LDN-193189对人去分化软骨肉瘤细胞系NDCS-1的抑制作用研究*
2016-03-31杨康汤小东郭卫
杨康 汤小东 郭卫
LDN-193189对人去分化软骨肉瘤细胞系NDCS-1的抑制作用研究*
杨康汤小东郭卫
摘要目的:检测骨形成蛋白(BMP)受体抑制剂LDN-193189对人去分化软骨肉瘤(DDCS)细胞系NDCS-1的抑制作用,探讨LDN-193189对去分化软骨肉瘤的抑癌机制。方法:以5 nmol/L的LDN-193189作用于NDCS-1细胞,MTT、平板克隆法检测LDN-193189对NDCS-1细胞的增殖抑制作用,Transwell法、划痕实验检测LDN-193189对NDCS-1细胞的侵袭抑制作用,Western blot检测BMPR2、p-Smad1/5及RUNX2的蛋白表达抑制情况。结果:药物处理后NDCS-1细胞增殖、侵袭被明显抑制;药物处理后NDCS-1细胞的BMPR2、p-Smad1/5及RUNX2蛋白表达下降。结论:LDN-193189通过抑制BMPR2-p-Smad1/5-RUNX2信号传导通路能有效抑制去分化软骨肉瘤细胞系NDCS-1的增殖侵袭能力。
关键词去分化软骨肉瘤LDN-193189 NDCS-1 BMPR2 p-Smad1/5 RUNX2
*本文课题受国家自然科学基金项目(编号:81172544)和教育部新世纪人才项目(编号:NCET-12-0007)资助
LDN-193189inhibits progression and induces apoptosis in human dedifferentiated chondrosarcoma cell line NDCS-1
Kang YANG, Xiaodong TANG, Wei GUO
Correspondence to: Xiaodong TANG; E-mail: tang15877@126.com
Department of Bone Oncology, Peking University, People's Hospital, Beijing 100044, China
This work was supported by the Natural Science Foundation of China(No.81172544)and the Program for New Century Excellent Talents in University(No.NCET-12-0007)
Abstract Objective: To clarify the effects of the BMP receptor inhibitor LDN-193189 in the dedifferentiated chondrosarcoma(DDCS)cell line NDCS-1 and to explore the anti-tumor mechanismof LDN-193189 in DDCS.Methods: NDCS-1 was treated with 5 nmol/L of LDN-193189.MTT assay and clone formation experiments were used to verify that LDN-193189 suppressed cell proliferation.Transwell and wound healing tests were performed to demonstrate that LDN-193189 inhibited cell invasion.Western blot detection was used to show that LDN-193189 inhibited the suppression of BMPR2, p-Smad1/5, and RUNX2 protein expression.Results: The BMPR2 signaling pathway was inhibited by LDN-193189; thus, cell viability and invasion were significantly suppressed.Conclusion: LDN-193189 induces the inhibition of progression in vitro via the BMPR2-p-Smad1/5-RUNX2 signaling pathway in the human DDCS cell line NDCS-1.
Keywords:dedifferentiated chondrosarcoma, LDN-193189, NDCS-1, BMPR2, p-Smad1/5, RUNX2
去分化软骨肉瘤是一种特殊的软骨肉瘤,约占所有软骨肉瘤的10%[1],软骨肉瘤一旦发生去分化改变,肿瘤侵袭性明显增高[2]。由于早期转移与放化疗不敏感,去分化软骨肉瘤预后极差,5年生存率为10%~24%[3-5]。以往实验室研究表明去分化软骨肉瘤中BMPR2、RUNX2表达明显高于普通软骨肉瘤[6-7],抑制BMPR2能够控制肿瘤的发生发展[8],而LDN-193189是BMP受体特异性抑制剂,因此本研究以去分化软骨肉瘤细胞系NDCS-1为研究对象,观察LDN-193189对NDCS-1细胞的作用并检测BMPR2及下游基因p-Smad1/5、RUNX2的表达,探讨LDN-193189对NDCS-1细胞的作用机制,为后续体内实验与临床应用提供前期研究基础。
1 材料与方法
1.1材料
人去分化软骨肉瘤细胞系NDCS-1由日本Akira Ogose教授惠赠;人Ⅱ级软骨肉瘤细胞系SW1353(美国ATCC公司);人Ⅰ级软骨肉瘤细胞系HCS2/8由日本Takigawa教授惠赠;RPMI-1640、L-15、DMEM培养基(美国Gibco公司);细胞胎牛血清(美国Gibco,Invitrogen公司);LDN-193189购自美国Selleck公司,用100%DMSO溶解;MTT购自美国SIGMA公司;Transwell小室购自美国Corning公司;双抗购自美国Gibco公司。小鼠抗人多克隆BMPR2抗体购自英国Abcam公司;小鼠抗人多克隆RUNX2、兔抗人多克隆GAPDH抗体购自美国Santa Cruz公司;p-Smad1/5、Smad1购自德国CST公司;辣根过氧化物酶标记羊抗小鼠IgG、免疫组织化学染色试剂盒购自北京中杉金桥生物公司。
1.2方法
1.2.1细胞培养NDCS-1与HCS2/8培养于含有5% CO2的37℃培养箱,SW1353培养于不含CO2的37℃培养箱。培养基分别为RPMI-1640、DMEM、L-15培养基,所有培养基均含10%胎牛血清及1%双抗,取对数生长期细胞用于实验操作。
1.2.2免疫组织化学染色对病理证实为去分化软骨肉瘤的蜡块进行切片,按照免疫组织化学染色试剂盒说明书操作:脱蜡,PBS清洗,抗原修复,3% H2O2孵育,封闭,BMPR2、RUNX2一抗37℃孵育3 h,二抗37℃孵育30 min,DAB显色。
1.2.3MTT法检测LDN-193189对NDCS-1细胞的增殖抑制作用取对数生长期NDCS-1细胞计数,以5000个细胞/孔的密度接种于96孔板,24 h后按照2.5、5、10、20 nmol/L浓度加入LDN-193189,设置5个复孔并以DMSO作对照。加药后培养48 h,每孔加入MTT (5 mg/mL)20 μL,继续培养4 h,吸去培养液,每孔加入DMSO150μL,酶标仪(波长570nm)测定每孔OD值。
1.2.4平板克隆法检测LDN-193189对NDCS-1细胞集落形成能力的影响取对数生长期NDCS-1细胞计数,按1 000个细胞/孔密度接种于6孔板,加入5 nmol/L的LDN-193189,设置3个复孔并以DMSO作对照。14 d后吸去培养液,每孔加入1 mL结晶紫培养30 min,观察并拍照。
1.2.5Transwell法检测LDN-193189对NDCS-1的迁移抑制作用取对数生长期NDCS-1细胞计数,按5×105/孔密度接种于Transwell小室上室,下室加入5 nmol/L的 LDN-193189,设置3个复孔并以DMSO作对照。加药后培养24 h,吸去培养液,0.1%结晶紫染色计数。
1.2.6划痕实验检测LDN-193189对NDCS-1的迁移抑制作用取对数生长期NDCS-1细胞接种于6孔板,待细胞汇合度达到80%时于6孔板划一直线,加入5nmol/L 的LDN-193189,DMSO作对照,于0、24 h拍照。
1.2.7Western blot检测BMPR2、p-Smad1/5、Smad1、RUNX2表达取对数生长期NDCS-1细胞接种于6孔板,待细胞汇合度达到80%时加入5 nmol/L的LDN-193189,DMSO作对照,药物作用48 h收集细胞,用RIPA+ 1%PMSF裂解液裂解细胞提取总蛋白,进行10% SDSPAGE分离电泳,湿转法转至PVDF膜,5%脱脂奶粉封闭1 h,一抗4℃培育过夜、次日二抗室温孵育1 h,化学发光试剂盒显色。所有抗体浓度均为1:1 000。
1.3统计学处理
采用SPSS 20.0统计软件进行统计学分析,均数比较采用单因素方差分析和独立t检验。P<0.05为差异有统计学意义。
2 结果
2.1BMPR2、RUNX2在不同级别软骨肉瘤中差异表达
免疫组织化学染色结果显示在去分化软骨肉瘤中BMPR2与RUNX2阳性表达,表达阳性率统计结果见表1,BMPR2、RUNX2在去分化软骨肉瘤中表达阳性率明显高于普通软骨肉瘤,有统计学意义(P<0.01,P<0.05)。在去分化软骨肉瘤细胞系NDCS-1中BMPR2、p-Smad1/5及RUNX2的蛋白表达水平明显高于普通软骨肉瘤细胞系SW1353及HCS2/8(图1,2)。

表1 临床病理等级与BMPR2或RUNX2表达阳性之间的关系Table 1 Association between clinicopathological characteristics and BMPR2 or RUNX2 expression

图1 去分化软骨肉瘤样本中BMPR2、RUNX2表达(免疫组织化学染色×200)Figure 1 Detection of BMPR2 and RUNX2 expression in DDCS samples with immunohistochemistry(immunohistochemistry×200)

图2 不同级别软骨肉瘤细胞系中BMPR2、p-Smad1/5、RUNX2蛋白表达Figure 2 Detection of BMPR2, p-Smad1/5, and RUNX2 protein levels in different grades of chondrosarcoma cell lines with Western blot analysis
2.2 LDN-193189对去分化软骨肉瘤NDCS-1细胞增殖影响
MTT结果显示,LDN-193189能有效抑制去分化软骨肉瘤细胞系NDCS-1细胞的增殖:2.5 nM增加到5 nM时细胞增殖能力显著降低,而5nM以上随着浓度的增加未见更加明显的抑制细胞增殖能力,因此后续实验均以5 nM为实验浓度。克隆形成实验表明5 nM的LDN-193189明显抑制NDCS-1细胞的集落形成能力(图3)。
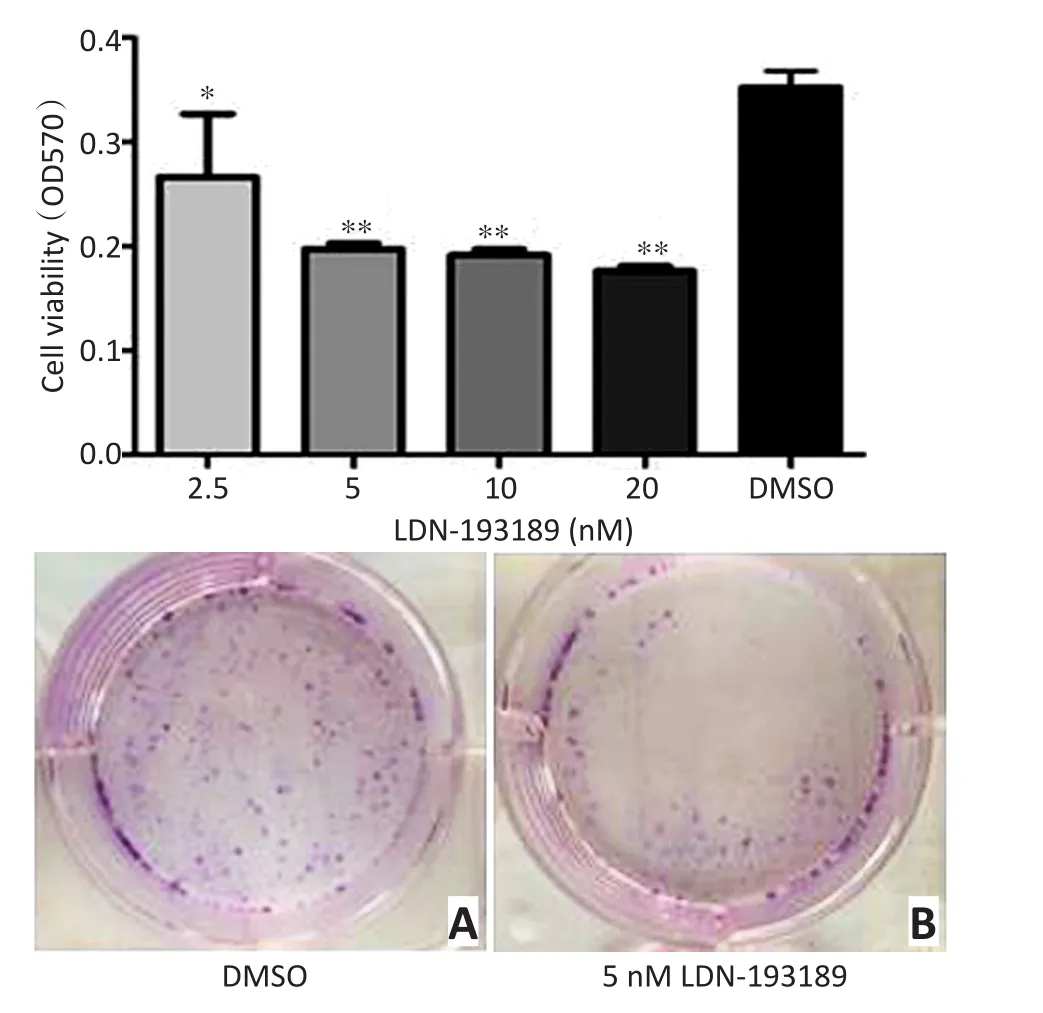
图3 LDN-193189处理细胞24 h后能明显抑制去分化软骨肉瘤细胞系NDCS-1的增殖能力Figure 3 LDN-193189 significantly inhibits proliferation of the DDCS cell line NDCS-1 after 24 h of treatment
2.3LDN-193189能明显抑制去分化软骨肉瘤NDCS-1细胞的侵袭能力
Transwell结果显示,LDN-193189能明显抑制去分化软骨肉瘤细胞系NDCS-1侵袭能力,划痕实验表明用药处理24 h后,能明显抑制NDCS-1的迁移能力(图4)。

图4 LDN-193189处理细胞24h后能明显抑制去分化软骨肉瘤细胞系NDCS-1的侵袭能力Figure 4 LDN-193189 significantly inhibits the invasive capacity of the DDCS cell line NDCS-1 after 24 h of treatment
2.4LDN-193189抑制BMPR2、p-Smad1/5及RUNX2的表达
经过5 nM LDN-193189处理后,与对照组相比,NDCS-1中Smad1蛋白表达水平无改变,而BMPR2、p-Smad1/5、RUNX2蛋白表达水平显著降低(图5)。

图5 Western blot结果显示LDN-193189抑制NDCS-1细胞中BMPR2、p-Smad1/5及RUNX2蛋白表达水平Figure 5 LDN-193189 down-regulates BMPR2, p-Smad1/5, and RUNX2 protein levels in the DDCS cell line NDCS-1
3 讨论
去分化软骨肉瘤是一种高度恶性的骨肿瘤,放化疗不敏感,术后极易转移复发,目前多采用手术切除[3-5]。去分化软骨肉瘤常由软骨肉瘤发生去分化改变形成,预后极差,多数患者发病2年内死于广泛转移[2]。
LDN-193189是合成的一种小分子化合物,能够选择性抑制BMP信号通路,通过抑制BMPⅠ型受体ALK2和ALK3的转录活性,进而抑制下游Smad1/5/8磷酸化及转录因子的表达,但是研究发现LDN-193189同样能够抑制BMPR2蛋白水平的表达。
本实验研究了LDN-193189对去分化软骨肉瘤细胞系NDCS-1的抑制增殖侵袭作用。MTT结果显示,LDN-193189有效抑制NDCS-1细胞的增殖能力并呈现剂量依赖效应;同时使用克隆形成实验,发现经过LDN-193189处理后,NDCS-1细胞集落形成能力明显降低。之后使用Transwell实验及划痕实验,发现LDN-193189可以明显抑制NDCS-1细胞的侵袭能力。
BMP是TGF-β超家族成员,其在调控生长发育过程中起着重要作用,如增殖、分化、迁移、细胞死亡等[9]。目前已发现将近20种BMP家族成员[10],BMP信号通路由细胞膜表面的BMP配体结合到BMPⅠ型和Ⅱ型受体所启动[9]。配体诱导的受体活化能够启动两条信号通路:第一种是依赖Smad信号通路,即受体特异性的磷酸化Smad1/5/8,之后磷酸化的Smad1/5/8与Smad4形成复合体,最终这一复合体进入核内并调控目的基因转录[11]。另一条信号通路是非依赖Smad信号通路,即有丝分裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK),包含p38、Jun激酶和ERK激酶信号通路[12-13]。BMPR2是BMP受体的一种,在BMP信号通路中起着重要作用,BMPR2的异常导致肺动脉高压的发生[14]。此外,多种恶性肿瘤中BMPR2常异常表达,如乳腺癌[8]。以往的研究认为BMPR2是肿瘤抑制基因,能够抑制肿瘤的转移与复发[15],但本课题组以往研究却发现BMPR2在软骨肉瘤中起着促进肿瘤转移与复发的作用[16]。此外,在前期应用免疫组织化学实验发现BMPR2及下游基因RUNX2在去分化软骨肉瘤中高表达。而LDN-193189是BMP受体抑制剂,也许能通过抑制BMPR2及下游基因从而抑制去分化软骨肉瘤的发生发展。在本研究中,经LDN-193189处理后,NDCS-1细胞中BMPR2、p-Smad1/5、RUNX2表达明显降低,而Smad1蛋白的表达无明显变化。结果表明,LDN-193189通过下调BMPR2-pSmad1/5-RUNX2信号通路有效抑制NDCS-1细胞的增殖侵袭并促进凋亡。但本研究的重点是BMPR2依赖Smad通路,是否LDN-193189也能通过非依赖Smad信号通路调控肿瘤的发生发展尚需进一步研究证实。
综上所述,LDN-193189作为一种实验药物,在体外可以有效抑制去分化软骨肉瘤细胞系的增殖侵袭能力。本实验为将来LDN-193189作为治疗去分化软骨肉瘤一种靶向药物奠定前期研究基础。
参考文献
[1] Sakamoto A.The molecular pathogenesis of dedifferentiated chondrosarcoma[J].Indian J Orthop, 2014, 48(3):262-265.
[2] Bharath G, Burrah R, Shivakumar K, et al.Dedifferentiated chondrosarcoma: an aggressive variant of chondrosarcoma[J].Asian Cardiovasc Thorac Ann, 2015, 23(2):221-223.
[3] Matsumoto Y, Takahashi Y, Harimaya K, et al.Dedifferentiated chondrosarcoma of the cervical spine: a case report[J].World J Surg oncol, 2013, 11:32.
[4] Yokota K, Sakamoto A, Matsumoto Y, et al.Clinical outcome for patients with dedifferentiated chondrosarcoma: a report of 9 cases at a single institute[J].J Orthop Surg Res, 2012, 7:38.
[5] Huang J, Jiang Z, Yang Q, et al.Benign looking giant cell component in dedifferentiated chondrosarcoma: benign or malignant? A case report[J].Int J Surg Pathol, 2013, 21(1):48-53.
[6] Guo W, Gorlick R, Ladanyi M, et al.Expression of bone morphogenetic proteins and receptors in sarcomas[J].Clin Orthop Relat Res, 1999, 365:175-183.
[7] Tang X, Lu X, Guo W, et al.Different expression of Sox9 and Runx2 between chondrosarcoma and dedifferentiated chondrosarcoma cell line[J].Eur J Cancer Prev, 2010, 19(6):466-471.
[8] Pouliot F, Blais A, Labrie C.Overexpression of a dominant negative typeⅡbone morphogenetic protein receptor inhibits the growth of human breast cancer cells[J].Cancer Res, 2003, 63(2):277-281.
[9] Zhang Y, Wang Y, Yang K, et al.BMP4 Increases the Expression of TRPC and Basal [Ca2+]i via the p38MAPK and ERK1/2 Pathways Independent of BMPRII in PASMCs[J].Plos One, 2014, 9(12):e112695.
[10] Chen D, Zhao M, Mundy GR.Bone morphogenetic proteins[J].Growth Factors, 2004, 22(4):233-241.
[11] von Bubnoff A, Cho KW.Intracellular BMP signaling regulation in vertebrates: pathway or network[J]? Dev Biol, 2001, 239(1):1-14.
[12] Nohe A, Hassel S, Ehrlich M, et al.The mode of bone morphogenetic protein(BMP)receptor oligomerization determines different BMP-2 signaling pathways[J].J Biol Chem, 2002, 277(7):5330-5338.
[13] Gallea S, Lallemand F, Atfi A, et al.Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells[J].Bone, 2001, 28(5):491-498.
[14] Diebold I, Hennigs JK, Miyagawa K, et al.BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension[J].Cell Metab, 2015, 21(4):596-608.
[15] Owens P, Pickup MW, Novitskiy SV, et al.Disruption of bone morphogenetic protein receptor 2(BMPR2)in mammary tumors promotes metastases through cell autonomous and paracrine mediators[J].Proceedings of the National Academy of Sciences, 2012, 109(8):2814-2819.
[16] Jiao G, Guo W, Ren T, et al.BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells[J].Cell Death and Disease, 2014, 5(12):e1571.
(2015-11-17收稿)
(2016-01-06修回)
(编辑:杨红欣)

杨康专业方向为骨肿瘤的临床诊治与基础研究。
E-mail:yzykchina@163.com
·临床研究与应用·
作者简介
通信作者:汤小东tang15877@126.com
doi:10.3969/j.issn.1000-8179.2016.02.293
作者单位:北京大学人民医院骨肿瘤科(北京市100044)
