Protective role of methylene blue in kidney and jejunum injuries of rats by paraquat
2016-02-14CHENWeiCHENJunliangCHUJianjunSUNJieyunFANHongbinZHANGPengDUBinHUMingzhuPANGQingfeng
CHEN Wei,CHEN Jun-liang,CHU Jian-jun,SUN Jie-yun,FAN Hong-bin,ZHANG Peng,DU Bin,HU Ming-zhu,PANG Qing-feng
(Jiangnan University,1.Emergency Department,Affiliated Wuxi Third People′s Hospital,Wuxi 214041,China;2.Wuxi Medical College,Wuxi 214122,China)
·ORIGINAL ARTICLES·
Protective role of methylene blue in kidney and jejunum injuries of rats by paraquat
CHEN Wei1,CHEN Jun-liang2,CHU Jian-jun2,SUN Jie-yun2,FAN Hong-bin2,ZHANG Peng2,DU Bin2,HU Ming-zhu2,PANG Qing-feng2
(Jiangnan University,1.Emergency Department,Affiliated Wuxi Third People′s Hospital,Wuxi 214041,China;2.Wuxi Medical College,Wuxi 214122,China)
OBJECTIVE To investigate the antidotal ability and mechanisms of methylene blue(MB)against paraquat(PQ)-poisoned kidney and jejunum injuries in rats.METHODS Male Sprague-Dawley rats were randomly divided into 6 groups(n=6):normal control group(0.2 mL 0.9%saline solution,ip),MB group(MB 2 mg∙kg-1,ip),PQ group(PQ 35 mg∙kg-1,ip),PQ+MB ig therapy group(PQ 35 mg∙kg-1,ip;MB 2 mg∙kg-1,ig),PQ+MB ip therapy group(PQ 35 mg∙kg-1,ip,and MB 2 mg∙kg-1,ip),and PQ+MB 2 h ip therapy group(PQ 35 mg∙kg-1,ip;2 h later MB 2 mg∙kg-1,ip).48 h later,the content of blood urea nitrogen(BUN)and creatinine(Cr),the activity of superoxide dismutase(SOD)and myeloperoxidase(MPO),the content of adenosine triphosphate(ATP)and malonaldehyde(MDA)in the kidney and jejunum tissues were determined.The pathological changes were detected by HE staining,apoptosis by terminal deoxyuridine triphosphate deoxynucleoitidyl transferase mediated nick end labeling(TUNEL)assay,and the expression of heme oxygenase-1(HO-1)protein in the kidney by immunohistochemistry.RESULTS Compared with normal control and MB group,the injury scores of kidney and jejunum in PQ group were increased(P<0.05),the content of BUN and Cr was increased(P<0.05),the activity of SOD and the content of ATP were decreased(P<0.05,P<0.01),the activity of MPO and the content of MDA were increased(P<0.05,P<0.01),and the apoptosis rate of the kidney and jejunum was increased(P<0.01).Compared with PQ group,the injury scores of the kidney and jejunum in all three MB treatment groups were decreased(P<0.05),the content of BUN and Cr was decreased(P<0.05),the activity of SOD and the content of ATP were increased(P<0.05,P<0.01),the activity of MPO and the content of MDA were decreased(P<0.05),the apoptosis rate of the kidney and jejunum was decreased(P<0.01),and the immunhistochemical score of HO-1 in kidney tissue was increased(P<0.05).CONCLUSION MB can alleviate PQ-poisoned kidney and jejunum injuries,which may be related to up-regulated expressions of HO-1.
paraquat;methylene blue;jejunum;kidney;antidote
Paraquat(PQ) poisonings are frequently fatal due to their severe acute toxicity and lack of antidotes.Afteringesting high dosesofPQ(>30 mg∙kg-1in humans),patients often die within 1 week due to multiple organ failure[1-2].Early detoxification methods,such as gastric lavage,acti⁃vated carbon adsorption,catharsis,hemoperfusion,corticosteroids and cyclophosphamide,have been employed in patients with acute PQ poisoning.How⁃ever,mortality remains considerably high[3].Effective therapies for PQ intoxication are urgently needed.
PQ induces its toxic effects mainly via oxidative stress-induced mechanisms.Therefore,researchers and clinicians have placed great emphasis on the use of antioxidants as a treatment modality for PQ toxicity.However,the results turn out to be quite disappointing.For example, the protective effects of superoxide dismutase and catalase are hindered due to their poor bioavail⁃ability,low stability,or rapid hydrolysis in the blood[4].Other antioxidants,such as α-tocopherol,provide limited protection because of their extreme insolubility[5].Thus,despite considerable effort,a suitable and effective antioxidant for PQ intoxi⁃cation remains to be developed.
Methylene blue(MB),as a new antioxidant agent,could alleviate the experimentally-induced hepatic injury in rats and the ischemia-reperfu⁃sion injury in lung transplantation[6-7].More⁃over,we found that MB could attenuate the pul⁃monary and hepatic injury induced by PQ[8-9]. However,it is still unknown whether MB attenuates PQ-induced jejunum and kidney injury in rats.In the present study,we further investigated the antidotal ability and mechanisms of MB against PQ-induced toxicity in rats.
1 MATERIALS AND METHODS
1.1 Drug,reagents and instruments
PQ(1,1-Dimethyl-4,4′-bipyridiniumdichloride) was obtained from Shanghai Pesticide Research Institute CO.,Ltd.(Shanghai,China). MB was obtained from Jumpcan Pharmaceutical CO.,Ltd.(Taizhou,China).Adenosine triphos⁃phate(ATP),superoxide dismutase(SOD),malondialdehyde(MDA)and myeloperoxidase(MPO) assaykitswereallobtainedfrom Jiancheng Bioengineering Institute CO.,Ltd.(Nanjing,China).Heme oxygenase-1(HO-1)polyclonal antibody(No.SC-12310,Santa Cruz Biotechnology,Canada).TUNEL detection kit was obtained from KeyGen Biotechnology Co.,Ltd(Nanjing,China).All other reagents were of analytical grade and commercially available.7170A automatic biochemical analyzer(Hitachi,Japan),IX51 fluorescence microscope(Olympus,Japan).
1.2 Animals and treatment
Male Sprague-Dawley(SD)rats,200-230 g,were supplied by the Animal Experimental Center of Jiangnan University.SCXK(Su)2014-0001.The SD rats were housed in a room with a 12 h light/ dark cycle.Rats were givenad libitumaccess to food and water.All animal experiment proce⁃dures were approved by and performed in accor⁃dance with the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee.
After a week of acclimation,the rats were randomly divided to six groups(n=6):① normal control group that was administered with a single ip of 0.2 mL 0.9%saline solution;② MB group that was administered with a single ip of MB 2 mg∙kg-1;③ PQ group that was administered with a single ip of PQ 35 mg∙kg-1[10];④PQ+MB ig group that was administered with MB 2 mg∙kg-1by ig immediately after PQ administration[11];⑤PQ+MB ip group that was ip administered with MB 2 mg∙kg-1immediately after PQ administration;⑥PQ+MB 2 h ip group that was ip administered with MB 2 mg∙kg-12 h after PQ administration.In the preliminary experiments,PQ+MB ig 2 h group (rats were ig administered with MB 2 mg∙kg-12 h after PQ administration)had no protective effect(data not shown).
1.3 Sample collection
Forty-eight hours after PQ administration,the rats were anesthetized with 10%chloral hydrate. The leftkidney was scissored and freezed inliquid nitrogen and stored in a-80℃ refrigerator for biochemical analysis while the right kidney was washed by PBS damping fluid,fixed in 4%parafor⁃maldehyde at 4℃ for 48 h and processed for his⁃tological and immunohistochemical analysis.The jejunum samples from each rat were divided into two parts,one for histological analysis and the other for biochemical analysis.
1.4 Hematoxylin and eosin(HE)staining for tissure injury detection
Paraffin sections(5 μm thick)were prepared using to routine methods and stained with HE methods.Tissues were examined under light microscopy by a blinded observer and scored using a Chiu system for jejunum tissue[12]and an arbitrary scale for kidney injury[13].
1.5 Biochemical analysis of serum and tissue
The blood samples were collected into tubes without anticoagulant,and serum was separated by centrifugation at 3200×gat 4℃for 5 min and stored in a freezer at-20℃until analysis.Blood urea nitrogen(BUN)and creatinine(Cr)were analyzed by an autoanalyzer.
The activity of MPO and SOD,and the con⁃tent of MDA and ATP in the kidney and jejunum tis⁃sue were determined using commercially prepared kits following the manufacturer′s instructions.
1.6 Apoptosis detection with terminal deoxy⁃uridine triphosphate deoxynucleoitidy transfer⁃ase mediated nick end labeling(TUNEL)method
Apoptosis in the jejunum and kidney tissues was determined by TUNEL method using anin situcell detection kit.The fluorescence microscope was used to observe positive cells.The cells with green fluorescent particles orfragmentswere deemed to apoptosis cells.Five microscope fields of each section were picked to calculate the apoptosis rate(AR)using the formula:AR(%)=account of positive cells/account of total cells×100%.
1.7 Immunohistochemical analysis of kidney and jejunum tissue
The kidney and jejunum tissue sections were immunostained with rabbit anti-rat HO-1 anti⁃body and goat anti-rabbit tissue second antibody(1∶200 in dilution)at 4℃overnight.The immuno⁃ complexes were visualized and scored using 3, 3′-diaminobenzidine.
1.8 Statistical analysis
Statisticalanalysiswasperformed using SPSS16.0 statistical software for windows.All values were expressed asx±s.Significant differences were determined using one-way analysis of vari⁃ance(ANOVA)by Student-Newman-Keels test.P<0.05 was considered statistically significant.
2 RESULTS
2.1 Effect of MB on histopathology and injury scores of kidney and jejunum tissue in PQ-poisoned rats

Fig.1 Effect of methylene blue(MB)on histopatho⁃logical changes in kidney tissue of rats exposed to paraquat(PQ)by HE staining(A)and injury scores (B).Male SD rats were injurid by PQ 35 mg∙kg-1and treated with or without MB 2 mg∙kg-1.48 h later,specimens of the right kidney were collected for pathological examination and injury scores.White arrows for the swollen glomerulus,and blank arrows for the edematous renal tubules.x±s,n=6.*P<0.05,compared with normal control group;#P<0.05,compared with PQ group.
Histopathological results are shown in Fig.1.After PQ treatment for 48 h,compared with normal and MB groups,kidney tissue in the PQ group exerted marked structuralalterations. For example,glomerular capillaries became ecchy⁃motic,tubularepithelialcells ofthe kidney swelled and the kidney injury score was increased(P<0.05).Compared with PQ group,alterations of kidney tissue in all the three MB treatment groups were drastically attenuated and injury scores were decreased(P<0.05).
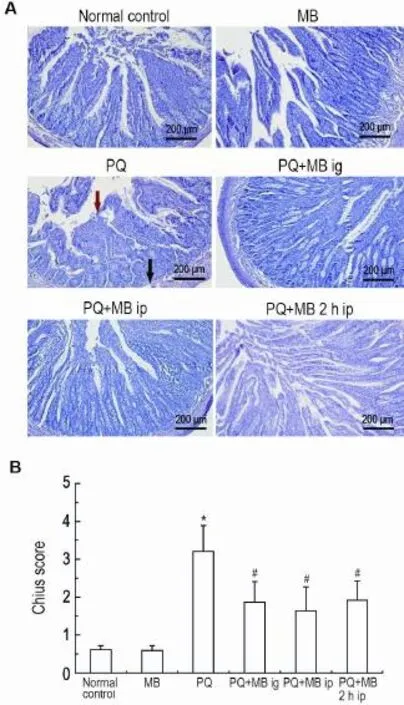
Fig.2 Effect of MB on histopathological changes in jejunum tissue of rats exposed to PQ by HE staining(A) and injury scores(B).See Fig.1 for the rat treatment.The red arrows show the lamina propria and submucosa mild separa⁃tion and the blank ones show the abruption of villus.x±s,n=6. *P<0.05,compared with normal control group;#P<0.05,com⁃pared with PQ group.
As shown in Fig.2,compared with the normal control and MB groups,jejunum injuries in PQ group were characterized by hemorrhage of mucosa,abruption of villus,gland damage,inflam⁃matory cell infiltration,lamina propria and submu⁃ cosa mild separation.Injury scores in PQ group was increased(P<0.05).Compared with PQ group,MB administration could alleviate such histological damage in each MB treatment group,and injury scores in these groups were decreased(P<0.05).
2.2 Effect of MB on content of BUN and Cr in serum of PQ-poisoned rats
As shown in Tab.1,compared with normal control and MB groups,contents of BUN and Cr in PQ group were increased(P<0.05).Compared with PQ group,MB treatment reduced the level of BUN and Cr in all the three MB treatment groups(P<0.05).

Tab.1Effect of MB on blood urea nitrogen(BUN)and creatinine(Cr)in serum of rats exporsed to PQ
2.3 Effect of MB on oxidative stress indexes in jejunum tissue of PQ-poisoned rats
As shown in Tab.2,compared with normal control and MB groups,the activity of MPO and the content of MDA in the kidney and jejunum tissue were increased while the activity of SOD and content of ATP were decreased in PQ group(P<0.05,P<0.01).Compared with the PQ group,the activity of MPO and the content of MDA were decreased while the activity of SOD and the content of ATP were increased in all the three MB treatment groups(P<0.05,P<0.01).
2.4 Effect of MB on apoptosis in kidney and jejunum tissue of PQ-poisoned rats
As shown in Tab.3,compared with normal con⁃trol and MB groups,the apoptosis rate of kidney and jejunum tissue in PQ group was increased(P<0.05). Compared with PQ group,MB treatment reduced the apoptosis rate of kidney and jejunum tissue in all the three MB treatment groups(P<0.01).

Tab.2 Effect of MB on biochemical indexes in kidney and jejunum tissue of rats exposed to PQ
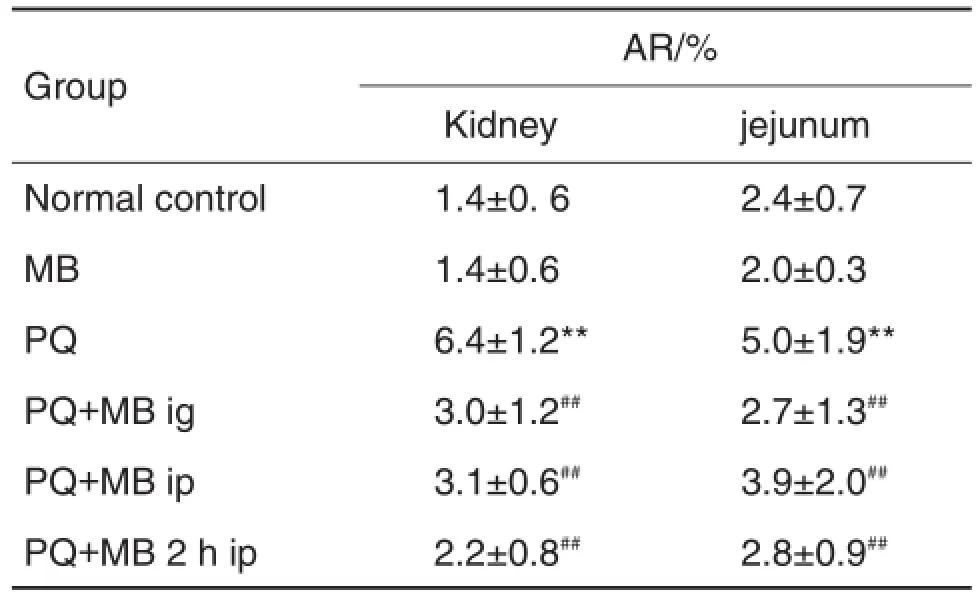
Tab.3 Effect of MB on apoptosis rate(AR)in kidney and jejunum tissue of rats exposed to PQ detected by terminal deoxyaridine triphosphate deoxynucleoitidyl transferasemediatednickendlabeling(TUNEL)method
2.5 Effect of MB on expression of HO-1 in kidney tissue of PQ-poisoned rats
Immunohistochemical analysis results are showninFig.3.InnormalcontrolandMB groups,HO-1 expression was not detectable. Compared with PQ group,MB treatment enhanced the expression ofHO-1 and increased the immunoreactive score in all the three MB treat⁃ment groups(P<0.05).
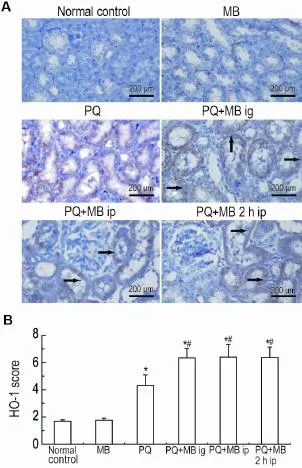
Fig.3 Immunohistochemical images(A)and immunereactive score(B)for heme oxygenase-1(HO-1)in kidney tissue of rats exposed to PQ.See Fig.1 for the rat treatment.The arrows for the positive cells.x±s,n=6.*P< 0.05,compared with normal group;#P<0.05,compared with PQ group.
3 DISCUSSION
This study has provided evidence that MB might be an effective antidote for PQ poisoning based on the following results:①MB could alle⁃viate the structural lesion and oxidative response induced by PQ challenge;② MB inhibited the PQ-induced apoptosis in the jejunum and kidney. Importantly,MB could proudce protective effect at different administration time(immediately and 2 h after PQ intoxication)and by various means of administration(ig and ip).These results sug⁃gest that MB could decrease the PQ toxicoity and act as a drug for pre-hospital emergence and hospital emergence treatment.
This is the first time that we have found that MB could alleviate the jejunum and kidney injuries induced by PQ.Multiple organ failure is a main killer for severe PQ-poisoned patients[14].There are few studies that investigate the toxical effect of PQ on gastro-intestine.In fact,gastro-jejunum injury is very universal in those ingested PQ-poi⁃soned patients.The postmortem of non-survivors display manyseriouspathologicalchanges,including mucosal lesions in the pharynx,esoph⁃agus and stomach,and intestine perforation[15]. The contribution of this gastrointestinal injury to mortality is probably under-estimated.In this study,we found that PQ led to significant jejunum injury,characterized by hemorrhage of mucosa,abruption of villus,gland damage,inflammatory cell infiltration,lamina propria and submucosa mild separation.These serious injuries in the gastro-intestine may have diminished the absorption function so that MB,ig administered 2 h after PQ intoxication,had no protective effect.We found that MB could alleviate the gastrointestinal lesion induced by PQ.Combined with our previous results[8-9],It may be concluded that MB could protect against the multiple organ failure induced by PQ.
PQ-induced toxicity is mediated through redox-cycling and subsequent generation of reac⁃tive oxygen species(ROS)[16-17].Subsequently,ROS-induced apoptosis also contributes to PQ-induced multiple organ injury.In view of our recent studies,the protective function of MB could be explained byseveralmechanisms involving the scavenging of ROS,inhibition of MPO activity,attenuation of the increase in cata⁃lase and glutathione peroxidase activities,downregulation of the apoptotic pathways.MB func⁃tions as an alternative electron carrier which effi⁃ciently shuttles electrons between the reduced form of nicotinamide-adenine dinucleotid and cytochrome c.As a result,this process reroutes electron transfer from complexⅠtoⅢ,reduces electron leakage,and attenuates ROS produc⁃tion.Furthermore,we observed that MB could directly induce HO-protein expression.Recent studies have also shown that the inducing expres⁃sion of HO-1 could alleviate the PQ toxicity[18]. HO-1 has anti-oxidative,anti-inflammatory and anti-apoptotic functions which prevent liver injury under different conditions,such as ischemicreperfusion injury,and diabetes[19-21].Our findings indicate that MB may prevent PQ-induced kidney injury by up-regulating HO-1 expression.
In this study,we found that both ig and ip have similar results on PQ-poisoned rats in the immediate administration of poisoning,which indicates that MB is an easy and practical anti⁃dote for PQ intoxication.We will go on to investi⁃gate the beneficial effect of MB on the survival rate in PQ-poisoned rats.
In conclusion,MB can decrease PQ toxicity through its anti-oxidative and anti-apoptosis property,which may be an effective antidote for PQ poisonings.
REFERENCCES:
[1]Wang WH,Zhang H,Yu YL,Jiang JH.Correlation of plasma endothelin and multiple organ dysfunction syndrome caused by acute paraquat poisoning[J].Chin Crit Care Med(中国危重病急救医学),2005,17(5):293-295.
[2]Weng CH,Hu CC,Lin JL,Lin-Tan DT,Huang WH,Hsu CW,et al.Sequential organ failure assess⁃ment score can predict mortality in patients with paraquatintoxication[J].PLoSOne, 2012,7(12):e51743.
[3]Gawarammana IB,Buckley NA.Medical management of paraquat ingestion[J].Br J Clin Pharmacol,2011,72(5):745-757.
[4]Samai M,Hague T,Naughton DP,Gard PR,Chatterjee PK.Reduction of paraquat-induced renal cytotoxicity by Manganese and Copper complexes of EGTA and EHPG[J].Free Radic Biol Med,2008,44(4):711-721.
[5]Zingg JM,Azzi A.Non-antioxidant activities of vitamin E[J].Curr Med Chem,2004,11(9):1113-1133.
[6]Bozkurt B,Dumlu EG,Tokac M,Ozkardes AB,Ergin M,Orhun S,et al.Methylene blue as an antioxidant agent in experimentally-induced injury in rat liver[J].Bratisl Lek Listy,2015,116(3):157-161.
[7]Abreu Mda M,Pazetti R,Almeida FM,Correia AT,Parra ER,Silva LP,et al.Methylene blue attenu⁃ates ischemia-reperfusion injury in lung transplan⁃tation[J].JSurg Res,2014,192(2):635-641.
[8]Chen JL,Dai L,Zhang P,Chen W,Cai GS,Qi XW,et al.Methylene blue attenuates acute liver injury induced by paraquat in rats[J].Int Immuno⁃pharmacol,2015,28(1):808-812.
[9]Tian ZG,Ji Y,Yan WJ,Xu CY,Kong QY,Han F,et al.Methylene blue protects against paraquatinduced acute lung injury in rats[J].Int Immuno⁃pharmacol,2013,17(2):309-313.
[10]Choi JS,Jou SS,Oh MH,Kim YH,Park MJ,Gil HW,et al.The dose of cyclophosphamide for treating paraquat-induced rat lung injury[J].Korean J Intern Med,2013,28(4):420-427.
[11]Aksu B,Umit H,Kanter M,Guzel A,Aktas C,Civelek S,et al.Effects of methylene blue in reducing cholestatic oxidative stress and hepatic damage after bile-duct ligation in rats[J].Acta His⁃tochem,2010,112(3):259-269.
[12]Chiu CJ,Scott HJ,Gurd FN.Intestinal mucosal lesion in low-flow states.Ⅱ.The protective effect of intraluminal glucose as energy substrate[J].Arch Surg,1970,101(4):484-488.
[13]Rifaioglu MM,SefilF,Gokce H,Nacar A,Dorum BA,Davarci M.Protective effects of caffeic acid phenethyl ester on the dose-dependent acute nephrotoxicity with paraquat in a rat model[J].Environ Toxicol,2015,30(3):375-381.
[14]Shi J,Gao YF,Huang P,Zeng RS.Clinical analysis of multiple organ dysfunction syndrome caused by acute paraquat poisoning[J].Chin J Ind Hyg Occup Dis(中华劳动卫生职业病杂志),2011,29(7):519-521.
[15]Dinis-Oliveira RJ,De Pinho PG,Santos L,Teixeira H,Magalhães T,Santos A,et al.Postmortem analyses unveil the poor efficacy of decontamination,antiinflammatoryandimmunosuppressivetherapies in paraquat human intoxications[J].PLoS One,2009,4(9):e7149.
[16]Drechsel DA,Patel M.Chapter 21 paraquat-induced production of reactive oxygen species in brain mitochondria[J].Methods Enzymol,2009,456:381-393.
[17]Lee HJ,Han J,Jang Y,Kim SJ,Park JH,Seo KS,et al.Docosahexaenoic acid prevents paraquatinduced reactive oxygen species production in dopaminergic neurons via enhancement of glutathione homeostasis[J].Biochem Biophys Res Commun,2015,457(1):95-100.
[18]Zerin T,Kim YS,Hong SY,Song HY.Quercetin reduces oxidative damage induced by paraquat via modulating expression ofantioxidantgenesin A549 cells[J].J Appl Toxicol,2013,33(12):1460-1467.
[19]Rao J,Qian X,Li G,Pan X,Zhang C,Zhang F,et al.ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury[J].Am J Transplant,2015,15(1):76-87.
[20]Xu D,Hu L,Xia X,Song J,Li L,Song E,et al. Tetrachlorobenzoquinone induces acute liver injury,up-regulates HO-1 and NQO1 expression in mice model:the protective role of chlorogenic acid[J].EnvironToxicol Pharmacol,2014,37(3):1212-1220.
[21]Salley TN,Mishra M,TiwariS,JadhavA,Ndisang JF.The heme oxygenase system rescues hepatic deterioration in the condition of obesity comorbid with type-2 diabetes[J].PLoS One,2013,8(11):e79270.
(本文编辑:贺云霞)
亚甲蓝对百草枯中毒大鼠肾和空肠损伤的保护作用
陈 炜1,陈俊良2,楚建军2,孙洁云2,范红斌2,张 鹏2,杜 斌2,胡明珠2,庞庆丰2
(江南大学 1.附属无锡市第三人民医院急救中心,江苏无锡 214041;2.无锡医学院病理生理学教研室,江苏无锡 214122)
目的 观察亚甲蓝(MB)对百草枯(PQ)中毒大鼠肾和空肠损伤的保护作用,并探讨其可能机制。方法 雄性SD大鼠分为6组(n=6):正常对照组(0.2 mL 0.9%生理盐水,ip)、MB组(MB 2 mg∙kg-1,ip)、PQ模型组(PQ 35 mg∙kg-1,ip)、PQ+MB ig治疗组(PQ 35 mg∙kg-1,ip;MB 2 mg∙kg-1,ig)、PQ+MB ip治疗组(PQ 35 mg∙kg-1,ip;MB 2 mg∙kg-1,ip)和PQ+MB 2 h ip治疗组(PQ 35 mg∙kg-1,ip,2 h后+MB 2 mg∙kg-1,ip);48 h后,取肾和空肠组织,进行HE染色及病理损伤评分;检测血尿素氮(BUN)含量、血肌酐(Cr)含量、超氧化物歧化酶(SOD)活性、髓过氧化物酶(MPO)活性、三磷酸腺苷(ATP)含量和丙二醛(MDA)含量;末端脱氧核苷酸转移酶介导dUTP缺口末端标记(TUNEL)法检测凋亡情况;免疫组化法检测肾组织中血红素加氧酶-1(HO-1)蛋白含量。结果 与正常对照组和MB组相比,PQ组大鼠肾和空肠组织的病理损伤评分增高(P< 0.05),BUN和Cr含量增加(P<0.05),SOD活性和ATP含量降低(P<0.05,P<0.01),MPO活性和MDA含量增加(P<0.05),大鼠肾和空肠组织凋亡率增高(P<0.01);与PQ组相比,所有MB治疗组大鼠肾和空肠组织病理损伤评分降低(P<0.05),BUN和Cr含量减少(P<0.05),SOD活性和ATP含量增高(P<0.05,P<0.01),MPO活性和MDA含量降低(P<0.05),大鼠肾和空肠组织凋亡率降低(P<0.05),肾组织HO-1免疫组化评分增高(P<0.05)。结论 MB能减轻PQ中毒大鼠的肾和空肠损伤,其保护机制可能与MB可提高HO-1表达有关。
百草枯;亚甲蓝;空肠;肾;解毒剂
国家自然科学基金面上项目(81270126);中央高校基本科研业务费专项资金资助(JUSRP51412B);江苏省普通高校研究生科研创新计划(KYLX_1174);江苏省普通高校研究生科研创新计划(KYLX15_1196);江南大学大学生创新实践项目(2016074Z)
庞庆丰,E-mail:qfpang@jiangnan.edu.cn,Tel:15052430172,Fax:(0510)85238406
2015-10-09接受日期:2016-07-03)
R285.1,R979.3 < class="emphasis_bold">Document code:A Article ID:
A Article ID:1000-3002-(2016)08-0815-08
10.3867/j.issn.1000-3002.2016.08.004
Foundation item:The project supported by National Natural Science Foundation of China(81270126);Fundamental Research Funds for the Central Universities(JUSRP51412B);Jiangsu Province Graduate Student Research Innovation Project(KYLX_1174);Jiangsu Province Graduate Student Research Innovation Project(KYLX15_1196);and Jiangnan University Students Innovation Training(2014317)
PANG Qing-feng,E-mail:qfpang@jiangnan.edu.cn,Tel:15052430172,Fax:(0510)85238406
Biography:CHEN Wei,male,Doctor,major research field is emergence medicine.
