化学改性氧化石墨烯交联的聚酰亚胺气凝胶
2015-12-29姚维尚张学同
梁 祎 卢 赟 姚维尚 张学同
(北京理工大学材料科学与工程学院,北京100081)
化学改性氧化石墨烯交联的聚酰亚胺气凝胶
梁 祎 卢 赟*姚维尚 张学同*
(北京理工大学材料科学与工程学院,北京100081)
聚酰亚胺(PI)气凝胶是一类密度低、机械性能好、隔热性能优异的多孔材料,通常使用昂贵的化学交联剂进行交联.氧化石墨烯(GO)是近年来广受关注的用于聚合物增强的纳米功能填料.以前报道的PI/GO复合材料多是纤维或膜的形式.为了获得PI/GO复合气凝胶,本文采用化学改性氧化石墨烯(m-GO)替代1,3,5-三(4-氨基苯氧基)苯(TAB)等常规的交联剂,使之与4,4′-二氨基二苯基醚(ODA)和3,3′,4,4′-联苯四羧酸二酐(BPDA)反应,制得了m-GO交联的PI气凝胶.GO的化学改性通过其与过量ODA在水热条件下反应实现.通过扫描电子显微镜(SEM)研究了PI/m-GO气凝胶的微观结构.分别通过氮气吸脱附测试、热重分析和热线法研究了m-GO对气凝胶的孔特性、热稳定性和热导率的影响.测试结果表明,所获得的PI/m-GO气凝胶保持了高的孔隙率、热稳定性和绝热性.压缩测试结果显示,与采用1.8%(质量分数,w)的TAB进行交联的PI气凝胶相比,仅用0.6%(w)的m-GO交联所获得的气凝胶具有更高的比杨氏模量(杨氏模量/密度)、比屈服强度(屈服强度/密度)和更小的体积收缩率.
聚酰亚胺气凝胶;氧化石墨烯;交联;机械性能;收缩率
Key Wo rds:Polyim ide aerogel;Graphene oxide;Crosslinking;Mechanicalproperty; Shrinkage ratio
1 Introduction
Aerogelsareultra-lightand highly porousnano-materialsassembled by nano-sized building units such as zero-dimensional (0D)nano-particles,one-dimensional(1D)nano-w ires,and twodimensional(2D)nano-sheets.Its consecutive open poursendow aerogels w ith unique properties and potential applications in various fields such asacousticaland thermal insulations,catalysis, etc.Since the firstcase on SiO2aerogelwas reported in 1931 by Kistler,1many kinds of inorganic and polymeric aerogels have been produced by far.2-6A lthough aerogels have aroused intense interestsallover theworld,they are notused asmuch as itwas expected.Besides the unique drying technique,this is ascribed to the fragileness for themost inorganic aerogelsand the relatively poor thermalstability for themostpolymeric aerogels.Formany applications,typically in the fields of aerospace,superior mechanical property and high thermalstability are both needed.In 2004,Rhine etal.7disclosed the preparation of linear polyimide aerogels that composed of aromatic dianhydrides and aromatic diamines or a combined aromatic and aliphatic diamines.However,the three-dimensional(3D)network structures of these linear aerogels are not covalently bonded butproduced through the physical interaction between polymer chains.Therefore,although themechanical property of these aerogels is good,they tend to exhibita large amountof shrinkage during the fabrication process.To further improve themechanical property,and to reduce the shrinkage,Meador etal.8-11in NationalAeronautics and Space Adm inistration(NASA)has synthesized a seriesof polyimide(PI)aerogels crosslinked withmultifunctionalamines such aseight(am ino)-silsesquioxane(OAPS)and 1,3,5-triam inophenoxy benzene(TAB).They also applied chem ical im idization at room temperaturew ith excess pyridine/acetic anhydride instead of heating im idization to obtain fully im idized aerogels.The crosslinked PIaerogels generally exhibited higher glass transition temperature,better mechanical property,and less amount of shrinkage than linear aerogels.These research resultsmake PI aerogelsbe potential candidates foraerospace applications.
In thisstudy,we reportPIaerogels crosslinkedwith chemically modified graphene oxides(m-GO).GO is a sheet-like carbon nano-materialwith superhigh Young'smodulus due to itsunique lattice structure.Bearing carboxylgroup,carbonylgroups,epoxy groups,and hydroxylgroupson itsbasalplanesand edges,12,13GO is solvent compatible and chemically active,whichmakes it a prominent candidate to fabricate high strength and functional resin-based composite.PI/GO composites,exhibiting enhanced mechanical properties,high decomposition temperatureand so on, have been extensively studied.14-19However,most reported PI/GO compositeswere studied in the form of filmsor fibers rather than aerogels.Investigation on the synthesisof the PI/GO aerogelmay providea clue to develop anovelporouscomposite.In thisarticle, GOmodified w ith 4,4′-oxydianiline(ODA)wasused to crosslink PIaerogels.The organic am ine groups derived from ODA endow them-GO sheets notonly to dissolve better in organic solvents, butalso to reactw ith the dianhydride.Thismay turn out to be an econom icmethod to crosslink the PIaerogelby avoiding the use of expensive traditional crosslinking agents.The effectsofm-GO on themorphology,pore property,thermal stability,shrinkage, thermal insulation,and mechanical property of the composite aerogelsw illbe investigated.
2 Experim en tal
2.1 Materials
4,4′-Oxydianiline(98%)was purchased from Sinopharm Chemical Reagent Co.,Ltd.;N-methyl-2-pyrrolidinone(NMP, 99.5%)was purchased from Beijing Tongguang Chem ical Company.Acetone(99.5%)waspurchased from Beijing Keweinuo ChemicalReagentCo.,Ltd.;Pyridine(99%)waspurchased from Beijing Chem ical Factory.These reagents were used w ithout further purification.3,3′,4,4′-Biphenyltetracarboxylic dianhydride(BPDA,98%)was purchased from Beijing Zhong Ke Chem ical Technology Co.Ltd.and dried at120°C for24 h prior to use.GOwassynthesized according to the steps reported in our previous study.201,3,5-Triaminophenoxy benzene(TAB)was prepared according to the procedure reported in the literature.21-23
2.2 Preparation o fm-GO
Them-GO was prepared approximately according to the method reported in the literature15except that hydrothermal reaction vesselwasused to conduct the reaction,which omitting the 24 h nitrogen purge.Briefly,to 150mLNMP,1.5 g GOwasadded and ultrasonication wasapplied to obtain uniform suspension.A solution of 2.3 g ODA in 15 m L NMPwas added to the GO suspension under stirat80°C.Then themixturewasmoved into hydrothermal reaction vesseland heated at80°C in oven for 24 h.A fter the reaction was finished,solvent-exchangewasapplied in acetone to remove the unreacted ODA.Them-GO powder obtained by filtration was re-dispersed into NMPby ultrasonication.The concentration ofm-GO in NMPwasdetermined to be 3.5mg·m L-1.
2.3 Syn thesis o f PI/m-GO aerogels
The PI/m-GO aerogels(PIaerogels crosslinked bym-GO)was synthesized as shown in Fig.S1(Supporting Information).As an example,the synthesisof the PI/m-GO aerogelw ith 0.2%m-GO content isdescribed as follows.To a solution of ODA(1.58 g,7.9 mmol)in 35.8m L of NMP,2.1m L concentratedm-GO suspension was added and ultrasonication wasapplied.After 15m in of ultrasonication,BPDA(2.395 g,8.15 mmol)was added under nitrogen.Acetic anhydride(6.15mL,65mmol,8:1molar ratio to BPDA)and pyridine(5.25 m L,65 mmol)were added under stirring after the BPDA was dissolved.Them ixturewas poured intomold immediately to form gel.The reaction scheme is shownin Fig.S1.The obtained gelwasaged for24 h at room temperature and then solvent-exchanged w ith ethanol.A fter that,the gelwas subject to supercritical CO2extraction.By changing the ratio of m-GO suspension to NMP,a series of PI/m-GO aerogelsw ith differentm-GO contents were prepared.For simplicity,these aerogelswere named as PI/x%m-GO,in which x%refers to the mass fraction ofm-GO.A PIaerogel crosslinked w ith 1.8%(w) TAB was prepared asa control sample named as PI/1.8%TAB. The synthesis of PI/1.8%TAB is sim ilar to the PI/0.2%m-GO. ODA(1.58 g,7.9mmol)and BPDA(2.395 g,8.15mmol)were dissolved in NMP(33m L)under nitrogen atmosphere.Then the solution of TAB(0.07 g,0.175mmol)in NMP(5.65m L)was added and ultrasonicationwasapplied.The subsequentoperations were gelation,ageing,solventexchange,and super critical CO2drying.A ll the PI/m-GO composite aerogels and the PI/TAB aerogelwere designed w ith the theoreticaldensity(ρ)of 75mg· cm-3.
2.4 Instrum en tations
Microstructure observationwas conducted by scanning electron m icroscope(SEM,Hitachi,S-4800,Japan)w ith an accelerating voltage of 5-10 kV after sputter coating of the specimensw ith platinum.Raman spectra(HORIBA Jobin Yvon S.A.S,RAM HR800,France)were recorded w ith an excitation laser wavelength of 632.8 nm.X-ray powder diffraction(XRD,Rigaku, Dmax 2200,Japan)was conducted w ith Cu Kαradiation(λ= 0.15416 nm).Fourier transform infrared(FTIR)spectrum was conducted on the Tensor 27 spectroscopy(Bruker Optics, Germany).The X-ray photoelectron spectroscopy spectrograph (XPS,Kratos,AXISUltra XPS,Japan)hasamonochromatic Al Kαradiation(hv=1486.71 eV)powered w ith 10mA and 15 kV. Brunauer-Emmett-Teller(BET)nitrogen sorption isothermswere investigated by ASAP 2010(M icromeritics,America)instrument at77 K,and the pore size distribution was calculated from the desorption branch of sorption isotherms by the Barrett-Joyner-Halenda(BJH)formula.Thermogravimetric(TG)analysiswas conducted on a TG209(Netzsch,Germany)instrument from room temperature to 850°Cw ith a heating rate of 10°C·m in-1under nitrogen atmosphere.The compression testswere carried on by a universal testmachine(6022,America)made in Instron.The compression velocity was30%of the sample length perminute and the testwould be stopped when the strain reached 80%of the sample length.The specimens for compression tests were prepared in accordancew ith ASTM D695-10,inwhich the sample sizesarenominally 1.5-1.8 cm in diameterand the length is close to the tw ice as long as the diameter.Thermal conductivity was taken at room temperature on a thermal conductivitymeasuring instrument(Xi'an Summer Creek Electronic Technology Co.,Ltd., TC 3010L,China)by thehotw iremethod.
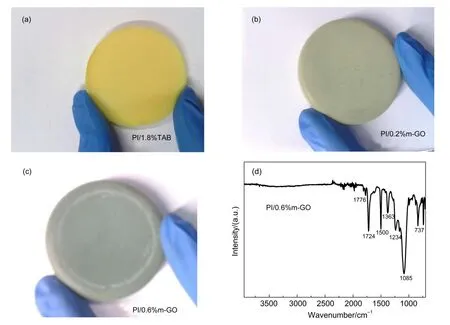
Fig.1 Digitalphotosof(a)PI/1.8%TAB aerogel,(b)PI/0.2%m-GO com positeaerogel,(c)PI/0.6%m-GO compositeaerogel,and(d)the FTIR spectrum of PI/m-GO aerogel
3 Resu lts and d iscussion
The image photos(Fig.S2),Raman spectra(Fig.S3(a)),XRD patterns(Fig.S3(b)),and FTIR spectra(Fig.S4)(Supporting In-formation)of the obtainedm-GO were investigated.The results are in accordance w ith those reported in the literature,15which confirms that the organic am ine groups have grafted onto the GO sheetby the reaction of the organic aminew ith the epoxy of GO. Moreover,differing from the literature,XPS spectra(Fig.S5 (Supporting Information))show none peak ascribed to carbonyl or carboxylgroups,suggesting thatahigher GO reduction degree (O%=25.2%)may be achieved by use of the hydrothermal reaction vessel.

Fig.2 SEM im agesof(a)PI/1.8%TAB aerogeland(b-d)PI/m-GO copositeaerogel
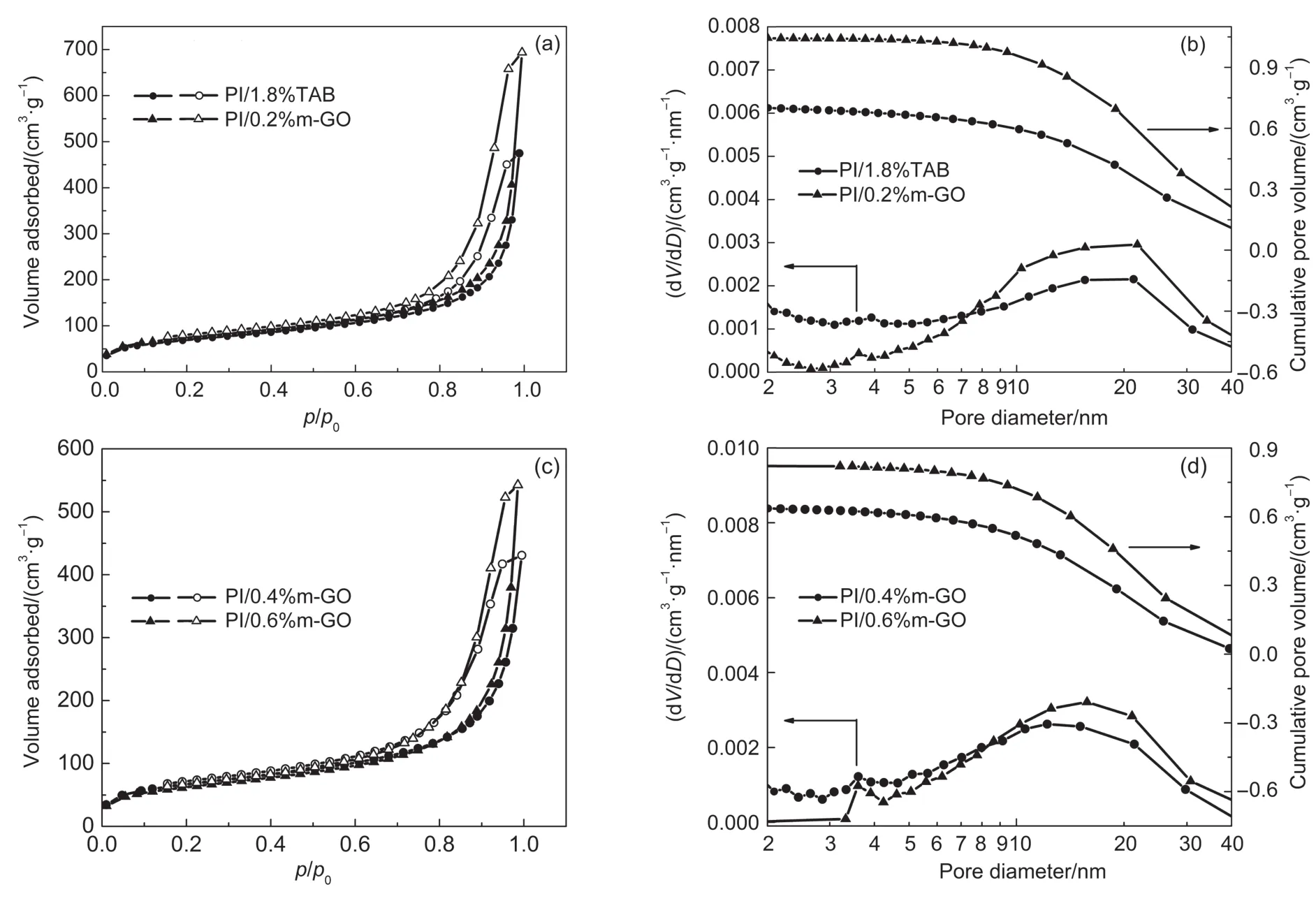
Fig.3 Typicalnitrogen adsorption-desorption isotherm s(a,c)and pore size distribution(b,d)of theobtained aerogels
To enable the poly(amid acid)(PAA)to reactw ith them-GO or TAB,the PAAwas formulatedw ith anhydrideas theend group by adjusting themolar ratio of BPDA to ODA to be(n+1):n,in which n is the number of repeated units.Eight-fold excess of acetic anhydride and pyridine was used to insure complete chemical im idization.The color of the PI/m-GO composite aerogels is totally different from thatof PI/1.8%TAB aerogel,and gets darkerw ith the increase of them-GO content,as shown in Fig.1(a-c).Fig.1(d)shows the FTIR spectrum of theobtained PI/ m-GO aerogel.The absence of bandsat~1800 and~1670 cm-1indicating theexistenceof isoimideand bandsat3200-3500 cm-1referring to―OH indicate that the im idization is complete.24-26
To investigate them icrostructure of the PI/m-GO aerogel,the SEM wasconducted.Fig.2(b-d)shows the SEM photosof the PI/ m-GO aerogelatvarious regionswith differentmagnifications.It can be seen from Fig.2(b)that them-GO sheetsarequite thick and have coarse surface,which is quite different from what they present before reacting w ith PAA(Fig.S2).Fig.2(c)is themagnified picture of the region w ithin the black circle in Fig.2(b), showing that the coarse surfaceofm-GO sheet is composed of PI aggregates.This indicates that the PIchains are able to propagate on them-GO sheetsby reactingw ith the am ine group onm-GO. The PIaggregatesonm-GO sheetsare similar to those composing of PImatrix in the PI/m-GO aerogel(Fig.2(d)),while the former presentshorter fibrous shape andmore compactingmorphology than the latter.The structure of PIparts in the PI/m-GO aerogel shown in Fig.2(d)seems the same as that of the PI/1.8%TABaerogelshown in Fig.2(a),both are porous frameworks composed of tangled fibrous PIaggregatesw ith diameter of about20 to 30 nm.
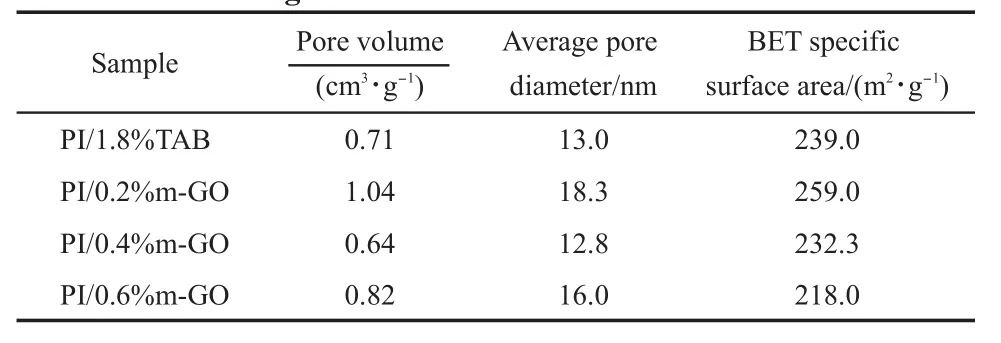
Tab le1 Pore structure data of PI/1.8%TAB and PI/m-GO aerogelsw ith differentm-GO contents

Fig.4 Stress-strain curvesof(a)PI/1.8%TAB,(b)PI/0.2%m-GO,(c)PI/0.4%m-GO,(d)PI/0.6%m-GO and the inset show ing the shapeof PI/0.6%m-GO aerogelbeforeand after com p ression

Table2 M echanicaldata of PI/1.8%TAB and PI/m-GO aerogels w ith differentm-GO contents

Fig.5 (a)Volum e shrinkage ratio and(b)density of theobtained aerogels
Since them-GO sheetsquite differ from the traditional crosslink agents in size,concern of whether thiswould damage the pore property andmechanicalproperty of the compositeaerogels was aroused.Curves of typical nitrogen adsorption-desorption isotherms of the obtained aerogels are shown in Fig.3.The pore structure data of PI/m-GO aerogels listed in Table 1were calculated by BJHmethod.According to IUPAC,all theadsorption and desorption curvesare type IV isothermswith a H1 hysteresis loop, which indicates that the PI/m-GO aerogels aremesoporousmaterials.9,27This result is consistentw ith the average pore diameter of 12.8-18.3 nm recorded in Table 1.Itcan be seen that the pore property of the PI/m-GO aerogels doesnotsignifucantly distinguish from thatof the PI/1.8%TAB aerogel,and no obvious trend of the pore property relative tom-GO content isobserved.
Compression tests for the obtained aerogelswere performed and the stress-strain curves are shown in Fig.4.The photo inserted in Fig.4 shows the shape of PI/0.6%m-GO aerogelbefore and after compression.The sample did not break during compression,and the radialdimension remained unchanged.Actually, all the composite aerogels acted in the sameway,indicating good toughness.All the stress-strain curves present three stages:(1) linear stage,inwhich the reversible deformation occurred and the slope of the curvewas considered asYoung'smodulus(hereinafter referred to as E),(2)yield stage,in which the framework of the aerogelbegan to collapse and irreversible deformation occurred, and(3)dense stage,inwhich the structure of theaerogelbecame dense and the stress increased sharply w ith the developing of strain.Although asmentioned above,all the aerogelswere prepared w ith the theoretical density of 75mg·cm-3,their actual apparent density(ρ)varied slightly due to the uncontrollable shrinkage.Therefore,to compare theirmechanical property appropriately,the concepts of specific Young'smodulus(E/ρ)and specific yield strength(yield strength/ρ)areused herein.Itcan be seen in Table2 that E and yield strength of the PI/m-GO aerogels both enhanced w ith the increase of them-GO contents.When the m-GO content reached 0.6%,the specific Young'smodulus and specific yield strength of the PI/m-GO aerogelwere higher than thoseof PI/1.8%TAB aerogel.The volume shrinkage ratiosof the obtained aerogels were calculated according to the follow ing equation:volume shrinkage Vs=(Vwetgel-Vaerogel)/Vwetgel×100%.As shown in Fig.5,the amountof shrinkage for PI/m-GO aerogels crosslinked w ith 0.2%-0.6%m-GO isabout26%,which is less than thatof PIaerogel crosslinkedw ith 1.8%TAB(34%).
GO isamaterialw ith good thermal conductivity.The thermalconductivitiesof the obtained aerogelsweremeasured by hotw ire method to investigate the effectofm-GO on the aerogels.The thermal conductivities of the PI/m-GO aerogels increased very slightly from 0.0302 to 0.0318W·m-1·K-1as them-GO content enhanced from 0.2%to 0.6%,and these valueswere very close to the thermal conductivity of the PI/1.8%TAB aerogel(Fig.6).This indicates that the use ofm-GO as crosslink agent for PIaerogel would notstrikingly increase the thermalconductivity,and the PI/ m-GO aerogelsstill remain excellent thermal insulating attribute.
Thermogravimetric(TG)analysis of the composite aerogels showsno surprising results comparingw ith PI/1.8%TAB aerogel. As it can be seen in Fig.S6(Supporting Information),the fast weight loss of all the obtained aerogels took place above the temperature of 520°C,revealing that the composite aerogels possessexcellent thermalstability.

Fig.6 Therm alconductivitiesof theobtained aerogels
4 Conc lusions
PIaerogels croslinkedwithm-GOwere prepared by the in-situ polymerization of ODA,BPDA,andm-GO in NMP followed by supercritical CO2drying.The SEM photos of the PI/m-GO aerogelsshow that the short fibrous PIaggregatesgrew on them-GO sheets,confirming the reaction betweenm-GO and PIoccurred.By crosslinking w ith only 0.6%m-GO,the superiormechanical propertiesand lessamountof shrinkage in comparison w ith PIaerogel crosslinked w ith 1.8%TABwereachieved,indicating that them-GO has significant reinforcing effect on PI aerogels.Actually,crosslink is critical to improvemechanical property and reduce the shrinkage.In our study,the sizeofm-GO ismuch larger than thatof the PIaggregates,whichmay lead to heterogeneity in network and limited crosslinking density.Hence, preparingm-GOwith smalldimensionmay bea clue to obtain PI/ m-GO aerogelswithmore superior propertiesby conquering the above issues.The results of nitrogen sorption test,TGA,and thermal conductivitymeasurement reveal that the PI/m-GO aerogels aremesoporousmaterials w ith average pore diameters of 12.8-18.3 nm,high specific areaof 218.0-259.0m2·g-1,and high initial decomposition temperature of about520°C,and the reported contentsofm-GO haveno significanteffectson the relative propertiesof the PI/m-GO compositeaerogels.
Suppo rting In fo rm ation: available free of charge via the internetathttp://www.whxb.pku.edu.cn.
(1)Kistler,S.S.Nature 1931,127,741.
(2)Tamon,H.;Ishizaka,H.;M ikami,M.;Okazaki,M.Carbon 1997,35(6),791.doi:10.1016/S0008-6223(97)00024-9
(3)Wang,Z.;Dai,Z.;Wu,J.;Zhao,N.;Xu,J.Adv.Mater.2013,25 (32),4494.doi:10.1002/adma.v25.32
(4)Xu,Z.J.;Ji,T.;Zhao,L.;Wang,W.Y.;Yang,C.Y.;Gan,L.H. Acta Phys.-Chim.Sin.2012,28(2),361.[徐子颉,吉 涛,赵蕾,王玮衍,杨春艳,甘礼华.物理化学学报,2012,28(2),361.] doi:10.3866/PKU.WHXB201112063
(5)Guo,X.Z.;Yan,L.Q.;Yang,H.;Li,J.;Li,C.Y.;Cai,X.B. Acta Phys.-Chim.Sin.2011,27(10),2478.[郭兴忠,颜立清,杨 辉,李 建,李超宇,蔡晓波.物理化学学报,2011,27 (10),2478.]doi:10.3866/PKU.WHXB20110925
(6)Xu,W.W.;Du,A.;Tang,J.;Chen,K.;Zou,L.P.;Zhang,Z.H.; Shen,J.;Zhou,B.Acta Phys.-Chim.Sin.2012,28(12),2958.[许维维,杜 艾,唐 俊,陈 珂,邹丽萍,张志华,沈 军,周斌.物理化学学报,2012,28(12),2958.]doi:10.3866/PKU. WHXB201209282
(7)Rhine,W.;Wang,J.;Begag,R.Production of Polyim ide Aerogel forCarbon Aerogel,InvolvesContacting Diamineand Aromatic Dianhydride Monomers in Solvent,Contacting Resulting Poly(amic acid)w ith Dehydrating Agent,and Drying Resulting Polyim ideGel.U.S.Patent2004/0132845A1,2004-07-08.
(8)Guo,H.;Meador,M.A.B.;M cCorkle,L.;Quade,D.J.;Guo,J.; Ham ilton,B.;Cakmak,M.;Sprow l,G.ACSAppl.Mater. Interfaces2011,3(2),546.doi:10.1021/am101123h
(9)Guo,H.;Meador,M.A.B.;M cCorkle,L.;Quade,D.J.;Guo,J.; Ham ilton,B.;Cakmak,M.ACSAppl.Mater.Interfaces2012,4 (10),5422.doi:10.1021/am301347a
(10)Meador,M.A.B.;Malow,E.J.;Silva,R.;Wright,S.;Quade, D.;Vivod,S.L.;Guo,H.;Guo,J.;Cakmak,M.ACSAppl. Mater.Interfaces2012,4(2),536.doi:10.1021/am2014635
(11)Meador,M.A.B.;W right,S.;Sandberq,A.;Nquyen,B.N.; Vankeuls,F.W.;Mueller,C.H.;Rodríguez-Solís,R.;M iranda, F.A.ACSAppl.Mater.Interfaces2012,4(11),6346.doi: 10.1021/am301985s
(12)Novoselov,K.S.;Jiang,D.;Schedin,F.;Booth,T.J.; Khotkevich,V.V.;Morozov,S.V.;Geim,A.K.Proceedingsof the NationalAcademy ofSciences2005,102(30),10451.doi: 10.1073/pnas.0502848102
(13)Mkhoyan,K.A.;Contryman,A.W.;Silcox,J.;Stewart,D.A.; Eda,G.;Mattevi,C.;M iller,S.;Chhowalla,M.Nano Lett. 2009,9(3),1058.doi:10.1021/nl8034256
(14)Shi,H.;Li,Y.;Guo,T.J.Appl.Polym.Sci.2013,128(5), 3163.doi:10.1002/app.v128.5
(15)Dong,J.;Yin,C.;Zhao,X.;Li,Y.;Zhang,Q.Polymer2013,54(23),6415.doi:10.1016/j.polymer.2013.09.035
(16)Huang,T.;Xin,Y.S.;Li,T.S.;Nutt,S.;Su,C.;Chen,H.M.; Liu,P.;Lai,Z.L.ACSAppl.Mater.Interfaces2013,5(11), 4878.doi:10.1021/am400635x
(17)Park,O.K.;Hwang,J.Y.;Goh,M.;Lee,J.H.;Ku,B.C.;You, N.H.Macromolecules2013,46(9),3505.doi:10.1021/ ma400185j
(18)Liao,W.H.;Yang,S.Y.;Wang,J.Y.;Tien,H.W.;Hsiao,S.T.; Wang,Y.S.;Li,S.M.;Ma,C.C.M.;Wu,Y.F.ACSAppl. Mater.Interfaces2013,5(3),869.doi:10.1021/am302494c
(19)Yoonessi,M.;Shi,Y.;Scheiman,D.A.;Lebron-Colon,M.; Tigelaar,D.M.;Weiss,R.A.;Meador,M.A.ACSNano 2012,6 (9),7644.
(20)Zhang,X.;Sui,Z.;Xu,B.;Yue,S.;Luo,Y.;Zhan,W.;Liu,B. J.Mater.Chem.2011,21(18),6494.doi:10.1039/c1jm10239g
(21)Ghani,M.A.A.;Abdallah,D.;Kazmaier,P.M.;Keoshkerian, B.;Buncel,E.Can.J.Chem.2004,82(9),1403.doi:10.1139/ v04-106
(22)Wang,G.Q.Water Resources Protection 2007,23(4),85.[王国贤.水资源保护,2007,23(4),85.]
(23)Chen,H.;Yin,J.1,3,5-Tri(4-amino phenoxy)Benzeneand Preparation Method Thereof.CN Patent1405145A,2003-03-26.[陈 焕,印 杰.1,3,5-三(4-氨基苯氧基)苯及其制备方法:中国,CN 1405145A[P].2003-03-26.]
(24)Chidambareswarapattar,C.;Larimore,Z.;Sotiriou-Leventis,C.; Mang,J.T.;Leventis,N.J.Mater.Chem.2010,20(43),9666. doi:10.1039/c0jm01844a
(25)Wu,W.;Wang,K.;Zhan,M,S.Ind.Eng.Chem.Res.2012,51 (39),12821.doi:10.1021/ie301622s
(26)Leventis,N.;Sotiriou-Leventis,C.;Mohite,D.P.;Larimore,Z. J.;Mang,J.T.;Churu,G.;Lu,H.Chem.Mate.2011,23(8), 2250.doi:10.1021/cm200323e
(27)Yang,S.;Feng,X.;Müllen,K.Adv.Mater.2011,23(31),3575. doi:10.1002/adma.201101599
Po lyim ide Aerogels Crosslinked w ith Chem ically Mod ified Graphene Oxide
LIANG Yi LU Yun*YAOWei-Shang ZHANG Xue-Tong*
(SchoolofMaterials Science&Engineering,Beijing Institute ofTechnology,Beijing 100081,P.R.China)
Polyim ide(PI)aerogels,which are generally crosslinked using expensive chem icalcrosslinking agents,are novelporousmaterials w ith high strength,high heat resistance,high porosity,and low density. Graphene oxide(GO)is a functionalnanofiller thathas aroused w ide interest in recentyears.The reported PI/ GO composites havemostly been in the form of fibers and film s.In this study,PI/GO composite aerogels were obtained using chemicallymodified graphene oxide(m-GO)as the crosslinking agent,instead of traditionalones such as 1,3,5-triam inophenoxybenzene(TAB),by reaction w ith 4,4′-oxydianiline(ODA)and 3,3′,4,4′-biphenyltetracarboxylic dianhydride(BPDA).The chem icalmodification ofGO was achieved by reacting GO w ith excess ODAusing a hydrothermalmethod.Them icrostructures of the PI/m-GO aerogels were investigated using scanning electronm icroscopy(SEM).Nitrogen sorption tests,thermogravimetric analysis,and a hot-w ire method were used to investigate the effects ofm-GO on the pore properties,thermalstabilities,and thermal conductivities,respectively,of the resulting aerogels.The results show that the PI/m-GO aerogels are highly porous,thermally stable,and heat insulating.Com pression tests showed that the PIaerogelprepared using 0.6%(mass fraction,w)m-GO instead of1.8%(w)TAB as the crosslinking agenthad a higher specific Young′s modulus[Young′smodulus/density(ρ)]and specific yield strength(yield strength/ρ),and less shrinkage.
O648
icle]
10.3866/PKU.WHXB201504146 www.whxb.pku.edu.cn
Received:December30,2014;Revised:April13,2015;Published onWeb:April14,2015.
∗Corresponding authors.LU Yun,Email:luyun@bit.edu.cn.ZHANG Xue-Tong,Email:zhangxtchina@yahoo.com;Tel:+86-10-68912370.
The projectwassupported by the NationalNatural Science Foundation of China(21373024)and Innovation Program of the Beijing Instituteof Technology,China.
国家自然科学基金(21373024)和北京理工大学创新项目基金资助
©Editorialofficeof Acta Physico-Chim ica Sinica