Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
2015-10-28GaneshNandhiniSistlaSujatha
Ganesh Nandhini, Sistla Sujatha
Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research , Puducherry, India- 605006
Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
Ganesh Nandhini, Sistla Sujatha*
Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research , Puducherry, India- 605006
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
Influenza
Pandemic
Post-pandemic
Influenza-like illness
India
Objective: To report the findings of influenza surveillance programme from Union territory of Puducherry and to document the clinical and epidemiological data of influenza viruses over a five year period from 2009-2013. Methods: Respiratory samples were collected from patients with influenza-like illness from 2009-2013 as part of routine diagnostic and surveillance activity. Detection of pandemic influenza A (H1N1) 2009, influenza A (H3N2) and influenza B was done using Real-time PCR. Results: Of the total 2 247 samples collected from patients with influenza-like illness during the study period 287 (12.7%) and 92 (4.0%) were positive for influenza A (H1N1) 2009 and influenza A (H3N2) respectively. A subset of 557 of these samples were also tested for influenza B and 24 (4.3%) were positive. Significantly higher positivity rate for both viruses was observed in adults when compared with children. The peak positivity of influenza A (H1N1) 2009 was observed in 2009 followed by 2012, while that of influenza A (H3N2) was more uniformly distributed with the exception of 2012. Overall mortality rate due to influenza A (H1N1) 2009 was 7.6% while it was 1% for influenza A(H3N2). Each year influenza-like illness and influenza virus activity coincided with period of high rainfall and low temperature except in the first half of 2012. Conclusions: As the sole referral laboratory in this region, the data provides a comprehensive picture of influenza activity. This information will be useful in future planning of the vaccine schedule and influenza pandemic preparedness.
1. Introduction
Influenza viruses have the potential to cause contagious respiratory illness ranging from mild flu to severe respiratory illness resulting in death. In April 2009, a new influenza A virus (H1N1) emerged abruptly and within a few weeks there was a global spread of this virus leading to more than 4 500 deaths by October 2009[1]. The first case of pandemic influenza in India was reported from Hyderabad in May 2009[2], immediately after which Influenza surveillance system was put in place with the support of National Center for Disease Control, New Delhi and World Health Organization. Twelve regionallaboratories were set up across India under Integrated Disease Surveillance Project network with the aim to provide information regarding national and local influenza activity and control efforts. As a part of this network, in July 2009, Regional Influenza Laboratory was set up at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, to diagnose influenza infection in patients referred from government and private hospitals in and around Union territory of Puducherry. From November 2011, this laboratory started sentinel surveillance activity with three sentinel centers; two from Puducherry (JIPMER hospital and Indira Gandhi Government General Hospital) and one from Karaikal (Karaikal Government General Hospital). As the sole referral laboratory from this part of the country, it gives a nearly complete picture of influenza activity in this region. To the best of our knowledge, there are no epidemiological studies on influenzaburden reported from this territory post-influenza A (H1N1) 2009 pandemic. Hence, the aim of this study was to report the findings of influenza virus surveillance from Union territory of Puducherry,and to document the clinical and epidemiological data of influenza viruses.
2. Materials and methods
2.1. Geographic details of Union territory of Puducherry
The Union territory of Puducherry has a hot and humid climate. It lies at latitude of 11º46´to 12º30´ North and a longitude of 79º36´to 79º52´East in the Southern part of India. The territory extends over an area of 479 Sq. km on the Coromandel Coast of Bay of Bengal. Total population is 1 244 464 as per the 2011 census. The average maximum temperature is 31.5 ℃ and average minimum temperature is 23.9 ℃. Summer is from March through July and winter is from December through February. The rainy months of this area are September to December. Meteorological (temperature and rainfall)data of Puducherry for the period between November 2011 and December 2013 were obtained from the Regional Meteorological Centre, Chennai [No. 8043/CS-(ER) -032 dated 30-07-2014].
2.2. Routine influenza testing during pandemic and postpandemic periods
Clinical specimens including nasal/throat/nasopharyngeal swabs/ tracheal aspirates were collected by physicians from patients with severe respiratory illness, admitted to government and private hospitals in and around Puducherry and sent to Regional Influenza laboratory at JIPMER in cold chain. Immediate testing for influenza A (H1N1) 2009, influenza A (H3N2) and influenza B virus identification was done by reverse transcriptase real-time polymerase chain reaction according to Centers for Disease Control and Prevention protocol[3].
2.3. Influenza-like illness (ILI) surveillance
Apart from routine influenza testing, according to the guidelines of National Influenza-like illness surveillance programme,nasopharyngeal samples were collected from patients (both children and adults) with symptoms of ILI defined as fever ( 38 ℃) plus either cough or sore throat, attending the outpatient care facilities in the 3 sentinel centers; JIPMER hospital, Indira Gandhi Government General Hospital and Karaikal Government General Hospital(Figure 1) In addition, respiratory specimens were also collected from hospitalized patients with severe acute respiratory illness(Figure 2)[4]. Patients with a history of acute respiratory infection in the preceding 30 d were excluded. Patient information including age, gender, clinical presentation, underlying conditions, outcome, travel and treatment details were recorded using a structured proforma.
2.4. Nucleotide sequencing and phylogenetic analysis
Sequencing of hemagglutinin (HA) gene and neuraminidase(NA) gene was done for representative swine influenza A (H1N1)2009 isolates of each year using gene-specific forward and reverse primers. The nucleotide sequence data from the study isolates were deposited in National center for Biotechnology information-GenBank with the accession numbers KM654292, KM654293,KM654294 and KM654295.
2.5. Statistical methods
The proportions were presented in percentages wherever applicable. The positivity rate across age groups, gender and inpatient-outpatient details were compared by the Chi-square test using Openepi software version 3.03. Statistical significance was concluded if the P-value was <0.05.
3. Results
3.1. ILI activity during 2009-2013
During the study period (May 2009-December 2013), respiratory samples were collected from 2 247 patients with symptoms of influenza-like illness. Among the total, 523 (23%), 429 (19%),153 (7%), 717 (32%) and 425 (19%) samples were collected in 2009, 2010, 2011, 2012 and 2013 respectively (Table 1). Out of 2 247 samples collected, 1314 (58%) were from out patients and 933 (42%) were from patients who were hospitalized with severe respiratory complications; 1 691 (75%) were adults (>13 years),556 (25%) children (A: C ratio, 3:1); 1 104 (49%) were male and 1 143 (51%) were female (M: F ratio, 1:1). Maximum numbers (52%)of patients were in the age group of 20-49 years, followed by 5-19 years (19%), 0-4 years (15%), 50-64 years (10%) and 65 years(4%). Major clinical symptoms in the patients were fever, cough,sore throat and dyspnea (Table 2).

Table 1 Details of influenza-like illness activity during 2009-2013 [n (%)].
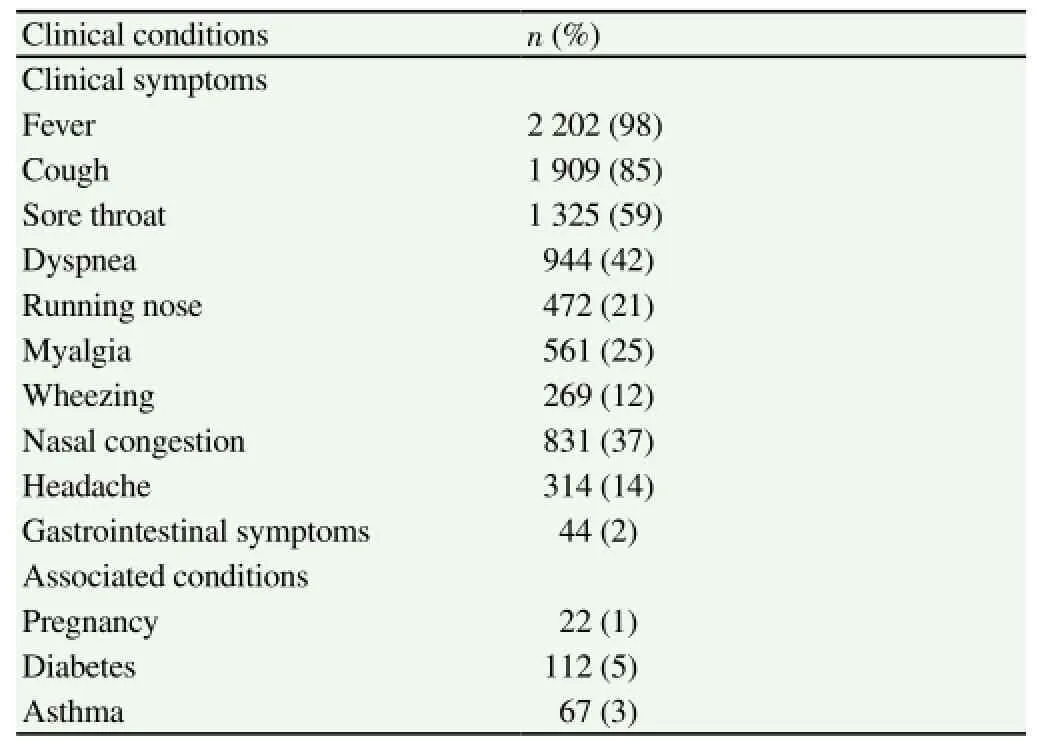
Table 2 Clinical presentation and underlying conditions of ILI patients.
3.2. Virological surveillance
During 2009-2013 influenza season, 379 (17%) patients tested positive for influenza A viruses using real-time polymerace chain reaction. Among them, 287 (76%) and 92 (24%) tested positive for influenza A (H1N1) 2009 and influenza A (H3N2) respectively(Table 3& 4). Starting from November 2011, a subset of 557 samples were also tested for influenza B with 24 (4.3%) testing positive; 13 were children and 11 were adults; 16 male, 8 female. No significant difference in the influenza positivity was observed based on the area of residence of ILI patients (Table 5).
Of the 287 influenza A (H1N1) positive patients, most were adults (79%). As expected, young and middle aged patients (20-49 years) were affected more by this pandemic strain (58%) followed by patients in 5-19 years age group (23%). Similarly, influenza A(H3N2) positivity was higher in adults (87%) particularly in 20-49 year age group (63%). No difference was observed in influenza infections among outpatients and inpatients. Both male and female patients were almost equally affected by influenza. Among the total influenza A (H1N1) 2009 positives, 9% reported history of travel in the last 8 d before the onset of symptoms and 18% had exposure to suspected/confirmed cases with respiratory symptoms.
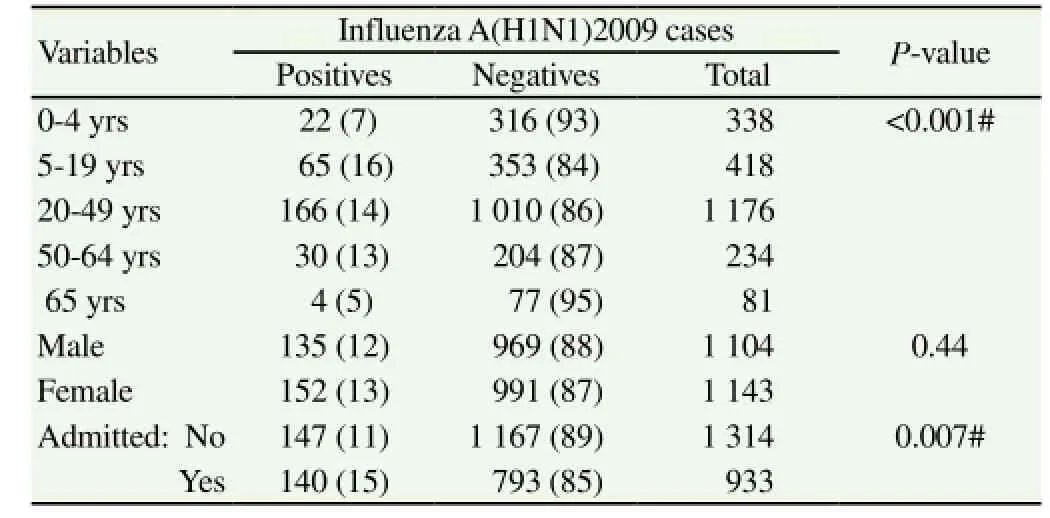
Table 3 Details of influenza A(H1N1)2009 during 2009-2013 [n (%)].

Table 4 Details of influenza A(H3N2) during 2009-2013 [n (%)].
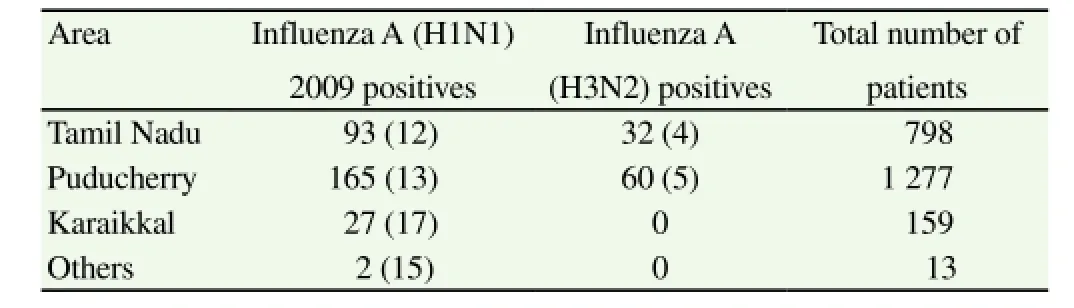
Table 5 Geographic details of the total number of ILI patients and influenza positives during 2009-2013 [n (%)].
3.3. Clinical complications and mortality
In the study duration of 5 years (2009-2013), 933 (42%) patients were hospitalized due to severe respiratory complications. Majority of the hospitalized patients were in the age group of 20-49 years(59%, 554/933); the most predominant reason for hospitalization was acute respiratory distress syndrome. Of the total hospitalized,184 (20%) patients tested positive for influenza A viruses; 140(15%), 39 (4%) and 5 (0.5%) tested positive for swine influenza A(H1N1)2009, seasonal influenza A(H3N2) and influenza B viruses respectively.
In total, 33 patients died during the study period due to severe respiratory complications of whom 23 had influenza A [22- influenza A (H1N1) 2009, 1-influenza A (H3N2)]; All the 22 patients who died of severe respiratory complications due to influenza A (H1N1)2009 infection were adults (Figure 3), 13 were female, 9 were male; 2 had diabetes mellitus and none were asthmatic or pregnant. Interestingly 13 patients had already received Oseltamivir before sample collection; however time and day of start of treatment were not recorded for all the cases.
自体输血也有禁忌证,以下患者不适合自体输血:采血可能诱发疾病加重者、菌血症患者、贫血、肝肾功能不良或严重心脏病等患者[20]。
3.4. Seasonal distribution of ILI and influenza
Influenza infection was at the peak during the pandemic outbreak of 2009 (August-December), where 40% (116/287) of the swine influenza A (H1N1) 2009 and 41% (38/92) of the seasonal influenza A (H3N2) cases were recorded (Figure 4). In 2010, there was a sharp peak of swine influenza positives in September and October (62 cases) after which in 2011, it came to a low with only 4 cases (1%). Again in 2012, there was increased swine influenza activity, where 35% (100/287) of the influenza A (H1N1) 2009 were observed. However, unlike in 2009 and 2010, where swine influenza cases peaked in the 2nd half of the year (August-December), 70% of the cases in 2012 were observed in 1st half (March-June) of the year(70/100). The number of seasonal influenza cases was less than swine influenza throughout the study period (1:3) and 2012 had no positive cases.
3.5. Sequencing
Sequencing of HA and NA gene was done for representative swine influenza A (H1N1) 2009 isolates of each year and all the sequences(KM654292, KM654293, KM654294 and KM654295) showed98%-99% homology with the clade Ⅰ reference strain Influenza A/ California/07/2009 and there were no significant mutations seen in these sequences.
4. Discussion
During this study, the number of ILI patients varied from year to year, more ILI cases were observed in 2009, in response to the alert created by the spread of influenza A (H1N1) 2009 pandemic throughout the world, as also in 2012, which again saw a sharp rise in the number of influenza A (H1N1) 2009 cases.
In our study, the contribution of influenza to ILI was approximately 21% which corroborates several reports from all over the world[5-7]. The predominant type of influenza A virus in this study was pandemic influenza A(H1N1)2009.This finding is consistent with other reports from Delhi and Lucknow[8,9]. However, contradictory reports of increased seasonal influenza A (H3N2) have been reported from China during the same period[10].
Mortality rate of influenza A (H1N1) 2009 infection was 1% and similar scenario was observed in state of Kolkata (1%) and it is lower than reports from Gujarat (19%), Brazil (11%)[17-19]. Low mortality rate could be because of timely admission, immediate initiation of oseltamivir prophylaxis in the suspected cases and surplus availability of oseltamivir due to the awareness created by the pandemic. Although the mortality rate was low, it is of concern that 13 of 22 patients who died of pandemic influenza infection had received oseltamivir prior to sample collection; hence studies are ongoing to detect mutations involved in resistance, if any. Oseltamivir is the oral anti-viral drug for the treatment of influenza A and B viruses and sporadic cases of oseltamivir resistant swine influenza A (2009) have been reported since 2009. A specific mutation causing a histidine to tyrosine substitution (H275Y) is known to confer oseltamivir resistance in these swine influenza viruses. In India, Potdar et al, reported a single isolate of pandemic influenza A (H1N1) resistant to oseltamivir in 2013 with H275Y mutation[20]. Although the sequencing analysis of the representative influenza isolates in this study showed no significant mutations in the HA and NA genes, continuous monitoring of genetic analysis for oseltamivir resistance is important as it is the most widely used antiviral drug against influenza.
Worldwide, it has been reported pandemic influenza A (H1N1)2009 infected young adults more than any other group of patients[21]. Similarly, in this study maximum number of pandemic flu positivity was observed in young adults. There are a few studies from western countries where more number of children were affected by pandemic influenza than adults[22,23], however in those studies the definition of children used was 18 years unlike in the present study here 13 years is used as cut off. In 2011-2012, there were reports of shift in the age distribution of pandemic influenza cases, and more elderly individuals aged >60 years were affected[24], but such association was not observed in our study, and consistently it was individuals aged 20-49 years who were affected more due to the pandemic influenza infection. Although it is proposed that children and the elderly are prone to seasonal influenza infection[25], no such association was observed between the ages and as such the number of cases of seasonal influenza were low throughout the study period in this region. The seasonal influenza A (H3N2) positivity was only 4% compared to a study from Pune, India where equal proportion of seasonal and pandemic influenza was reported[26]. This observation cannot be explained unless a detailed analysis of climatic factors like humidity, pressure and healthy seeking behavior of patients for mild upper respiratory infections is studied.
Each year influenza-like illness activity was found to be consistently high during the months of lower mean temperature and maximum rainfall in this region (September-November), except in 2012 when the first phase of influenza positives came in peak summer season (March-May) followed by the second phase in rainy months. Also, it was observed that the number of patient visits was higher during the periods of increased rainfall in this region. The pattern of influenza observed here showed a unimodal peak during the monsoon season and this pattern is reported in other studies from India and other tropical state like Brazil[9,27]. The reason may be due to crowding in houses and increased risk of transmission of influenza.
To the best of our knowledge, the present study is the first to report prevalence and seasonality of influenza viruses, in and around Union territory of Puducherry from 2009 to 2013. Influenza A viruses are still considered to be a threat to human society due to their frequent mutations, hence continuous monitoring of antigenic variants of influenza isolates and trend of association with seasonal factors is necessary to help in future pandemic preparedness.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We acknowledge National Center for Disease Control, New Delhi for technical support and Integrated Disease Surveillance Programme, New Delhi for financial support.
[1] Pandemic (H1N1) 2009 - update 69. World Health Organization; 2014.[Online] Available from: http://www.who.int/csr/don/2009_10_09/en/
[2] First confirmed case of swine flu in India. The times of India. [Online]Available from: http://timesofindia.indiatimes.com/india/Firstconfirmedcase-of-swine-flu-in-India/articleshow/4538930.cms.[Accessed on 2009 May 16]
[3] CDC protocol of realtime RTPCR 2009. [Online] Available from: http:// www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRpr otocol_20090428.pdf.
[4] WHO surveillance case definitions for ILI and SARI World Health Organization; [Online]Available from: http://www.who.int/influenza/ surveillance_monitoring/ili_sari_surveillance_case_definition/en/.[Accessed on 2014 Jan]
[5] Maman I, Badziklou K, Landoh ED, Halatoko AW, Nzussouo TN,Defang GN, et al. Implementation of Influenza-like illness Sentinel Surveillance in Togo. BMC Public Health 2014; 14(1): 981.
[6] Chadha MS, Hirve S, Dawood FS, Lele P, Deoshatwar A, Sambhudas S, et al. Burden of seasonal and pandemic influenza-associated hospitalization during and after 2009 A (H1N1) pdm09 pandemic in a rural community in India. PLoS ONE 2013; 8(5): e55918.
[7] Bellei N, Carraro E, Perosa A, Granato C. Patterns of influenza infections among different risk groups in Brazil. Braz J Infect Dis 2007; 11(4):399-402.
[8] Choudhry A, Singh S, Khare S, Rai A, Rawat DS, Aggarwal RK, et al. Emergence of pandemic 2009 influenza A H1N1, India. Indian J Med Res 2012; 135(4): 534-537.
[9] Dangi T, Jain B, Singh AK, Mohan M, Dwivedi M, Singh JV, et al. Influenza virus genotypes circulating in and around Lucknow, Uttar Pradesh, India, during post pandemic period, August 2010-September 2012. Indian J Med Res 2014; 139(3): 418-426.
[10] Chen JF, Sun BC, Yuan J, Zhang RS, Ou XH. Surveillance of influenza virus during 2010-2012 in Changsha, China. Southeast Asian J Trop Med Public Health 2014; 45(2): 319-325.
[11] Broor S, Chahar HS, Kaushik S. Diagnosis of influenza viruses with special reference to novel H1N1 2009 influenza virus. Indian J Microbiol 2009; 49(4): 301-307.
[12] Shrikhande S, Tenpe S, Deogade N, Bhoyar S. Epidemiology of pandemic H1N1 strains in a tertiary hospital of Maharashtra. Indian J Public Health 2012; 56(3): 242.
[13] Mehta AA. Clinical profile of patients admitted with swine-origin influenza a (H1N1) virus infection: an experience from a tertiary care hospital. J Clin Diagn Res 2013; 7(10): 2227-2230.
[14] Sarkar M, Agrawal AS, Sharma Dey R, Chattopadhyay S, Mullick R, De P, et al. Molecular characterization and comparative analysis of pandemic H1N1/2009 strains with co-circulating seasonal H1N1/2009 strains from eastern India. Arch Virol 2011; 156(2): 207-217.
[15] Elliot AJ, Powers C, Thornton A, Obi C, Hill C, Simms I, et al. Monitoring the emergence of community transmission of influenza A/ H1N1 2009 in England: a cross sectional opportunistic survey of self sampled telephone callers to NHS Direct. BMJ 2009; 339: b3403-b3403.
[16] H1N1 in post-pandemic period. World Health Organization. [Online]Available from: http://www.who.int/mediacentre/news/statements/2010/ h1n1_vpc_20100810/en/[Accessed on 2010 Aug].
[17] Biswas DK, Kaur P, Murhekar M, Bhunia R. An outbreak of pandemic influenza A (H1N1) in Kolkata, West Bengal, India, 2010. Indian J Med Res 2012; 135(4): 529-533.
[18] Rana H, Parikh P, Shah AN, Gandhi S. Epidemiology and clinical outcome of H1N1 in Gujarat from July 2009 to March 2010. J Assoc Physicians India 2012; 60: 95-97.
[19] Pariani E, Martinelli M, Canuti M, Jazaeri Farsani SM, Oude Munnink BB, Deijs M, et al. Influenza and other respiratory viruses involved in severe acute respiratory disease in northern italy during the pandemic and postpandemic period (2009-2011). BioMed Res Int 2014; 2014: 1-5.
[20] Potdar VA, Padbidri VV, Chadha MS. Oseltamivir-resistant influenza A(H1N1) pdm09 virus: first reported case from India. WHO South-East Asia J Public Health 2013; 2(1): 6.
[21] Delaney JW, Fowler RA. 2009 influenza A (H1N1): a clinical review. Hosp Pract 1995. 2010; 38(2): 74-81.
[22] Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010; 375(9720): 1100-1108.
[23] Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational populationbased study. Lancet 2010; 376(9755): 1846-1852.
[24] Chowell G, Echevarría-Zuno S, Viboud C, Simonsen L, Grajales Muñiz C, Rascón Pacheco RA, et al. Recrudescent wave of pandemic A/H1N1 influenza in Mexico, winter 2011-2012: Age shift and severity. PLoS Curr 2012; 4: RRN1306.
[25] Lagacé-Wiens PRS, Rubinstein E, Gumel A. Influenza epidemiologypast, present, and future. Crit Care Med 2010; 38: e1-e9.
[26] Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza(H1N1) 2009 is associated with severe disease in India. PLoS ONE 2010;5(5): e10540.
[27] Mello WA de, Paiva TM de, Ishida MA, Benega MA, Santos MC dos,Viboud C, et al. The dilemma of influenza vaccine recommendations when applied to the tropics: The Brazilian case examined under alternative scenarios. PLoS ONE 2009; 4(4): e5095.
15 June 2015
Sistla Sujatha, Department of Microbiology, JIPMER,Puducherry, India- 605006.
Tel: +91 9894058062
E-mail: sujathasistla@gmail.com
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of CXCR4 pretreated with ultrasound-exposed microbubbles on accelerating homing of bone marrow mesenchymal stem cells to ischemic myocardium in AMI rats
- Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma
- Anti-proliferation and radiosensitization effects of chitooligosaccharides on human lung cancer line HepG2
- Clinical significance of microRNA-130b in osteosarcoma and its role in cell growth and invasion
- Effect of microRNA-208a on mitochondrial apoptosis of cardiomyocytes of neonatal rats
- Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins
