一步湿化学法合成超细金纳米线
2015-12-29徐海英阚彩侠王长顺刘津升柯军华
徐海英 阚彩侠 王长顺 倪 媛 刘津升 徐 伟 柯军华
(1南京航空航天大学理学院应用物理系,南京211106;2南京工程学院数理部,南京211167)
一步湿化学法合成超细金纳米线
徐海英1,2阚彩侠1,*王长顺1倪 媛1刘津升1徐 伟1柯军华1
(1南京航空航天大学理学院应用物理系,南京211106;2南京工程学院数理部,南京211167)
以氯金酸(HAuCl4)为前驱物,油胺同时作为溶剂、表面稳定剂和还原剂,通过简单的一步湿化学法合成超细金纳米线.制备出的超细金纳米线不仅产量高、纯度高,而且纵横比大,纳米线平均直径~2 nm,长度可达数十微米.如果添加另一种还原剂油酸并调节油胺和油酸的体积比为1:1,将生成直径为~9 nm的金纳米线.通过改变反应温度和还原剂用量,对该种超细金纳米结构的生长机制进行阐述说明:以油胺为模板,在油胺和一价金卤化物(AuCl)亲金键合形成的一维聚合链作用下,被还原的金原子附着在已成核颗粒表面,一维地生长成超细金纳米线.
准一维;超细;金纳米线;湿化学法;生长机制
1 In troduction
One-dimensional(1D)metallic nanostructures have continuously drawn significant research attention in recentyears due to their unique electrical,optical,mechanical properties and their potentialapplications.1-11For the face-centered cubic(fcc)structured noblemetals,Au and Ag have very close lattice constants(0.4078 and 0.4086 nm,respectively).However,researcheson Au and Ag nanostructuresshow that theirmorphologiesvary a lot through the same synthesismethod.For example,in the same polyol process (w ith the same reaction temperature,solvent,and surfactant),large size of Au nanoplates(micrometers in lateralsize)12and long Ag nanow ires(NWs,micrometers in length)13are formed,respectively from their precursors.Ifwater isused assolvent,small sized Au nanorods14,15and Ag nanoplates16w ill be formed. Therefore,the synthesis of long AuNWs and large sized Ag nanoplates is stilla challenging topic.
Quasi-one-dimensionalAu nanostructures have been synthesized through anion exchange resin,template-assisted electrochemicaldeposition.17-24Ultrafine Au nanostructureshave caused extensive attention,25-27since chemical technology wasapplied to synthesize successfully ultrafine AuNWs by Yang et al.28For special studies,electron-beam irradiation and suspensionmethods are only ever proposed for the fabrication of AuNWsby gentle contactof Au film or tip based on an electronicmicroscopeunder ultrahigh vacuum conditions.29,30These techniquesmainly resulted in the production of thick(>10 nm in diameter)or polycrystalline AuNWs,and even procedures are rather complicated.Inmany reports,the production of single-crystalline AuNWs required intermediate step or a long reaction time.5,30,31There thusneed to develop a simplemethod to produce high-quality AuNWs.
Studieshave predicted that thin AuNWs,especially w ith sub-10 nm in diameter,have good electrical conductivity and are promising candidates for futuremolecular connectionsof thenanoelectronic aspects32-35aswellas active components in nanoscale electric andmechanicaldevices,ow ing to theirexcellentelectrical andmechanical proterties.36-38Herein,we reporta simplewet chem icalmethod for the production of high-quality ultrafine AuNW s.With the improvement to chem ical process,ultrafine AuNW s can be obtained w ith~2 nm in diameter and tens of m icrometer in length through chemical reaction between chloroauric acid(HAuCl4)and oleylamine,in which oleylamine is used as the reducing agent and surfactant.The diameters of AuNWs can be tuned by changing the volume ratio of oleylamine to oleic acid.
2 Experim en tal
2.1 Material
The chem icals including oleylamine(C18H37N,40.0%),oleic acid(C18H34O2,99.0%),hexane(CH3(CH2)4CH3,86.18%),and tetrachloroaurate(HAuCl4·4H2O,99.9%)were purchased from ShanghaiChem ical Reagent Co.Ltd.Acetone(CH3COCH3, ≥99.5%)and alcohol(CH3CH2OH,≥99.7%)were purchased from Sinopharm Chemical Reagent Co.Ltd.All the chemical reagents used were of analytical purity(purity level:AR)and were used w ithout further purification.
2.2 Syn thesis o f u ltrafine AuNWs
In a typical synthesis,0.1 g HAuCl4wasm ixed w ith 1 m L oleylamine in 1m L hexane,themixturewas injected into 9m L oleylamine at 85°C under vigorousmagnetic stirring.In the process,hexane was evaporated under nitrogen atmosphere. HAuCl4(orange color)reacted with oleylamine and formed organic halogen compound AuCl(colorless).The color changes of them ixturewere shown in Fig.1(A,B).Thenmagnetic stirring wasstopped after10min and the reaction processwaskeptat85°C for 5 h until the color gradually changed to dark red(Fig.1C). After cooling the solution to room temperature,the colloid was dispersed in amixed solution(acetone and ethanolw ith volume ratio of 1:3),and an obvious flocculent agglomeration was formed,as shown in Fig.1D.At last the agglomeration was redispersed in hexane for severalhours(see Fig.1E),the dissolved productwas separated several timesw ith hexane to remove the excessoleylamine(see Fig.1F),and ultrasonication is required to dissipate the product in hexane(see Fig.1G)for further investigation.
2.3 Charac terization
The sampleswerewashed and deposited on a carbon-coated copper grid and dried under ambient condition for furthermeasurements.Transm ission electronm icroscope(TEM)and highresolution transm ission electron m icroscope(HRTEM)images were obtained using JEOL-100CX and JEOL-2011m icroscope, respectively.

Fig.1 Photographsof the reaction system and products in separation p rocess
3 Resu lts and d iscussion
Fig.2(A,B)are TEM images of the product.From themorphologieswe can see that the product consists of uniform and continuousAuNWs,togetherw ith a smallnumber of large-sized AuNWsand nanoparticles.Mostof theAuNWsareultrafinewith ~2 nm in diameter and severalmicrometers in length.And these ultrafine AuNWsare flexible on TEM grid.Inset in Fig.2B clearly indicates that the nanow iresare easily looped orbenton the grid edge.
The diameter of the AuNWs is likely to be further tuned by controlling solvent combination during the synthesis.AuNWs w ith~2 nm in diameter can be synthesized when only oleylam ine is used as the solvent,and whereas AuNWs w ith~9 nm in diameterareobtained from amixture solventof 5m L oleylamine and 5mL oleic acid.Fig.2C is the HRTEM image of two individualAuNWswith~9 nm in diameter,which are synthesized by adding oleic acid replacing partof oleylam ine asanother reductant.Obviously,the diameter of AuNW s becomes significantly larger.The using of oleylam ine,in otherword,is better thanoleic acid in the synthesis process.From Fig.2C,we can see that mostof the AuNWs are single crystalline,and layer fault is also observed.Fig.2D is the HRTEM image of the single crystalline part of Fig.2C.It shows that single crystalline AuNWs grow along<111>direction,the ordered crystal fringes perpendicular to<111>directionw ith relatively smooth surface.The inter-fringe distance ismeasured to be 0.235 nm(Fig.2D),which is corresponding to(111)lattice spacing(0.23 nm)of the fcc Au crystal.
Thesesynthesized AuNWs tend to form largebundlesand selfassemble into densely packed bundles.Most of the observed ultrafine AuNWs are found to be self-assemblied w ith~2 nm distance because of the surface coverage of oleylam ine linker between two adjacentnanowires.39As described in the experiment, the flocculent agglomeration should be the packed network of AuNWs dissolved in hexane.In addition,itcan be seen that the ultrafine AuNW s are easily to be melted or fractured by the electron beam irradiation for a long time under TEM measurement,as indicated by Fig.2(A,B).

Fig.3 Schem atic illustration for the formation of theu ltrafine AuNW s
In the procedure,the solvent oleylam ine serves both as the reducing agentand soft template for stabilizing ultrafine AuNWs by forming 1D polymeric chain,31,40on which there is a strong aurophilic interaction from the oleylamine-AuCl complexes.As can be seen in Fig.3,the saltprecursor(Au3+)ispartly reduced at the beginning of the reaction and small gold clusters(Au+)are formed sequentially.Then,crystals nucleate from the continuous reduction ofAu+inside the chain-likestructureofoleyamine-AuCl complexes.Because these crystalshave differentsurface energies on different facets,the density of oleylamine adsorbed on different surfaces varies,which directs the anistropic grow th along the direction w ith lower packing density into ultrafine AuNWs.Inevitably,the continuous nucleation w ill form nanoparticle and thicker nanowirewhen higher gold concentrationsare provided.31Aurophilic bond of oleylam ine-AuCl complexes plays a critical role in forming of ultrafine AuNWs.Schematic illustration for the formation of the ultrafine AuNW template shows that the strong interactions between amine molecules adsorbed on AuNWs, which directs the1D developmentof theultrafineAuNWs.
In order to further study the aurophilic bonding-assisted grow th mechanism of AuNWs,we investigated the influences of reaction temperature and molar ratio of the reaction system onmorphologiesofAu nanostructures.Theexperiment is carried outatdifferent temperatures(70,80,85,90°C),and themorphologiesof Au nanostructures are shown in Fig.4.Only Au nanoparticles w ith~12 nm in diameter are obtained at70°C,as indicated in Fig.4A.HRTEM image(inset of Fig.4A)shows the five-fold tw inned structure of the nanoparticle,which is the common and stable structureof fccmetalnanocrystal.Itcan be seen from the Fig.4B,nanow ires alongside spheroidalnanoparticles appear in the productwhen the temperature is 80°C.Comparatively,the diameter of nanow ires is about equal to the size of the nanoparticles.When the temperature increases to 85°C(Fig.4C),ultrafine AuNWs(~2 nm in diameter)w ith high quality and high purity were formed,compared to Fig.4B.However,the ultrafine AuNWs are usually assembled in bundles possibly due to the strong interaction between aminemolecules adsorbed on the surface of AuNWsand theevaporation of solvent in the preparation of TEM sample.The product,obviously,is dominated by many Au nanoparticles and shortnanow ires(~200 nm in length)together w ith a few of ultrafine AuNW swhen the temperature continues up to 90°C.These results indicate that80-85°Cmore or less is suitable for thegrow th of thenanow ires,and considerably,85°C is the optimal temperature to produce ultrafine AuNWsw ith the highestyield.If the temperature is higher than 100°C,only Au nanoparticlesareobtained,possibly due to the thermaldegradationwhichmakes thatAuNWs cannotkeep their shapessteady.27
In short,theobserved variationsmightbe related to the stateof Au.Themonovalent Au ions are gradually reduced to form a small amount of Au particles at the beginning and then slow ly grow up to large and uniform particles.With increasing the reaction temperature,the nanoparticleswillbecome smallerbecause of reconstruction and Ostwald ripening phenomenon,and AuNWsw illbe produced in the1D polymeric template.40
Fig.5(A-D)are TEM imagesof synthesized productby changing the volume of oleylamine(7-10m L)at85°C w ith other conditionsunchanged.Fig.5A is themorphology of the productwhen 7 m L oleylam ine is applied.It can be seen that branched Au nanostructures are formed under this condition,and no nanoparticlesand nanow ires can beobserved.It isquite possible that the short chain of oleylamine combined w ith the reduced Au atoms from the oleylamine-AuCl complex and self-assembled into discontinuous chain-like structures.Then the nanoparticles aggregated into thebranched nanostructure.With an increase of the volume of oleylamine,more oleylaminemolecules coated on the surfaceofAu,and the reduction of the chain-like structure formed the longer soft template,resulting in the formation of continuous chains.Because of atomic diffusion,aggregation,and recombination,the branched nanostructures began to grow into nanostructures such as nanotrees,long nanorods and nanow ires,as shown in Fig.5B.
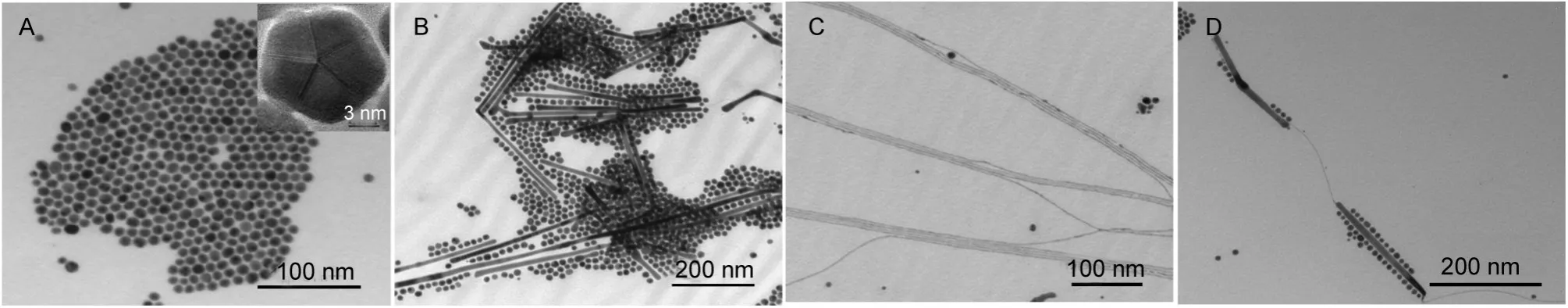
Fig.4 TEM im agesof productssynthesized at different reaction tem peratures (A)70°C,(B)80°C,(C)85°C,(D)90°C

Fig.5 TEM imagesof products synthesized by changing theoleylam ine volum e (A)7m L,(B)8m L,(C)9m L,(D)10m L
When thevolumeof oleylamine is8mL,itindicates thatahigh yield of Au long nanorods is formed.TheseAu long nanorodsare thin,continuous,w ith a diameter of~5 nm and the length up to several hundreds nanometers(see Fig.4C).Nano-shaped tips (enlarged head-shaped/tadpole-shaped)are observed at the end of these nanorods.The generated nanorods by this process are generally much thinner than the head of the rods.There is no obvious decrease in the size of the head of the rods,which roughly retains their original spherical shape.41,42When the oleylam ine achieves a certain volume,the product is dom inated by ultrafine AuNW s w ith diameter of~2 nm and the length up to several m icrometers.These ultrafine AuNWsare uniform,high yield and self-assembly in bundles(see Fig.4D).
The above results,for thegrow thmechanism,indicate that the obtained productsare confined by the chain-like template of the oleylamine-AuCl complex,as schematically illustrated by Fig.3. And themolar ratio of the oleylamineand HAuCl4isalso critical for the formation of ultrafine AuNW s.Itcan be understood that long hydrophobic chains w ith am ino groups combine strongly w ith Au,finally leading to the formation of ultrafine AuNWs. Oleylaminealso playsa roleof a surfactant,which providesa soft template for the oriented combination and recombination of Au particles,elongating the nanow ires.The greater the volume of oleylam ine in a certain range,the longer the tail chain,the higher theaspect ratio of nanow ires.
4 Conc lusions and ou tlook
This paper presents a simple synthesis of ultrafine AuNWs in an oil-bath at85°C for5 h by reduction of HAuCl4in oleylamine. The ultrafine AuNWs are high yield,high aspect ratio with diameter of~2 nm.The as-synthesized ultrafine AuNW s could easily self-assemble into closely packed bundles possibly due to the strong interaction of the adsorbed amine on the surface of AuNWs,aswellas theevaporation of solvent in the preparation of TEM sample.With adjusting the reaction temperature and the volume of the oleylam ine,various shapes of Au nanostructures were formed by reconstruction from Au atoms or their agglomeration.In thismethod,oleylamine hasmultifunctional roles as the solvent,surfactant,and reductant.The surfactant induces anisotropic grow th by adsorbing on the specific Au crystalline surface.The grow thmechanism of using oleylam ine as the soft templatehasbeen utilized to explain the formation of nanow ires. This work is important for both fundamental study of their quantum properties and applications including sensors,waveguide,transparent conductive electrode,and nano-connector in electronic devices.
References
(1)Xia,Y.N.;Xiong,Y.J.;Lim,B.;Skrabalak,S.E.Angew.Chem. Int.Edit.2009,48,60.doi:10.1002/anie.200802248
(2)Cui,Y.;Wei,Q.Q.;Park,H.K.;Lieber,C.M.Science 2001, 293,1289.doi:10.1126/science.1062711
(3)Hu,S.;Wang,X.Chem.Soc.Rev.2013,42,5577.doi:10.1039/ c3cs00006k
(4)Wiley,B.;Sun,Y.G.;Xia,Y.N.AccountsChem.Res.2007,40, 1067.doi:10.1021/ar7000974
(5)Halder,A.;Ravishankar,N.Adv.Mater.2007,19,1854.
(6)Hu,Y.;Lu,L.H.;Liu,J.H.;Chen,W.J.Mater.Chem.2012,22, 11994.doi:10.1039/c2jm31483e
(7)Li,C.C.;Cai,W.P.;Kan,C.X.;Zhang,L.D.Mater.Lett.2004, 58,196.doi:10.1016/S0167-577X(03)00444-0
(8)Lacroix,L.M.;Arenal,R.;Viau,G.J.Am.Chem.Soc.2014, 136,13075.doi:10.1021/ja507728j
(9)Takahata,R.;Yamazoe,S.;Koyasu,K.;Tsukuda,T.J.Am. Chem.Soc.2014,136,8489.doi:10.1021/ja503558c
(10)Kempa,T.J.;Kim,S.K.;Day,R.W.;Park,H.G.;Nocera,D. G.;Lieber,C.M.J.Am.Chem.Soc.2013,135,18354.doi: 10.1021/ja411050r
(11)Long,Y.T.;Zhang,M.N.Sci.China Chem.2009,52,731.
(12)Kan,C.X.;Wang,C.S.;Li,H.C.;Qi,J.S.;Zhu,J.J.;Li,Z.S.; Shi,D.N.Small2010,6,1768.doi:10.1002/sm ll.201000600
(13)Kan,C.X.;Zhu,J.J.;Zhu,X.G.JournalofPhysicsD-Applied Physics2008,41,155304.doi:10.1088/0022-3727/41/15/ 155304
(14)Murphy,C.J.;Thompson,L.B.;Chernak,D.J.;Yang,J.A.; Sivapalan,S.T.;Boulos,S.P.;Huang,J.Y.;A lkilany,A.M.; Sisco,P.N.CurrentOpinion in Colloid&Interface Science 2011,16,128.
(15)Li,C.C.;Sun,L.;Sun,Y.Q.;Teranishi,T.Chem.Mater.2013, 25,2580.doi:10.1021/cm400392e
(16)M illstone,J.E.;Hurst,S.J.;Metraux,G.S.;Cutler,J.I.; M irkin,C.A.Small2009,5,646.doi:10.1002/sm ll.v5:6
(17)Dertli,E.;Coskun,S.;Esenturk,E.N.J.Mater.Res.2013,28, 250.doi:10.1557/jm r.2012.407
(18)Sinha,A.K.;Basu,M.;Sarkar,S.;Pradhan,M.;Pal,T. Langmuir2010,26,17419.doi:10.1021/la102387x
(19)Kim,J.U.;Cha,S.H.;Shin,K.;Jho,J.Y.;Lee,J.C.Adv.Mater. 2004,16,459.
(20)Wang,J.G.;Tian,M.L.;Mallouk,T.E.;Chan,M.H.W. J.Phys.Chem.B 2004,108,841.doi:10.1021/jp035068q
(21)Forrer,P.;Schlottig,F.;Siegenthaler,H.;Textor,M.J.Appl. Electrochem.2000,30,533.doi:10.1023/A:1003941129560
(22)Wang,J.Faraday Discuss.2013,164,9.doi:10.1039/ c3fd00105a
(23)Li,Y.;Koshizaki,N.;Cai,W.P.Coord.Chem.Rev.2011,255, 357.doi:10.1016/j.ccr.2010.09.015
(24)Dar,F.I.;Habouti,S.;M inch,R.;Dietze,M.;Es-Souni,M. J.Mater.Res.2012,22,8671.
(25)Morita,C.;Tanuma,H.;Kawai,C;Ito,Y.;Imura,Y.;Kawai,T. Langmuir2013,29,1669.doi:10.1021/la304925e
(26)M izoguchi,D.;Murouchi,M.;Hirata,H.;Takata,Y.;Niidome, Y.;Yamada,S.J.Nanopart.Res.2011,13,6297.doi:10.1007/ s11051-011-0555-0
(27)Kura,H.;Ogawa,T.J.Appl.Phys.2010,107,074310.doi: 10.1063/1.3369441
(28)Huo,Z.Y.;Tsung,C.K.;Huang,W.Y.;Zhang,X.F.;Yang,P. D.Nano Lett.2008,8,2041.doi:10.1021/nl8013549
(29)Ohnishi,H.;Kondo,Y.;Takayanagi,K.Nature 1998,395, 780.doi:10.1038/27399
(30)Kondo,Y.;Takayanagi,K.Science 2000,289,606.doi:10.1126/ science.289.5479.606
(31)Pazos-Perez,N.;Baranov,D.;Irsen,S.;Hilgendorff,M.;Liz-Marzan,L.M.;Giersig,M.Langmuir2008,24,9855.doi: 10.1021/la801675d
(32)Wang,C.;Hu,Y.;Lieber,C.M.;Sun,S.J.Am.Chem.Soc. 2008,130,8902.doi:10.1021/ja803408f
(33)Oo,T.Z.;Mathews,N.;Xing,G.C.;Wu,B.;Xing,B.G.; Wong,L.H.;Sum,T.C.;Mhaisalkar,S.G.J.Phys.Chem.C 2012,116,6453.doi:10.1021/jp2099637
(34)Pud,S.;Kisner,A.;Heggen,M.;Belaineh,D.;Tem irov,R.; Simon,U.;Offenhausser,A.;Mourzina,Y.;Vitusevich,S.Small 2013,9,846.doi:10.1002/sm ll.v9.6
(35)Yoshihira,M.;Moriyama,S.;Guerin,H.;Ochi,Y.;Kura,H.; Ogawa,T.;Sato,T.;Maki,H.Appl.Phys.Lett.2013,102, 203117-1.doi:10.1063/1.4807806
(36)Wang,C.;Sun,S.H.Chem.Asian J.2009,4,1028.doi: 10.1002/asia.v4:7
(37)Lu,Y.;Song,J.;Huang,J.Y.;Lou,J.Adv.Funct.Mater.2011, 21,3982.doi:10.1002/adfm.v21.20
(38)Lu,W.;Lieber,C.M.Nat.Mater.2007,6,841.doi:10.1038/ nmat2028
(39)Feng,H.;Yang,Y.;You,Y.;Li,G.;Guo,J.;Yu,T.;Shen,Z.; Wu,T.;Xing,B.Chem.Commun.2009,1984.
(40)Lu,X.M.;Yavuz,M.S.;Tuan,H.Y.;Korgel,B.A.;Xia,Y.N. J.Am.Chem.Soc.2008,130,8900.doi:10.1021/ja803343m
(41)Huang,X.;Li,S.;Wu,S.;Huang,Y.;Boey,F.;Gan,C.L.; Zhang,H.Adv.Mater.2012,24,979.doi:10.1002/ adma.201104153
(42)He,J.;Wang,Y.;Feng,Y.;Qi,X.;Zeng,Z.;Liu,Q.;Teo,W.S.; Gan,C.L.;Zhang,H.;Chen,H.ACSNano 2013,7,2733.doi: 10.1021/nn4001885
Ultrafine Au Nanow ires Syn thesized via One-Step Wet Chem icalMethod
XU Hai-Ying1,2KAN Cai-Xia1,*WANG Chang-Shun1NIYuan1LIU Jin-Sheng1XUWei1KE Jun-Hua1
(1DepartmentofApplied Physics,College ofScience,Nanjing University ofAeronauticsand Astronautics,Nanjing 211106, P.R.China;2DepartmentofMathematicsand Physics,Nanjing Institute ofTechnology,Nanjing 211167,P.R.China)
Ultrafine Au nanowires(AuNWs)were synthesized in high yields by a one-step wetchem icalmethod using oleylam ine as the solvent,surfactant,and reductant.The obtained AuNWswere ofhigh purity and had a high aspect ratio,w ith diameters of~2 nm and lengths of tens ofm icrometers.AuNWs ofdiameter~9 nm were also obtained in the presence ofoleic acid,atan oleic acid:oleylam ine volum e ratio of1:1.The form ation of AuNWs was studied by changing the reaction tem perature and the volume ofoleylam ine.It is proposed that the grow thmechanism of the Au nanostructures involves strong aurophilic interactions from oleylam ine-AuCl complexes;the reduced Au atoms agglomerate and attach to preformed particles,and the oleylam inemolecular layeracts as a soft tem p late,leading to one-dimensionalgrow th ofAu atom s into AuNW s.
Quasi-one-dimension;Ultrafine;Au nanow ire;Wetchem icalm ethod; Grow thmechanism
O648
icle]
10.3866/PKU.WHXB201504012 www.whxb.pku.edu.cn
Received:January 19,2015;Revised:March 30,2015;Published onWeb:April1,2015.
∗Corresponding author.Email:cxkan@nuaa.edu.cn;Tel:+86-25-52113852.
The projectwassupported by the NationalNaturalScience Foundation of China(11274173),FundamentalResearch Funds for the Central Universities,China(NZ2015101,NJ20140005),Funding of Jiangsu Innovation Program forGraduate Education,China(KYZZ_0091),and Qing Lan Projectof Jiangsu Province,China.
国家自然科学基金(11274173),中央高校基本科研专项资金(NZ2015101,NJ20140005),江苏省研究生培养创新工程(KYZZ_0091)及江苏省青蓝工程资助项目
©Editorialoffice of Acta Physico-Chim ica Sinica