δ-catenin与Cdc42在非小细胞肺癌中表达的相关性
2016-06-17王恩华
张 迪, 王恩华
1.中国医科大学基础医学院病理教研室,辽宁 沈阳110122;2.中国医科大学附属第一医院病理科,辽宁 沈阳110001
δ-catenin与Cdc42在非小细胞肺癌中表达的相关性
张 迪1,2, 王恩华1,2
1.中国医科大学基础医学院病理教研室,辽宁 沈阳110122;2.中国医科大学附属第一医院病理科,辽宁 沈阳110001
[摘要]背景与目的:δ-catenin是p120 catenin 亚家族中的成员,可与细胞膜上的E-cadherin直接结合,形成E-cadherin/catenin复合体。δ-catenin还可以通过调节Cdc42(Small GTP酶)活性以影响细胞骨架装配。该研究检测非小细胞肺癌(non-small cell lung cancer,NSCLC)中δ-catenin及Cdc42的表达情况并探讨了二者表达的相关性。方法:采用免疫组织化学方法检测122例NSCLC标本中δ-catenin与Cdc42的表达。采用蛋白[质]印迹法(Western blot)及逆转录聚合酶链反应(reverse transcription polymerase chain reaction,RT-PCR)法检测肺癌组织中δ-catenin及Cdc42的蛋白及mRNA表达情况。在肺癌细胞系中,分别上调或干扰δ-catenin的表达后,利用G-LISA及Transwell小室法检测Cdc42活性以及肺癌细胞侵袭能力的改变。结果:δ-catenin和Cdc42在肺癌组织中其蛋白及mRNA表达明显高于正常肺组织。而在122例NSCLC病例中,δ-catenin阳性表达率为65.57%(80/122),Cdc42过表达率为68.03%(83/122)。δ-catenin阳性表达和Cdc42的过表达具有较好的相关性(P<0.001)。δ-catenin和Cdc42的协同表达与肺癌的高临床分期、低分化、病理类型为腺癌和淋巴结转移相关(P<0.05),并且与肺癌患者的不良预后明显相关。在肺癌细胞系中通过调节δ-catenin表达,改变Cdc42的表达及活性,影响肺癌细胞的侵袭能力。结论:在肺癌组织中δ-catenin和Cdc42的表达具有相关性,而二者协同表达与患者不良预后相关。
[关键词]δ-catenin; Cdc42; Small GTP酶;非小细胞肺癌;预后
Correspondence to: WANG Enhua E-mail: wangeh@hotmail.com
δ-catenin作为p120catenin(p120ctn)亚家族中的重要成员(包括p120ctn、δ-catenin、ARVCF、p0071和plakophilins 1/2/3)[1]。最早发现中枢神经系统表达δ-catenin,并具有促进突触生长的功能[2]。δ-catenin与同家族的p120的结构及作用相似[1],具有9个Arm重复序列[3-4],与p120共同在细胞膜上的E-cadherin(E-cad)拥有相同的近膜结构域(juxtamembrane domain,JMD)结合位点[5],可以通过与E-cad直接结合形成E-cadherin/catenin复合体,调节细胞间的粘附。
据报道,δ-catenin还可以通过对Small GTP酶活性的调节来影响细胞骨架生长[6-7]。Cdc42 是Small GTP 的核心成员,它们在代谢中存在与GTP结合的活化型和与GDP结合的失活型,两种形式好像分子开关般动态调节actin细胞骨架的装配,并引发各种生物学反应,例如细胞的形态、趋化和细胞运动等[8-9]。
因此,推测在肺癌组织中,δ-catenin与Cdc42之间很可能存在着联系。本研究检测了122例非小细胞肺癌(non-small cell lung cancer,NSCLC)样本中δ-catenin及Cdc42的表达情况并探讨了它们的表达与临床病理因素的关系。同时,在肺癌细胞系中分别过表达或干扰δ-catenin进而影响肺癌细胞的侵袭和转移。
1 材料和方法
1.1标本来源与患者资料
122例具有随访资料的原发性肺鳞癌、腺癌来自1998—2005年在中国医科大学第一附属医院行外科手术切除的标本存档蜡块。其中男性62例,女性60例,年龄33~80岁,中位年龄为59岁。按2004年WHO肺肿瘤组织学分类标准,其中鳞癌55例,腺癌67例。高分化30例,中分化58例,低分化34例。按照国际抗癌联盟(Union for International Cancer Control,UICC)于2002年修订的肿瘤pTNM分期标准,Ⅰ期18例,Ⅱ期40例,Ⅲ期57例,Ⅳ期7例。所有患者术前均未接受放化疗,术后常规化疗。标本均经4%中性甲醛溶液固定,石蜡包埋。此外,切除35例新鲜肺癌及癌旁正常肺组织,立即放入-70 ℃环境下保存,以备将来提取蛋白及RNA。
另外,对122例中的60例进行了详实的术后随访,生存时间的计算是从手术日期到由于复发或转移而死亡的日期或末次随访日期为止。
1.2免疫组织化学染色与结果判定
标本组织制成4 μm连续切片,二甲苯脱蜡和梯度酒精水化后,经柠檬酸盐缓冲液(pH 为6.0)高压抗原修复,再经3%H2O2灭活内源性过氧化物酶活性,非免疫血清封闭非特异性位点,之后切片与单克隆抗体δ-catenin (l∶50,美国Santa公司)和Cdc42 (1∶100,美国Santa公司)在4 ℃条件下温育过夜。再加生物素标记的山羊抗小鼠二抗(1∶500),采用DAB显色。PBS代替一抗做阴性对照。
对122例NSCLC标本采用免疫组织化学SP方法检测δ-catenin与Cdc42的表达以及与各临床病理因素之间的关系,此外还检测60例随访标本中δ-catenin与Cdc42的表达与患者预后的关系。观察δ-catenin在肿瘤组织的表达,计数400个肿瘤细胞并计算其中阳性染色细胞的百分数。δ-catenin的评分标准[10]:免疫染色的强度(0为阴性,1为弱阳性,2为中等阳性,3为强阳性);免疫染色的阳性肿瘤细胞数(阴性记0分,1%~25%记1分,26%~50%记2分,51%~75%记3分,大于等于76%记4分)。最终分数即为两项评分的乘积。为了便于统计,将评分结果分为两组:小于2为阴性表达;大于等于2为阳性表达。对Cdc42进行免疫组织化学检测,每张切片随机选取5个高倍视野,每个视野计数100个瘤细胞,无着色为-,阳性细胞数小于等于25%为+,26%~75%为++,大于等于76%为+++。Cdc42细胞质表达-~+定义为表达正常,将++~+++定义为过度表达[11]。将δ-catenin阳性表达和Cdc42的过表达同时出现的情况,称为协同表达。
1.3细胞培养与质粒构建转染
人肺腺癌细胞系H460、LK2(美国Manassas公司),分别培养于含10%灭活胎牛血清、2.3 g/L NaHCO3、100 U/mL青链霉素的RPMI-1640和高糖DMEM(美国Gibco公司)培养液中,于37 ℃、CO2体积分数为5%的湿润空气培养箱中培养。每2 d用0.25%胰酶消化传代。
利用脂质体LipofectamineTM2000(美国Invitrogen公司)将含人源性全长δ-catenin cDNA的质粒(pCMV5-FLAG/δ-catenin),导入δ-catenin表达较低的LK2细胞系,并以空载体作为阴性对照。另将设计的δ-catenin siRNA (5’-CUACGUUGACUUCUACUCAUU-3’,5’-UGAGUAGAAGUCAACGUAGUU-3’),利用脂质体L i p o f e c t a m i n eT M2 0 0 0导入δ-catenin表达较高的H460细胞系,同时以非特异性的乱序s i R N A作为阴性对照(5’-UUCUCCGAACUUGUCACAUUU-3’,5’-AUGUGACAAGUUCGGAGAAUU -3’)。
1.4蛋白[质]印迹法(Western blot)检测
取适量肺癌组织和细胞加入裂解液,在冰浴下超声处理,低温高速离心(4 ℃,21 400×g,30 min),收集上清液。将总蛋白的裂解产物进行SDS-PAGE电泳后,转印至PVDF膜。加入单克隆抗体δ-catenin (1∶200)、Cdc42(l∶300),4 ℃温育过夜,用山羊抗小鼠二抗37 ℃温育2 h,ECL发光并曝光胶片,经BioImaging System测定蛋白条带灰度值。以GAPDH为内对照,并取其与δ-catenin的比值做为相对表达量。
1.5逆转录聚合酶链反应(reverse transcription polymerase chain reaction,RT-PCR)检测
采用TRIzol试剂(美国Invitrogen公司)提取肺癌组织和细胞总RNA,利用RNA PCR Kit(AMV)Ver. 3.0试剂盒[宝生物工程(大连)有限公司]进行RT-PCR检测,引物序列和反应条件见表1。RT-PCR产物于1.5%琼脂糖凝胶电泳,用自动电泳凝胶成像分析系统(Bio-imaging Systems),测定扩增带灰度值,以GAPDH为内参照,得到mRNA的相对表达量。

表 1 引物序列和反应条件Tab. 1 Primer sequences and reaction conditions
1.6Cdc42活性的检测
采用Cdc42 G-LISATM比色法试剂盒(美国Cytoskeleton公司)分析Cdc42活性。将预备好的各细胞系裂解液加入可特异结合活性Cdc42(绑定GTP)的孔板小室中,缓冲液清洗其他成分后,利用分光光度计(BD转导实验室)检测活性Cdc42在490 nm波长处的吸收度。
1.7基质胶侵袭实验(Transwell小室法)
按照BD公司说明操作,上室内加入100 μL 1∶4稀释的Matrigel基质胶(美国BD Biosciences公司),在37 ℃、CO2体积分数为5%的条件下放置2 h。取100 μL细胞悬液滴入上室内(3×105个/mL),下室加入含有10%胎牛血清的RPMI-1640培养液,上、下室之间用孔径为8 μm孔径的Transwell微孔滤膜(美国Corning公司)分开,转染后24 h,瘤细胞被接种到基质胶,将小室置37 ℃、CO2体积分数为5%的条件下分别放置24 h。弃去上室内液体,擦尽膜上Matrigel基质胶,用100%甲醇固定30 min,常规苏木精染色,显微镜下随机计数5个视野(×400)的侵袭到微孔膜下表面的细胞。
1.8统计学处理
应用SPSS for Windows 13.0 (SPSS Inc.,Chicago, IL, USA)软件进行数据分析。免疫组织化学结果采用Pearson’s Chi-Square检验。Western blot和RT-PCR检测结果采用Student’s t检验。患者生存分析采用Kaplan-Meier法,logrank检验患者生存率的差别。P<0.05为差异有统计学意义。

图 1 δ-catenin和Cdc42在正常肺组织和肺鳞癌、腺癌中的表达Fig. 1 δ-catenin and Cdc42 expression in normal lung tissue, squamous cell carcinoma and adenocarcinoma (SP, ×200)δ-catenin (A) and Cdc42 (B) were expressed at low levels in the bronchial epithelial cells of the adjacent normal lung tissues. The expression of δ-catenin was positive in the cytoplasm of lung squamous cell carcinoma and adenocarcinoma (C and E). Cdc42 was over expressed in the cytoplasm of lung squamous cell carcinoma and adenocarcinoma cells (D and F)
2 结 果
2.1δ-catenin的阳性表达与Cdc42的过表达一致性和相关性检测结果
本研究采用免疫组织化学方法检测发现,δ-catenin和Rac1在正常支气管上皮细胞的细胞质中呈弱表达或不表达,而在肺癌组织中,二者表达均明显增高。在122例NSCLC病例中,δ-catenin阴性表达为42例,阳性表达为80例,阳性表达率为65.57% (80/122);Cdc42在肺癌组织中的表达明显强于支气管黏膜上皮细胞,其过表达率为68.03%(83/122)(图1)。Cdc42的过表达与δ-catenin的阳性表达具有较好的相关性(表2,P<0.001)。本研究又分析了在肺癌组织中,δ-catenin阳性表达而同时Cdc42过表达(协同表达)的病例与临床病理因素的关系(表3)。结果发现,肺腺癌中的协同表达率为64.18%(43/67),明显高于鳞癌的45.45%(25/55,P<0.05);低分化中的协同表达率为70.59%(24/34),明显高于高中分化的50.00%(44/88,P<0.05);Ⅲ~Ⅳ期的协同表达率为65.63%(42/64),显著高于Ⅰ~Ⅱ期的44.83%(26/58,P<0.05);有LN转移的病例协同表达率是62.35%(53/85),也明显高于无LN转移病例的40.54%(15/37,P<0.05)。但协同表达与患者年龄和性别均未见关联性(P>0.05)。

表 2 δ-catenin与Cdc42在肺癌中表达的相关性Tab. 2 Correlation between δ-catenin and Cdc42 in lung cancer
2.2δ-catenin和Cdc42的协同表达与患者的临床病理因素及不良预后的关系
当δ-catenin和Cdc42协同表达时,患者的平均生存时间和生存率明显低于其在阴性表达时患者 (图2,P<0.05)。因而,δ-catenin和Cdc42协同表达与患者的不良预后明显相关。
2.3肺癌组织中δ-catenin和Cdc42的表达情况
本研究利用Western blot法检测δ-catenin和Cdc42的蛋白表达情况。结果显示,在癌旁正常肺组织中可见δ-catenin蛋白表达量较低,而在肺癌组织中其表达明显增强。Cdc42在肺癌组织中的表达也明显高于正常肺组织(图3 A,B)。RT-PCR检测进一步证实了我们在Western blot中观察到的结果,在肺癌组织中δ-catenin和Cdc42的表达增均明显增强(图3 C,D)。

表 3 δ-catenin阳性表达和Cdc42过表达(协同表达)与临床病理因素的关系Tab. 3 The relationship between δ-catenin and Cdc42 expression and clinicopathological factors

图 2 Kaplan-Meier生存分析法检验δ-catenin和Cdc42协同表达与患者的不良预后的相关性Fig. 2 Kaplan-Meier was used for examining the relationship between co-expression of δ-catenin and Cdc42 and poor prognosis of patients
2.4过表达及沉默δ-catenin对Cdc42表达及活性的影响
为了探讨δ-catenin对Rac1的影响,本研究分别检测了过表达与沉默δ-catenin后Cdc42的表达及活性变化。分析显示,在H460细胞系中沉默δ-catenin后, Cdc42表达及活性下降(图4,P<0.05);而在LK2细胞系过表达δ-catenin后,Cdc42表达及活性明显增加(图5,P<0.05)。
2.5δ-catenin过表达和表达下调对肺癌细胞侵袭的影响
利用基质胶侵袭实验检测δ-catenin过表达或干扰其表达后肺癌细胞侵袭能力的变化(图6)。δ-catenin过表达后48 h,平均侵袭的细胞数为48.38个,明显高于对照组的20.55个(P<0.05);而在干扰δ-catenin后48 h,平均侵袭的细胞数为27.70个,显著低于相应对照组的47.13个(P<0.05)。以上结果表明,上调δ-catenin表达可以促进肺癌细胞的增殖与侵袭能力,而下调δ-catenin表达则抑制肿瘤细胞的增殖与侵袭能力。

图 3 在肺癌组织中(T1~T3)中δ-catenin和Cdc42的蛋白及mRNA表达明显高于癌旁正常肺组织(N1~N3)Fig. 3 The protein and mRNA expressions of δ-catenin and Cdc42 in lung cancer tissues (T1-T3) were significantly higher than those in normal lung tissues (N1-N3)

图 4 Western blot、RT-PCR及G-LISATM检测H460细胞系中沉默δ-catenin后Cdc42的蛋白及mRNA的表达情况及GTP-Cdc42活性Fig. 4 The protein and mRNA expressions of Cdc42 after knocking down δ-catenin in H460 cell line, and the GTP-Cdc42 activity detected by Western blot, RT-PCR and G-LISATM
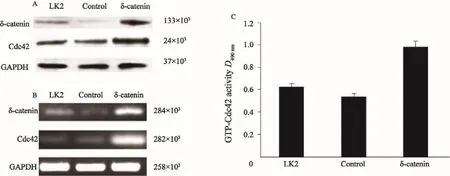
图 5 Western blot、RT-PCR及G-LISATM检测LK2细胞系中过表达δ-catenin后Cdc42的蛋白及mRNA的表达情况及GTP-Cdc42活性Fig. 5 The protein and mRNA expressions of Cdc42 after δ-catenin up-regulation in LK2 cell line, and the GTP-Cdc42 activity detected by Western blot, RT-PCR and G-LISATM

图 6 Transwell检测δ-catenin过表达及表达下调对肺癌细胞的侵袭的影响Fig. 6 The effect of δ-catenin up-regulation and down-regulation on the invasion of lung cancer cells detected by Transwell test
3 讨 论
δ-catenin和Cdc42在恶性肿瘤组织中的表达情况已有报道[12-19]。但是二者在肺癌组织中的表达是否具有相关性,以及这种相关性与临床病理因素和患者预后的关系等还未见报道,因而,本研究对122例NSCLC病例进行了检测分析。结果发现,在肺癌组织中,δ-catenin在细胞质中的表达增强,其蛋白及mRNA表达水平明显高于正常肺组织,而我们检测到Cdc42在肺鳞癌和腺癌中也存在过表达。同时δ-catenin阳性表达和Cdc42的过表达具有较好的相关性和一致性,其协同表达与肺癌的高临床分期、低分化、腺癌和淋巴结转移相关,并且与肿瘤的不良预后明显相关。
本研究检测到δ-catenin阳性表达与Cdc42过表达存在明显的相关性,同时δ-catenin表达增高时,Cdc42的表达及活性也明显增强。已知Cdc42是Small GTP酶家族中的核心成员[20],它们存在着与GTP结合的活化状态和与GDP结合的失活状态的可能。Cdc42的激活可以影响肿瘤细胞间的粘附功能[5,11],导致细胞伪足的延展,这必将导致肿瘤细胞具有更强的移动能力,有利于细胞向远处的游走[21-22]。这提示δ-catenin可通过调节Cdc42的表达与活性,促进肿瘤的侵袭转移。
总之,本研究在肺癌组织中发现δ-catenin 与Cdc42存在协同表达,而这种协同表达也与NSCLC患者的临床病理因素及预后不良密切相关。此外,δ-catenin可以调节Cdc42的表达与活性,影响肺癌细胞侵袭能力。
[参 考 文 献]
[1] HATZFELD M. The p120 family of cell adhesion molecules [J]. Eur J Cell Biol, 2005, 84(2-3): 205-214.
[2] KOUTRAS C, LESSARD C B, LÉVESQUE G. A nuclear function for the presenilin 1 neuronal partner NPRAP/ δ-catenin[J]. J Alzheimers Dis, 2011, 27(2): 307-316.
[3] KOSIK K S, DONAHUE C P, ISRAELY I, et al. Delta-catenin at the synaptic-adherens junction[J]. Trends Cell Biol, 2005, 15(3): 172-178.
[4] KAWAMURA Y, FAN Q W, HAYASHI H, et al. Expression of the mRNA for two isoforms of neural plakophilin-related arm-repeat protein/delta-catenin in rodent neurons and glial cells[J]. Neurosci Lett, 1999, 277(3): 185-188.
[5] LU Q, PAREDES M, MEDINA M, et al. Delta-catenin, an adhesive junction-associated protein which promotes cell scattering[J]. J Cell Biol, 1999, 144(3): 519-532.
[6] KIM K, SIROTA A, CHEN YH Y H, et al. Dendrite-like process formation and cytoskeletal remodeling regulated by delta-catenin expression[J]. Exp Cell Res, 2002, 275(2):171-184.
[7] ABU-ELNEEL K, OCHIISHI T, MEDINA M, et al. A deltacatenin signaling pathway leading to dendritic protrusions [J]. J Biol Chem, 2008, 283(47): 32781-32791.
[8] VEGA F M, RIDLEY A J. Snap shot: Rho family GTPases [J]. Cell, 2007, 129(7): 1430.
[9] SAHAI E, MARSHALL C J. RHO-GTPases and cancer [J]. Nat Rev Cancer, 2002, 2(2): 133-142.
[10] ZHANG J Y, WANG Y, ZHANG D, et al. Delta-catenin promotes malignant phenotype of non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in cytoplasm[J]. J Pathol, 2010, 222(1): 76-88.
[11] ZHANG J Y, ZHANG D, WANG E H. Overexpression of small GTPases directly correlates with expression of δ-catenin and their coexpression predicts a poor clinical outcome in nonsmall cell lung cancer[J].Mol Carcinog, 2013, 52(5):338-347.
[12] BURGER M J, TEBAY M A, KEITH P A, et al. Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer[J]. Int J Cancer, 2002, 100(2): 228-237.
[13] LU Q, DOBBS L J, GREGORY C W, et al. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer[J]. Hum Pathol, 2005, 36(10): 1037-1048.
[14] LU Q, ZHANG J, ALLISON R, et al. Identification of extracellular delta-catenin accumulation for prostate cancer detection[J]. Prostate, 2009, 69(4): 411-418.
[15] FANG Y, LI Z, WANG X, et al. Expression and biological role of δ-catenin in human ovarian cancer[J]. J Cancer Res Clin Oncol, 2012, 138(10): 1769-1776.
[16] LI X Y, LIU S L, CHA N, et al. Transcription expression and clinical significance of dishevelled-3 mRNA and δ-catenin mRNA in pleural effusions from patients with lung cancer[J]. Clin Dev Immunol, 2012, 2012: 904946[Epub 2012 Feb 23].
[17] DAI S D, WANG Y, ZHANG J Y, et al. Upregulation ofδ-catenin is associated with poor prognosis and enhances transcriptional activity through Kaiso in non-small cell lung cancer[J]. Cancer Sci, 2011, 102(1): 95-103.
[18] WANG M, DONG Q, ZHANG D, et al. Expression of delta catenin is associated with progression of human astrocytoma [J]. BMC Cancer, 2011, 11: 514. doi: 10.1186/1471-2407-11-514.
[19] GAO M, LIU L, LI S, et al. Inhibition of cell proliferation and metastasis of human hepatocellular carcinoma by miR-137 is regulated by CDC42[J]. Oncol Rep, 2015, 34(5): 2523-2532.
[20] NOBES C D, HALL A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia[J]. Cell, 1995, 81(1): 53-62.
[21] LANE J, MARTIN T, WEEKS H P, et al. Structure and role of WASP and WAVE in Rho GTPase signalling in cancer[J]. Cancer Genomics Proteomics, 2014, 11(3): 155-165.
[22] ZINS K, LUCAS T, REICHL P, et al. A Rac1/Cdc42 GTPasespecific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice[J]. PLoS One, 2013, 8(9): e74924.
Correlation between δ-catenin and Cdc42 expression in non-small cell lung cancer
ZHANG Di1,2,WANG Enhua1,2(1.Department of Pathology, the College of Basic Medical Sciences, China Medical University, Shenyang 110122, Liaoning Province, China; 2. Department of Pathology, the First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China)
[Key words]δ-catenin; Cdc42; Small GTPase; Non-small cell lung cancer; Prognosis
[Abstract]Background and purpose: δ-catenin is a member of the p120 catenin subfamily, which can directly bind to E-cadherin on the cell membrane, forming E-cadherin/catenin complex. δ-catenin can also affect the cytoskeleton assembly by regulating the activity of Cdc42 (Small GTPase). Therefore, this study detected the expression of δ-catenin and Cdc42 in non-small cell lung cancer (NSCLC) and investigated the relationship between them. Methods: The expressions of δ-catenin and Cdc42 in 122 cases of NSCLC were detected by immunohistochemistry. This study also used Western blot and real-time fluorescent quantitative polymerase chain reaction (RTFQ-PCR)to detect the protein and mRNA expressions of δ-catenin and Cdc42 in lung cancer tissues. After up-regulating or downregulating δ-catenin in lung cancer cell line, the activity of Cdc42 and invasion ability of lung cancer cells were detected by G-LISA and Transwell. Results: The mRNA and protein expression of δ-catenin and Cdc42 in lung cancer tissues was significantly higher than that in normal lung tissues. In 122 NSCLC cases, the δ-catenin positive expression rate was 65.57% (80/122), and the Cdc42 overexpression rate was 68.03% (83/122). There was a good correlation between δ-catenin positive expression and Cdc42 overexpression (P<0.001). The co-expression of δ-catenin and Cdc42 was related to the high clinical stage, poor differentiation, adenocarcinoma and lymph node metastasis of lung cancer(P<0.05), and was significantly associated with poor prognosis in patients with lung cancer. In the lung cancer cell line,the expression and the activity of Cdc42 were changed by regulating the δ-catenin expression, which affected invasion ability of the lung cancer cells. Conclusion: The δ-catenin expression was significantly correlated with the Cdc42 expression. The co-expression of δ-catenin and Cdc42 in lung cancer was correlated with the poor prognosis of lung cancer.
DOI:10.3969/j.issn.1007-3969.2016.03.004
中图分类号:R734.2
文献标志码:A
文章编号:1007-3639(2016)03-0221-09
基金项目:国家自然科学基金(81101779)。
通信作者:王恩华 E-mail:wangeh@hotmail.com
收稿日期:(2015-10-03 修回日期:2016-02-20)
