Hydrothermal synthesis and crystal structure of an organic-inorganic hybrid Keggin-type silicotungstate[FeII(phen)3]2[α-SiW12O40]·H2O
2015-11-27CHAIYunSHAOBoLUOJie
CHAI Yun,SHAO Bo,LUO Jie
(College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,Henan,China)
Polyoxometalates(POMs)are a particular family of metal-oxygen anionic clusters with multitudinous structures and intriguing properties[1].More and more interests have been paid on POMs because of latent functionalities in catalysis,medicine,material science,magnetism and so on[2].In this domain,the exploration and preparation of organic-inorganic hybrid POM-based materials(OIHPBMs)have attracted increasing interest over the past several decades.Integrating transition metals and organic ligands with inorganic POM building units has been developed as an effective approach to manufacture novel OIHPBMs[3].As we know,Keggin POMs are the most important building units and play a crucial role in the development history of POM chemistry.Thus,a series of OIHPBMs have been obtained such as[Cu(bpmpn)(H2O)][GeW12O40{Cu(bpmpn)}]·8H2O(bpmpn=N,N′-dimethyl-N,N′-bis-(pyridine-2-ylmethyl)-1,3-diaminopropane)[4],[HTA]3[α-PW12O40]·6H2O(TA=1,2,4-triazole)[5],[Ag2(4,4′-bpy)2(4,4′-Hbpy)(H2O)][PW12O40](4,4′-bpy=4,4′-bipyridine)[6],[Cu2Cl(phen)4][PW12O40][7]and so on.At the same time,some organicinorganic hybrid Keggin-type silicotungstates(STs)have also been reported.In 2006,KONG et al discovered a series of ST-based coordination polymers{[Cu(2,3-Me2pz)(2,5-Me2pz)0.5]4(SiW12O40)(2,5-Me2pz)}n(2,3-Me2pz=2,3-dimethylpyrazine;2,5-Me2pz=2,5-dimethylpyrazine),{[Cu2(4,4′-bipy)4(H2O)4](SiW12O40)(H2O)18}n(4,4′-bipy=4,4′-bipyridine),{[Cu(pz)1.5]4(SiW12O40)(H2O)3}n(pz=pyrazine)and{[Cu2(4,4′-bipy)4(H2O)4](SiW12O40)(4,4′-bipy)2(H2O)4}nby direct incorporation of POMs into the voids of the 2Dnetwork[8].In 2013,an OIHPBM[CuILo]4[SiW12O40]·5H2O containing copper ions and bichelate bridging ligands(Lo=bis(3-(2-pyridyl)pyrazole-1-ylmethyl)benzene)was addressed by LI et al by the POM-induced assembly approach[9].Since 2012,we have been exploiting the system of the trilacunary[α-SiW9O34]10-precursor,transition-metal and lanthanide cations in the presence of organic ligands by the hydrothermal technique with the goal of discovering novel transition-metal-lanthanide heterometallic STs.Thus,a library of unusual 1Ddouble-chain OIHPBMs[Cu(dap)2(H2O)]2{Cu(dap)2[α-H2SiW11O39Ln(H2O)3]2}·xH2O(Ln=CeIII,GdIII,ErIII,x=9;Ln=PrIII,NdIII,SmIII,EuIII,x=10;Ln=TbIII,DyIII,x=8)(dap=1,2-diaminopropane)[10]and three 3Dheterometallic STs NaH[Cu(dap)2(H2O)][Cu(dap)2]4.5[Ln(α-SiW11O39)2]·7H2O(Ln=SmIII,DyIII,GdIII)built by 1:2-type[Ln(α-SiW11O39)2]13-fragments and[Cu(dap)2]2+bridges[11]have been synthesized in our laboratory.When using phen to take the place of dap and iron ions to repalce copper ions,we obtained an organic-inorganic hybrid Keggin-type ST[FeII(phen)3]2[α-SiW12O40]·H2O(1)(CCDC:1045922).In this paper,we report the synthesis and structural characterization of 1.
1 Experimental
1.1 Physical measurements
Na10[α-SiW9O34]·18H2O was prepared in line with the literature method[12].All other chemicals were obtained from commercial resources and used without further purification.The IR spectrum was recorded from solid sample powder which was palletized with KBr on a Nicolet 170 SXFT-IR spectrometer(4 000-400cm-1).XPS spectra were recorded on an Axis Ultra X-ray photoelectron spectrometer.
1.2 Synthesis of 1
Na10[α-SiW9O34]·18H2O(0.150g,0.054 mmol),Fe2(SO4)3(0.600g,1.500mmol),2,3-pyrazine dicarboxylic acid(0.030g,0.178mmol)and phen(0.030g,0.163mmol)were suspended in 10mL of distilled water(554mmol)under stirring,and ethyl alcohol(85.9mmol)was added.The mixture was stirred for 6.5hat the room temperature,sealed in a Teflon-lined stainless steel autocalve(25mL),heated for 6dat 160℃,and then cooled to ambient temperature.Purple block crystals were obtained.Yield:ca.41%(Based on Na10[α-SiW9O34]·18H2O).Anal.calcd.(%)for C72H50Fe2N12O40SiW12:C,21.25;H,1.24;N,4.13.Found(%):C,21.20;N,1.36;N,4.08.
1.3 X-ray crystallography
Intensity data of 1 were collected on Bruker APEX-II CCD detector at 296(2)K with Mo Kαradiation(λ=0.071 073nm).Its structure was solved by utilizing direct methods and the heavy atoms were found using full-matrix least-squares with the SHELXTL-97software[13].The remaining atoms were found from successive Fourier syntheses.H atoms associated with C and N atoms were placed in calculated positions using a riding model and were refined isotropically using the default SHELXTL parameters.Those H atoms attached to O1Wwere not located.All the non-H atoms were refined anisotropically except for C8,C10,C18,C19,O16,O17and O1W.Table 1provides the crystal data of 1.

Table 1 Crystal data and structural refinements of 1
2 Results and discussion
2.1 Synthesis
With the development of POM chemistry,organic-inorganic hybrid transition-metal containing POMs play a crucial role in the research area of POM chemistry.Recently,we have been devoted to initiate the exploration on the hydrothermal reaction of the[α-SiW9O34]10-precursor with iron cation.By trial and error,a saturated Keggin-type OIHPBM1 was successfully separated.It is obvious that,in the formation of 1,the precursor must undergo the change from trilacunary Keggin[α-SiW9O34]10-unit to saturated Keggin[α-SiW12O40]4-unit.As a matter of fact,in our previous experiments[14],we have encountered this evolution.The bond valence sum(BVS)value for the Fe atom is 1.92,indicating that the oxidation state of Fe atom is+2,which is further confirmed by the XPS spectra.As Fe2(SO4)3was added into the preparation process,the Fe2+ion must result from the reduction of Fe3+ion with phen as a reductive agent.Similar phenomenon has been previously observed under hydrothermal environments[15].
2.2 Description of crystal structure
1 crystallizes in the triclinic space group P-1 and its molecular unit consists of one plenary Keggin[α-SiW12O40]4-anion,two discrete[FeII(phen)3]2+cations and one lattice water molecule(Fig.1a).There are comparatively stronger electrostatic interactions between[α-SiW12O40]4-anions and discrete[FeII(phen)3]2+cations in the solid state,which can be further consolidated by the results of IR spectra.The[α-SiW12O40]4-unit(Fig.1b)is built up of four corner-sharing W3O13groups and a central SiO4group.Each W3O13group is formed by three edge-sharing WO6octahedra.Four W3O13groups tetrahedrally surround the central Si heteroatom giving rise to the idealized Tdsymmetry of the[α-SiW12O40]4-unit.The Si-O distances fall in the range of 0.149(3)-0.174(4)nm and the W-O bond lengths are between 0.163 7(18)and 0.253(3)nm.It’s somewhat different from mono-,bi-and multi-metal substituted Keggin clusters such as[SiW11O39Ni(H2O)]6-[16],[{β-Ti2SiW10O39}4]24-[17]and[{γ-SiW8O31Cu3(OH)(H2O)2(N3)}3(N3)]19-[1].It can be clearly seen from Fig.1athat two discrete[FeII(phen)3]2+coordination cations are symmetrically located on both sides of the[α-SiW12O40]4-unit.In the[Fe(phen)3]2+cation,the Fe12+cation is defined by six N atoms from three phen ligands,resulting in the octahedral configuration of the Fe12+cation(Fig.1c).The Fe-N distances vary from 0.192 9(18)to 0.199(2)nm.More interestingly,all the[FeII(phen)3]2+and[α-SiW12O40]4-ions are aligned regularly in the three-dimensional space.As shown in Fig.2,discrete[Fe(phen)3]2+coordination cations are distributed in the clearance formed by[α-SiW12O40]4-units.Such distribution mode is favorable to impair the steric hindrance and create a closest packing of crystal structure.

Fig.1 (a)The molecular structure of 1.Lattice water molecule is omitted for clarity.Symmetry code A:2-x,1-y,2-z;(b)The structure of the saturated Keggin-type[α-SiW12O40]4-unit in 1;(c)The octahedral geometry of the Fe12+ion

Fig.2 The packing of 1viewed along the baxis
2.3 IR spectra
Fig.3 exhibits the IR spectra of 1,phen(2)and Na10[α-SiW9O34]·18H2O(3).Four characteristic vibration peaks resulting from the Keggintype[α-SiW12O40]4-unit are observed in the lowwavenumber region in the IR spectrum of 1.Four characteristic stretching vibration peaks,namely,ν(W-Oc),ν(W-Ob),ν(Si-Oa)andν(W-Ot)are observed at 793,875,917and 966cm-1,respectively[18].As to 3,ν(W-Oc),ν(W-Ob),ν(Si-Oa)andν(W-Ot)appear at 797,866,935 and 985cm-1,respectively.The difference of the IR spectra in the low-wavenumber region of 1 and 3 confirms the conversion of the[α-SiW9O34]10-unit to the[α-SiW12O40]4-unit.In addition,two vibration peaks of 2 at 3 066cm-1is assigned to ν(Ar-H)and 1 421cm-1corresponds to the characteristic of the skeletal vibration of aromatic ring,which are also observed at 3 058and 1 425cm-1in1,indicating the presence of phen in the structure of 1.Other else,the wide vibration band centered at 3 432cm-1in 1 is indicative of the lattice water molecule.

Fig.3 The IR spectra of 1,2and 3
2.4 XPS spectrum
In order to identify the oxidation state of the Fe2+ion,XPS analysis of 1 was carried out.The peak of Fe2p3/2at 706.4eV(Fig.4a)indicates that the Fe ion is+2,which is in agreement with previous result[19].Moreover,two characteristic XPS peaks at 33.4and 35.5eV which are ascribed to W4f7/2and W4f5/2(Fig.4b)confirm the presence of the W(VI)centers in 1 .
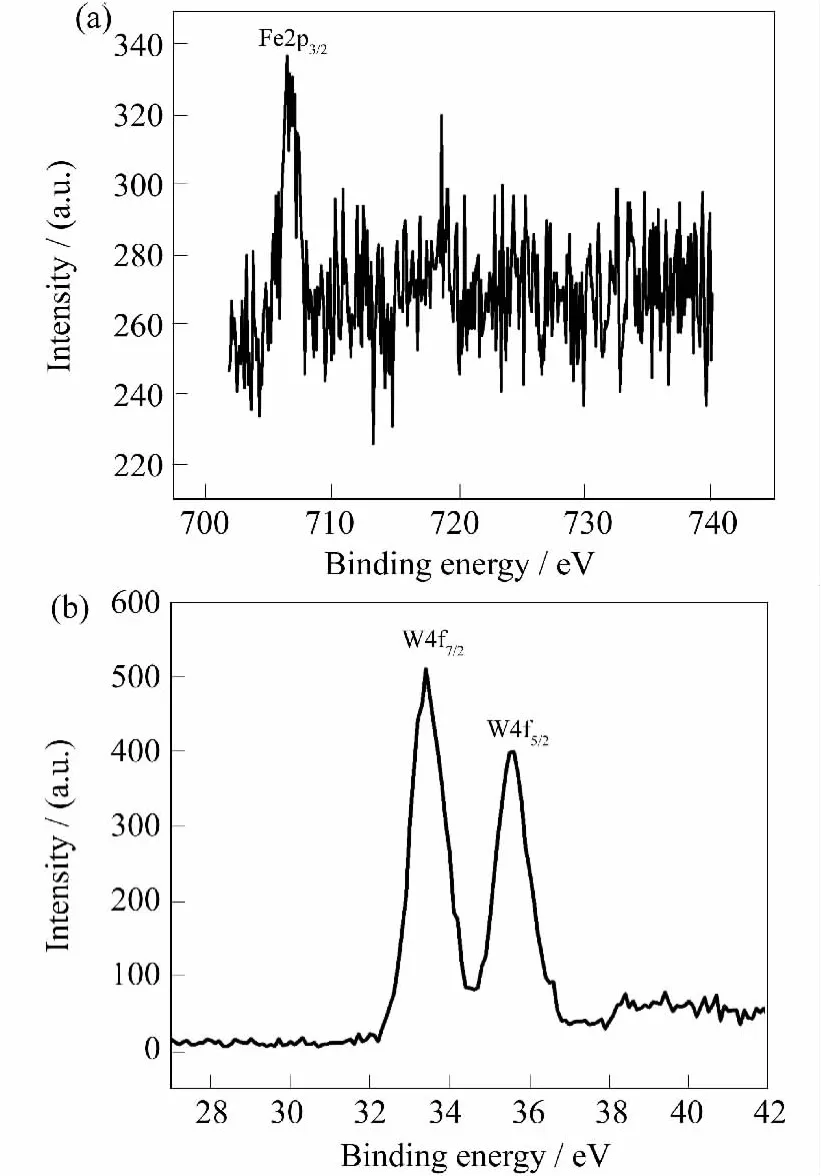
Fig.4 XPS spectrum of 1
3 Conclusion
In conclusion,an OIHPBM[FeII(phen)3]2[α-SiW12O40]·H2O has been hydrothermally made and its structure has been elucidated by IR spectrum,X-ray crystallography and XPS spectrum.In the following time,we will spare no effort to exploit the reactions of different POM precursors with transition-metal and lanthanide cations in the different functional organic ligands.
[1]PIERRE M,ANNE D,JÉRÔME M,et al.A nonanuclear copper(II)polyoxometalate assembled around aμ-1,1,1,3,3,3-azido ligand and its parent tetranuclear complex[J].Chem Eur J,2005,11:1771-1778.
[2]PROUST A,MATT B,VILLANNEAU R,et al.Functionalization and post-functionalization:a step towards polyoxometalate-based materials[J].Chem Soc Rev,2012,41:7605-7622.
[3]WEI M L,CHENG H,SUN Q Z,at al.Zeolite ionic crystals assembled through direct incorporation of polyoxometalate clusters within 3Dmetal-organic frameworks[J].Inorg Chem,2007,46:5957-5966.
[4]AMAIA I,LEIRE S F,SANTIAGO R,et al.Reversible dehydration in polyoxometalate-based hybrid compounds:a study of single-crystal to single-crystal transformations in Keggin-type germanotungstates decorated with copper(II)complexes of tetradentate N-donor ligands[J].Cryst Growth Des,2014,14:2318-2328.
[5]齐秀丽,赵浩哲,张景丽,等.有机-无机复合磷钨酸盐[HTA]3[α-PW12O40]·6H2O的合成与晶体结构[J].化学研究,2014,25(1):8-12.
[6]YANG H X,GAO S Y,LÜJ,et al.pH-dependent syntheses and crystal structures of a series of organic-inorganic hybrids constructed from Keggin or Wells-Dawson polyoxometalates and silver coordination compounds[J].Inorg Chem,2010,49:736-744.
[7]YANG H X,GUO S P,JUN T,et al.Hydrothermal syntheses,crystal structures,snd magnetic properties of a series of complexes constructed from multinuclear copper clustersand polyoxometalates[J].Cryst Growth Des,2009,9:4735-4744.
[8]KONG X J,REN Y P,ZHENG P Q,et al.Construction of polyoxomatelates-based coordination polymers through direct incorporation between polyoxometalates and the voids in a 2Dnetwork[J].Inorg Chem,2006,45:10702-10711.
[9]WANG X,ZHANG M M,HAO X L,et al.Polyoxometalate-induced new self-assemblies based on copper ions and bichelate-bridging ligands[J].Cryst Growth Des,2013,13:3454-3462.
[10]ZHAO J W,LUO J,CHEN L J,et al.Novel 1-D double-chain orgnic-inorgnic hybrid polyoxometalates constructed from dimeric copper-lanthanide heterometallic silicotungstate units[J].CrystEngComm,2012,14:7981-7993.
[11]LUO J,LENG C L,CHEN L J,et al.Three 3Dorganic-inorganic hybrid heterometallic polyoxotungstates assembled from 1:2-type[Ln(α-SiW11O39)2]13-silicotungstates and[Cu(dap)2]2+linkers[J].Synth Met,2012,162:1558-1565.
[12]HERVÉA,TÉZÉG.Study ofα-andβ-enneatungstosilicates and-germanates[J].Inorg Chem,1977,16:2115-2117.
[13]SHELDRICK,G.M.SHELXS 97,Program for crystal structure solution[CP].Germany:University of Göttingen,1997.
[14]LUO J,MA X,CHEN L J,et al.Synthesis,structure and properties of an organic-inorganic hybrid independent 1-D double-chain Keggin-type silicotungstate with mixed ligands[J].Inorg Chem Commun,2015,54:25-30.
[15]ZHAO J W,CHENG Y M,SHANG S S,et al.Syntheses,structures and properties of three new two-dimensional CuI-LnIIIheterometallic coordination polymers based on 2,2′-dipyridyl-5,5′-dicarboxylate ligands[J].Spectrochim Acta Part A:Mol Biomol Spectr,2013,116:348-354.
[16]LIU H S,GÖMEZ-GARCÍA C J,PENG J,et al.3Dtransition metal mono-substituted Keggin polyoxotungstate with an antenna molecule:synthesis,structure and characterization[J].Dalton Trans,2008:6211-6218.
[17]HUSSAIN F,BASSIL B S,BI L H,et al.Structural control on the nanomolecular scale:self-assembly of the polyoxotungstate wheel[{β-Ti2SiW10O39}4]24-[J].Angew Chem Int Ed,2004,43:3485-3488.
[18]LUO X Z,LI F Y,XU B B,et al.Enhanced photovoltaic response of the first polyoxometalate-modified zinc oxide photoanode for solar cell application[J].J Mater Chem,2012,22:15050-15055.
[19]CHEN W L,Li Y G,WANG Y H,et al.One-pot assembly of a new mixed-valent aggregate based on inorganic polyoxometalate ligands[J].Z Anorg Allg Chem,2009,635:1678-1687.