L, ZHOU Z F, XIA S Q, YI B, WEN J, SHEN J X,MA C Z, TU J X. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Molecular Biology,2010, 72: 111-123.
[24] DUN X L, ZHOU Z F, XIA S Q, XIA S Q, WEN L, YI B, SHEN J X,MA C Z, TU J X, FU T D. BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. The Plant Journal, 2011, 68: 532-545.
[25] ZHOU Z F, DUN X L, XIA S Q, SHI D Y, QIN M M, YI B, WEN J,SHEN J X, MA C Z, TU J X, FU T D. BnMs3 is required for tapetal differentiation and degradation, microspore separation, and pollen wall biosynthesis in Brassica napus. Journal of Experimental Botany,2012, 63: 2041-2058.
[26] YUAN W Y, LI X W, CHANG Y X, WEN R Y, CHEN G X, ZHANG Q F, WU C Y. Mutation of the rice gene PAIR3 results in lack of bivalent formation in meiosis. The Plant Journal, 2009, 59(2):303-315.
[27] ZHOU S, WANG Y, LI W, ZHAO Z G, REN Y L, WANG Y, GU S H, LIU Q B, WANG D, JIANG L, SU N. Pollen semi-sterility1 encodes a kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. The Plant Cell, 2011, 23(1): 111-129.
[28] 樊建青, 张立平, 赵昌平, 许晨光, 王灵云, 苑少华. 光温敏核雄性不育小麦BS366花粉母细胞减数分裂的细胞学研究. 中国细胞生物学学报, 2011, 33(6): 622-628. FAN J Q, ZHANG L P, ZHAO C P, XU C G, WANG L Y, YUAN S H. Studies on mieosis pollen mother cells in photoperiod-temperature sensitive genic male sterile wheat line BS366. Chinese Journal of Cell Biology, 2011, 33(6): 622-628. (in Chinese)
[29] 梁小红, 仪治本, 赵威军, 段运平, 崔贵梅, 孙毅. 高粱A2型细胞质雄性不育系小孢子发生的细胞学观察和减数分裂染色体行为分析. 作物学报, 2006, 32(8): 1107-1110. LIANG X H, YI Z B, ZHAO W J, DUAN Y P, CUI G M, SUN Y. Cytological observation of microsporogenesis and its chromosomal behavior in meiosis of A2 cytoplasmic-male sterile line in sorghum. Acta Agronomica Sinica, 2006, 32(8): 1107-1110. (in Chinese)
[30] 王芳, 卫保国, 李贵全, 李艳花. 大豆光敏雄性不育株 88-428BY-827小孢子母细胞的细胞学观察. 中国农业科学, 2004, 37(8):1110-1113. WANG F, WEI B G, LI G Q, LI Y H. A cytological observation of the pollen mother cells of the photoperiod-sensitive male sterile soybean plant of 88-428BY-827. Scientia Agricultura Sinica, 2004, 37(8):1110-1113. (in Chinese)
[31] 樊云芳, 胡胜武, 董彩华, 郭学兰, 刘胜毅. 一种甘蓝型油菜双隐性细胞核雄件不育的细胞学观察. 中国油料作物学报, 2006, 28(4):403-407. FAN Y F, HU S W, DONG C H, GUO X L, LIU S Y. Cytological investigation of microsporogenesis in a digenic recessive GMS line of Brassica napus. Chinese Journal of Oil Crop Sciences, 2006, 28(4):403-407. (in Chinese)
[32] BISHOP D K, PARK D, XU L, KLECKNER N. DMC1: A meiosisspecific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell, 1992, 69(3): 439-456.
[33] KOU Y J, CHANG Y X, LI X H, XIAO J H, WANG S P. The rice RAD51C gene is required for the meiosis of both female and male gametocytes and the DNA repair of somatic cells. Journal of Experimental Botany, 2012, 63(14): 5323-5335.
[34] KHOO K H P, ABLE A J, ABLE J A. The isolation and characterisation of the wheat molecular ZIPper I homologue, TaZYP1. BMC Research Notes, 2012, 5(1): 106.
[35] CHEN H H, WONG Y H, GENEVIERE A M, FAN M J. CDK13/ CDC2L5 interacts with L-type cyclins and regulates alternative splicing. Biochemical and Biophysical Research Communications, 2007, 354(3): 735-740.
[36] DONG X, HONG Z, SIVARAMAKRISHNAN M, MAHFOUZ M, VERMA D P S. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. The Plant Journal, 2005, 42(3): 315-328.
[37] HUANG X Y, NIU J, SUN M X, ZHU J, GAO J F, YANG J, ZHOU Q, YANG Z N. CYCLIN-DEPENDENT KINASE G1 is associated with the spliceosome to regulate CALLOSE SYNTHASE5 splicing and pollen wall formation in Arabidopsis. The Plant Cell, 2013, 25(2):637-648.
[38] SHI X, SUN X, ZHANG Z, FENG D, ZHANG Q, HAN L D, WU J X, LU T G. GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant and Cell Physiology, 2014, 56(3):497-509.
(责任编辑 李莉)
Cytological Researches on the Anther Development of a Thermo-Sensitive Genic Male Sterile Line TE5A in Brassica napus
LI Ke-qi1,2, ZENG Xin-hua1, YUAN Rong1, YAN Xiao-hong1, WU Gang1
(1Oil Crops Research Institute, Chinese Academy of Agricultural Sciences/Key Laboratory of Biology and Genetic Improvement of Oil Crops, the Ministry of Agriculture, Wuhan 430062;2Graduate School of Chinese Academy of Agricultural Science,Beijing 100081)
Abstract:【Objective】The aim of this paper is to detect the vital abortion periods and cytological characteristics of the anther development in a thermo-sensitive genic male sterile line TE5A in Brassica napus. The results will provide a theoretical basis for further study on the mechanism of male sterility.【Method】The sterile line TE5A was used as test material and was conducted withtwo different treatment temperatures (16℃ and 22℃). Then the fertility and characteristics of the floral organs of the plants were observed, respectively. The differences of anther development between the sterile and the normal plants of the sterile line TE5A were observed and compared by using toluidine blue, aniline blue and sudan black B to stain the semi-thin sectionings. Further observations and comparisons of cell ultrastructure of the anther development were carried out by transmission electron microscopy.【Result】The flowers of the plants were observed to be sterile, after seven days when the fertile rapeseed plants were moved to the light incubator of 22℃ from the one of 16℃. After the sterile rapeseed plants were moved to the light incubator of 16℃ from the one of 22℃, it was observed that the sterile flowers were opened first and the fertile flowers were opened after. Compared with the fertile flowers, the size and characteristic of petals of sterile plants in the sterile environment displayed no obvious difference. But the filaments of the sterile plants were significantly shorter than the fertile's and the sterile anthers withered without pollen grains in them. The pollen abortion of the sterile line TE5A in the sterile environment occurred at the pollen mother cell meiosis stage. The pollen mother cells could not pass the meiosis, with no dyads and tetrads formed in TE5A,insteading of some "pseudo microspores". The callose was normal deposited around the pollen mother cells, however, the callose hadn't been degraded until the mature pollen stage. The tapetum cells which could secret liposomes were observed to be normal in the fertile line. The pollen exines of the "pseudo microspores" were abnormal. The special bacula and tectum structure of the pollen exine couldn't formed, so the pollen exine couldn't combine with sporopollenins, no liposomes and other substances combined. With the development of the anthers, the "pseudo microspores" were decayed gradually and only empty shells remained at last.【Conclusion】Compared with the fertile flowers, the size and characteristic of petals of sterile plants in the sterile line TE5A displayed no obvious difference. But the filaments of the sterile plants were significantly shorter and the sterile anthers withered without pollen grains in them. The vital abortion of the sterile line TE5A occurred at the pollen mother cell meiosis stage. The pollen mother cells couldn't pass the meiosis, and neither dyads nor tetrads were formed. The abnormal degradation of the callose also play an important role in the anther development. The cytological characteristics of the thermo-sensitive genic male sterile line TE5A in B. napus were different from the previous materials. So these indicated that TE5A is a novel thermo-sensitive genic male sterile line.
Key words:Brassica napus; thermo-sensitive genic male sterile; anther abortion; meiosis
收稿日期:2016-03-14;接受日期:2016-05-20
基金项目:国家自然科学基金(31400243、31201152)、湖北省自然科学基金(2014CFB320)
