金属/AP颗粒混合物燃烧特性的光谱分析
2016-03-29胡春波杨建刚朱小飞蔡玉鹏
邓 哲,胡春波,杨建刚,朱小飞,蔡玉鹏
(西北工业大学燃烧、流动和热结构国家级重点实验室,陕西西安710072)
金属/AP颗粒混合物燃烧特性的光谱分析
邓哲,胡春波,杨建刚,朱小飞,蔡玉鹏
(西北工业大学燃烧、流动和热结构国家级重点实验室,陕西西安710072)
摘要:为了使Al/AP双组元粉末火箭发动机密度比冲最大化,将燃烧室特征长度由2.31m增至12.62m进行了Al/AP粉末火箭发动机点火测试。采用光谱仪、CCD相机、CO2激光点火器等对Al/AP混合物在1.01325×105Pa的氮气环境中的点火延迟、燃烧时间、燃烧平稳性等燃烧性能进行了研究。测量了Al颗粒的表观堆积密度。作为一种替代燃料,对镁颗粒也进行了研究。结果表明,增加燃烧室特征长度至12.62m时,可以得到最大燃烧室压强振荡幅度±2.43%的平稳燃烧性能。含粒径1μm铝粉的Al/AP混合物其燃烧过程的光强远大于含粒径10μm铝粉的样品,并且其在波长568nm发射光谱的光子数强度超过了光谱仪检测上限(65000数)。而含粒径10μm铝粉样品燃烧过程的568nm发射光谱信号出现间断且其全程强度低于19036数。粒径10μm铝粉点火延迟时间为粒径1μm铝粉点火延迟时间的3.65倍,燃烧时间为3.03倍以上,最大RAlO却比1μm铝粉少14.3%,密度低21.3%,说明粒度小的铝粉具有更好的燃烧性能,但是其堆积密度也更低。虽然Mg/AP的理论比冲为Al/AP的95.6%,但是其堆积密度比粒径1μm铝粉高8%,其点火延迟时间比粒径10μm铝粉短90.3%。火焰照片也表明镁粉可在很大程度上减少凝相沉积。
关键词:分析化学;发射光谱;燃烧特性;激光点火;金属颗粒
Received date:2015-08-21;Revised date:2015-10-12
Foundation:National Natural Science Foundation of China (51266013); Foundation of Northwestern Polytechnical University for Basic Research (JC20110205).
Biography:DENG Zhe(1987-), male, doctoral candidate. Research field: Aerospace propultion. Email: mail_express@163.com
Introduction
The Al/AP bipropellant powder rocket engine[1]employs micron-sized particles fluidized by a small quantity of gas (N2usually). And a dense gas-solid two phase flow results powder propellant feed system characteristics similar to liquid propellant system. The powder propellant offers a combination of no leakage, pre-packaged, storability and flexibility which are not completely achieved with liquid, solid and hybrid rockets. Powders are easily throttled and restarted[2]. There are different powder propellant formulations developing for different aims like weapon power system[1], Mars exploration[3], scramjet[4]and water ramjet[5].
Bell Aerospace Company[1]has conducted Al/AP powder rocket engine research with a combustion chamber with characteristic length (L*) of 2.31m in technologies as fluidization, packing and combustion. They transported particles fluidized by N2. A specific impulse performance efficiency maximum of 87% was obtained. Low frequency combustion pressure oscillations with amplitudes of ±88% of pressure of combustion chamber(Pc) , were encountered. Previous work mentioned above demonstrates that combustion of Al/AP powder propellant requires more research to improve combustion efficiency and smoothness.
Metal Particle size gives a contrary effect on combustion efficiency and packing density. Larger metal particle size increases packing density of particles in propellant tank, that results a higher density specific impulse, while lessen burning rate of particles, resulting a larger volume of combustion chamber. In this study, effect of particle size of Al on Al/AP mixture combustion was researched by using CO2laser igniter. A spectrometer and a CCD camera were also used to record emission signals and images of combustion behaviors of metal/AP mixtures in a constant volume explosion (CVE). Combustion and packing performances were analyzed. Results could be referenced to determine powder fuel.
1Experiments
1.1Equipments and samples
Ignition and combustion characteristics of Al/AP mixture are critical on combustor design of Al/AP bipropellants powder rocket engine. In our present work, the interior ballistic curve (Fig.1) indicates that increasingL*from Bell Company′s 2.31m[1]to 12.62m could improve the smoothness ofPcoscillation amplitude from ±88% to ±2.43%. However, combustion chamber would not be even big with more passive quality and volume. Decreasing particle size or using other energetic material instead maybe the feasible optimization method to reduce the engine size. Usually, the evaporation time of Al particles are much longer than the pyrolysis time of AP particles. So the particle size of Al dominates the combustion process.

Fig.1 Interior ballistic curve of Al/AP bipropellants withcharacteristic length of 12.62m
Al and AP particles are fluidized from different storage tanks and injected into combustion chamber through a mixing unit. Then, they are ignited instantly when flow pass flame front with a very high temperature of nearly 3000K. Ignition of metal particles is initialed with a fast heating rate. However, thermo analysis methods like TG and DSC with a slow heating rate could not reveal ignition delay, burning time and other combustion mechanism of Al/AP mixtures well. So we must attempt other experimental method instead.
Equipments combined of spectrometer, CCD camera and CO2laser igniter could satisfy the research requirement. A schematic diagram of the equipments used here[6]is Fig.2, with a CO2laser igniter which can provide a fast heating rate, including an Avaspec-2048 spectrometer that is the topic of the present paper. The spectrometer plugs directly into the computer via a USB port for data acquisition and was controlled by a NI 6008 board which also triggers CO2laser at the same time. Emitted light from the burning sample passes through the sidewall quartz window port and into the spectrometer via a fiber optic cable. The spectrometer with an upper limitation of detect of 65000 photon counts, allowed for accurate data collection at 1.05ms intervals and at 0.6nm wavelength resolution. A SONY CCD camera was used to record combustion and flame images. The fiber optic cable of the spectrometer was positioned horizontally at the sidewall, in-line with the sample. The CO2laser, which provided vertical heating beams in power density of 480-510W/cm2on samples, worked as an igniter for 500ms. The same initial mass loading of 20mg was used and conducted in 1.01325×105Pa nitrogen environment for all samples.

Fig.2 Schematic diagram of the equipments
1.2Preparation of Metal/AP mixtures
The formulations and properties of metal/AP mixtures are shown in Table 1. Either 10μm Mg or 1μm Al particles has a lower packing density than 10μm Al. Samples which were used in experiments and put compactly in alumina crucible, were composed of metal particles (Shanghai St-nano Science & Technology Co.,LTD) and AP particles (Liming Research Institute of Chemical Industry), uniformly mixed in mass ratio of 1∶3, milling for 5min. Enlarged views of metal particles were obtained by using scanning electron microscopy (SEM) in Fig.3. 10μm Mg particles show a higher spherical morphology than both 10μm and 1μm Al particles while show a greater size distribution difference.

Table 1 Formulations and properties of metal/AP mixtures

Fig.3 SEM images of metal particles
Note:Isp, specific impulse;Δ,packing density.1.3Analysis method
The appearance time and duration of emission signals at 568nm[7]and 500nm[8], which indicate heat release from all exothermic reaction, were used to determine ignition delays and burn times of Al and Mg particles, respectively. The burning rate of aluminum particle in form of vapor-phase reaction is extremely faster than that in form of surface reaction. So whether aluminum particles of different samples react with oxidizer in form of vapor phase is significant to study. The issue is how to contrast the combustion performance between two samples. Though the absolute value of emission signal indicates intensity of combustion, but it is determined with many factors like position of fiber optics probe, mass loading and ignition delay and so on, making it unstable. The relative value as a ration of two signals in different wavelength but same test is much more precise. Emission signals at 568nm indicate heat release from all exothermic reaction. The 486nm wavelength emission signals, only emitted as a result of AlO generation, were selected to track one of the strongest AlO emission bands as an indicator of the intensity of the vapor-phase reactions[9], according to the famous 9 steps reaction below. The strength of vapor-phase reactions was evaluated using the ratio of the signal intensities measured at 486nm and 568nm[7]:
RAlO=I486/I568
Surface reaction:
Al(l)→Al(g)
Al(l)+AlO(g)→Al2O(g)
Gas phase reaction:
Al(g)+O2→AlO+O
AlO+O2→AlO2+O
O+O→O2
Dissociation reaction:

Condensation:

Al2O+O2→Al2O3(l)
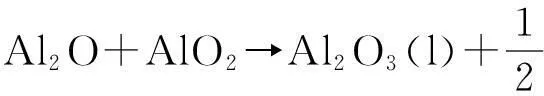
2Results and Discussions
2.1Combustion performance of the mixtures

Fig.4 Typical flame images of the samples in combustion
Because of the strong overexposure caused by metal combustion, each well-discerned image was shot clearly only by using 5% neutral density filter. Contrasting combustions between 10μmAl/AP and 1μmAl/AP in Fig 4, luminous streaks (condensed phase) occurred, and 1μmAl/AP, due to their larger specific surface areas than 10μm Al particles, gave out denser and brighter streaks, indicating a higher flame temperature and faster energy release. There were no streaks appeared in 10μm Mg particles tests, indicating that Mg particles burned fast and may lessen condensed phase deposition in the combustion chamber of powder rocket engine. It was well deduced that 10μmAl/AP has the slowest heat release rate for the lowest flame luminosity.Fig.5 is the typical spectra of the samples. In general, the features seen in the spectra in Fig.5 are representative of what was seen for all experiments. The most prominent features of the spectrum are the peaks at 387, 471, 486, 500, 518, 589, 769nm. Some of these peaks are attributed to well-known atomic lines. The lines near 589nm and 769nm, those appear in both 1μmAl/AP and 10μmMg/AP tests correspond to the famous sodium(589.0 and 589.6nm) and potassium(766.5 and 769.9nm) lines[6], indicating impurities existence. They appeared in the spectra herein as a single line due to 0.6nm resolution of the spectrometer. The 471 and 486 nm[10]peaks which appeared in 1μm Al particles tests correspond to the intense generation of AlO. The 500nm and 518nm peaks represent production of MgO and magnesium steam[8], respectively. A broadband at 378nm peak is most likely due to emission of MgOH(372~385nm).
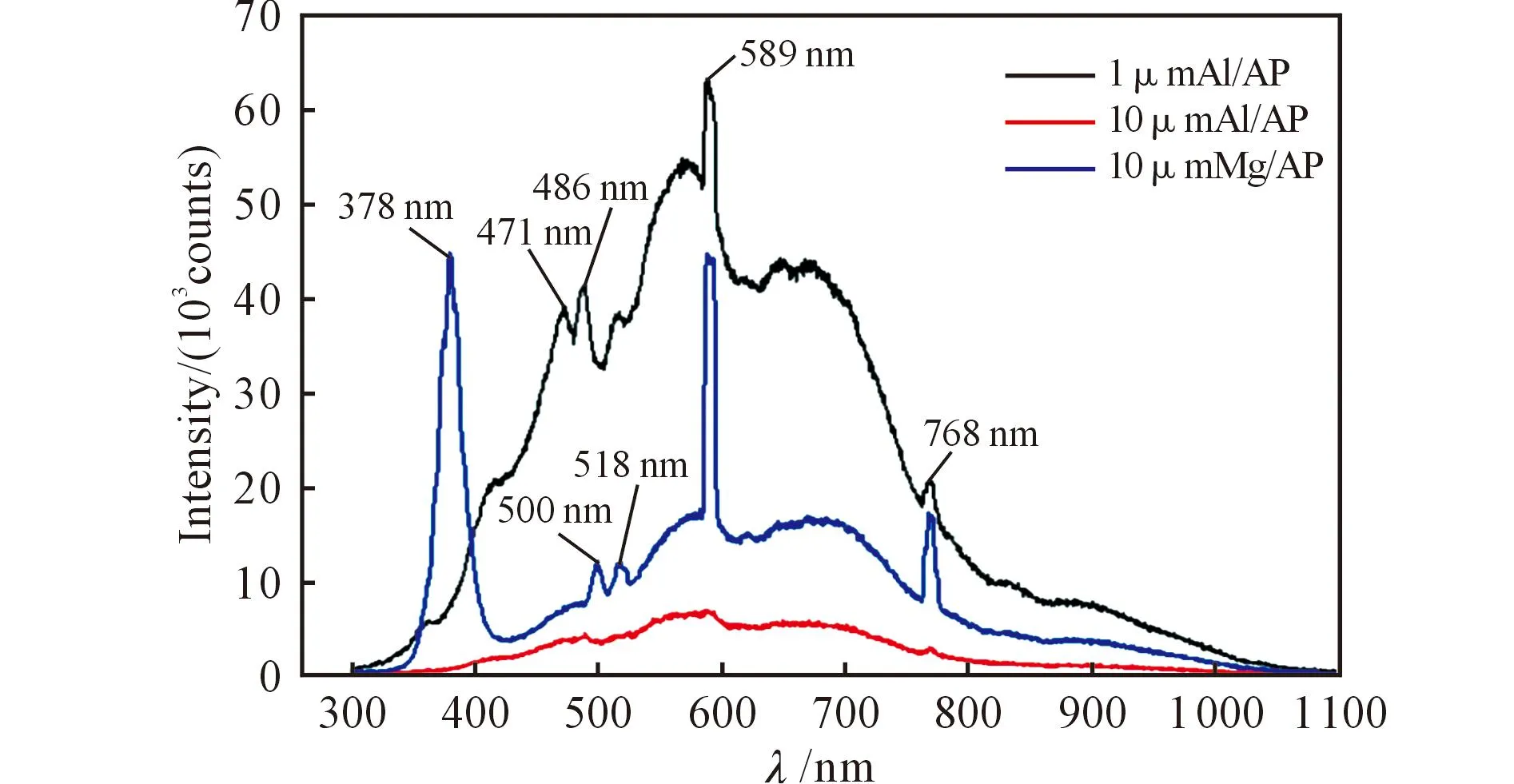
Fig.5 Typical emission spectra of metal/AP mixturesin discernible time
Typical emission traces measured at 568nm and 500nm are shown in Fig.6 and the combustion performance parameters were shown in Table 2.

Fig.6 Emission signals of the metal/AP mixtures at differentwavelength variety with time
It can be easily seen from Table 2 that 10μmAl/AP have the longest ignition delay of 65.10ms, and the burn time is 430ms but not limited, because surplus sample left after testing for CO2laser ignition stopping at 500ms of horizontal axis. Entire emission signals recorded at 568nm of 10μmAl/AP combustion present an intermittent trend and lower level emission below 19036 counts (Fig.6) than 1μmAl/AP of above 65000 counts in Table 3, indicating a lower burning rate. The possible mechanism of 10μmAl/AP combustion is that heat released from prior ignited particles which positioned on the upper of the crucible cannot support a sustained ignition to lower Al particles rapidly. When the products of burned particles diffusing away as a gas phase, lower particles exposure to laser beam, which is the main driving force for sustained combustion, and repeat the ignition process. The intervals with duration from 20 to 60ms alternately appeared among the whole combustion process. The combustion oscillations in Ref.1 are most likely due to lower burning rate of larger sized particles.

Table 2 Experiment results of combustion performance
Note:τ, ignition delay time;t,burn time;Nmax, maximum intensity;tr, interval range; RAlo, represents the strength of vapor-phase reaction on Al particles.
2.2Ignition process of the mixtures
Comparing with 10μmAl/AP, the shorter ignition delay and burn time (Table 2) on 1μmAl/AP indicates that small particles benefit the reduction of combustion chamber volume for Al/AP powder rocket engine. Higher specific surface area of 1μmAl/AP particles makes their activation energy lower. No intervals appearance in 1μmAl/AP (Fig. 6(b)) transferred the main driving force from laser igniter to rapid heat release of combustion products for sustained combustion. The only two disadvantages of small sized Al particles are low packing density and low activated aluminium content, because of relative thicker oxide layer in a individual particle. 10μmMg/AP has excellent performance (Table 3) both at combustion and packing density, except for low theoretical specific impulse. They should be an ideal fuel for small scaled engine with both low propellant charge and small combustion chamber. Combustion behaviors of 1μmAl/AP and 10μmMg/AP with short burn times and no intervals could stabilize flame front and smooth interior ballistic for the powder rocket engine.
RAlOratio on 1μmAl/AP was not useful after 26.25ms, because emission signals, both at 568nm and 486nm, exceeded 65000 photon counts, which is the upper detection limitation of the spectrometer. ButRAlOratio of 1μmAl/AP and 10μmAl/AP in ignition stage could be used to make a comparison. Higher-resolution views of ignition features for burning Al particles are shown in Fig.7, including typical emission traces measured at 486nm, 568nm and ratioRAlOcharacterizing intensity of AlO emission. When a strong AlO line is observed at 486nm, theRAlOratio becomes greater, indicating the vapor-phase combustion.RAlOmeasurements are no longer meaningful before ignition because of drastic oscillation caused by nearly zero emission signals. The measuredRAlOratios are both observed to begin increasing until particles ignited, indicating a transformation from surface combustion to vapor-phase combustion. The burning rate of vapor-phase combustion dominated by Al steam diffusion rate is very faster than that of surface combustion dominated by chemistry kinetics. So the maximumRAlOratio of 0.77 of 1μmAl/AP observed in Table 3 and Fig.7 indicated the faster burning rate than that of below 0.66 of 10μmAl/AP.

Fig.7 Detailed view of ignition features for burning Al particles
3Conclusions
(1)Maximum amplitude of ±2.43% atPcoscillation was obtained from the present fire tests on Al/AP bipropellant powder rocket engine by using a combustion chamber with 12.62m characteristic length. The result demonstrates that increasing chamber characteristic length promotes stable combustion.
(2)Though 1μmAl/AP has 27% lower packing density than 10μmAl/AP, its combustion performance is more excellent. 1μmAl/AP has less ignition delay and burn time than 10μmAl/AP. HigherRAlOratio of 1μmAl/AP indicates that small sized Al particle burns with higher proportion of vapor-phase reaction and burns much faster. Larger particle size may worsen combustion performance of burning rate and smoothness of chamber pressure.
(3)Contrasting with Al particles combustion, no streaks were observed in 10μmMg/AP combustion. Mg particles
could lessen condensed phase in the combustion chamber. Packing density and combustion performance of 10μmMg/AP are better than 1μmAl/AP and 10μmAl/AP, respectively. Mg powder is a worth considering fuel in small thruster.
Reference:
[1]Loftus H J, Montanino L N. Powder rocket feasibility evaluation, AIAA-1972-1162[R]. New York: AIAA, 1972.
[2]Goroshin S, Higgihs A J. Powdered magnesium-carbon dioxide propulsion concepts for Mars missions AIAA-1999-2408[C]∥35th AIAA/ASME/SAE/ASEE Joint Prop Conference and Exhibitlsion. New York: AIAA, 1999.
[3]Shafirovich E, Varma A. Metal-CO2Propulsion for Mars missions: current status and opportunities[J]. Journal of Propulsion and Power, 2008, 24(3): 385-394.
[4]Goroshin S, Higgins A J, Kamel M. Powdered metals as fuel for hypersonic ramjets, AIAA-2001-3919[R]. New York: AIAA, 2001.
[5]Waters D F, Cadou C P. Quantifying unmanned undersea vehicle range improvement enabled by aluminum-water power system[J].Journal of Propulsion and Power, 2013, 29(3): 675-685.
[6]Petersen E, Arvanetes J. Monitoring strand burner combustion products using emission spectroscopy, AIAA-2007-5767[R]. New York: AIAA, 2007.
[7]Yasmine A, Hoffman V K,Schoenitz M. Reactive, Mechanically alloyed Al·Mg powders with customized particle sizes and compositions[J]. Journal of Propulsion and Power, 2014, 30(1): 96-104.
[8]Brzustowski T A. Vapor-phase diffusion flames in the combustion of magnesium and aluminum: II. Experimental observations in oxygen atmospheres, AIAA-1963-490[R]. New York: AIAA, 1963.
[9]Liang Yongjun, Beckstead W M. Numerical simulation of unsteady, single Aluminum particle combustion in air, AIAA-1998-3825[R]. New York: AIAA, 1998.
[10]Bazyn T, Krier H. Shock tube measurements of combustion of nano aluminum, AIAA-2006-1157[R]. New York: AIAA, 2006.
