Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins
2015-10-28JinYangChenXiaoZhouMouXiaoChunDuCharlieXiang
Jin-Yang Chen, Xiao-Zhou Mou, Xiao-Chun Du, Charlie Xiang,*
1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, College of Medicine, Zhejiang University,Hangzhou 310003, China
2S-Evans Biosciences, Hangzhou 311121, China
3Clinical Research Institute, Zhejiang Provincial People's Hospital, Hangzhou 310014, China
Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins
Jin-Yang Chen1, Xiao-Zhou Mou3, Xiao-Chun Du2, Charlie Xiang1,2*
1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, College of Medicine, Zhejiang University,Hangzhou 310003, China
2S-Evans Biosciences, Hangzhou 311121, China
3Clinical Research Institute, Zhejiang Provincial People's Hospital, Hangzhou 310014, China
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
Adipose
Umbilical cord
Menstrual blood
Mesenchymal stem cell
Objective: To invest the differences among mesenchymal stem cells (MSCs) derived from different tissues and their impacts on clinical applications. Methods: In this study, MSCs were isolated from adipose tissue (AD), umbilical cord tissue (UC), and menstrual blood(Men) and comparedtheir biological characteristics in terms of proliferation capacity, passage capacity, colony formation, and surface markers were compared. Results: The stem cells(SCs) obtained from different sources were all characterized as MSCs, but demonstrated some differences. Umbilical cord-derived MSCs (UCMSCs) were able to overcome density inhibition. The proliferation rate decreased in the order UCMSCs> MenSCs> ADSCs, while the colony-forming ability decreased in the order MenSCs> ADSCs> UCMSCs. Based on gene-expression data for MSCs from different sources within the same donor, 768 MenSC genes were found that were specifically upregulated or downregulated compared with bone marrow-derived MSCs and UCMSCs, most of which were involved in cell function-related pathways. In addition, MenSCs appeared to be superior in terms of immune inflammation,stress response, and neural differentiation potentials, but weaker in terms of osteogenic and chondrogenic differentiation capacities, compared with UCMSCs and bone marrow-derived MSCs. Conclusions: MenSCs have higher extraction efficiency, colony-forming ability, and long time passage capacity. Although the proliferation capacity is inferior to UCMSCs.
1. Introduction
Mesenchymal stem cells (MSCs) are a class of adult stem cells with characteristics of self-renewal and pluripotency. MSCs can not only differentiate into mesenchymal cell lineages, but also into nonmesenchymal cell lineages, including astrocytes, oligodendrocytes,and neurons. Studies on the pluripotency of MSCs have laid a solid foundation for their clinical application in the field of regenerative medicine[1-3].
Current research on MSCs is mainly focused on their selfrenewal capacity, multi-lineage differentiation potential, surface markers, and immune regulation. Several studies have shown that MSCs derived from different tissues demonstrate a certain extent differences in terms of some of the above-mentioned aspects.
Although the immunophenotypes of MSCs comply with the minimum standards of the International Society for Cellular Therapy, there is no agreement on the expression of other molecular markers. For example, amniotic fluid-derived MSCs sustainably expressed embryonic stem cell-specific markers such as Nanog,SSEA-4 and OCT-4. OCT-4 expression levels were 9.4 times higher than in bone marrow-derived MSCs (BMSCs), while SSEA-4 expression was low in BMSCs but high in amniotic fluidderived MSCs[4]. Menstrual blood-derived stem cells (MenSCs)are novel stem cells with the basic characteristics of MSCs, but expressing different molecular markers. Meng et al found that MenSCs expressed OCT-4, but not NANOG or SSEA-4[5-7], whileRossignoli et al[8] reported that 19.4% of MenSCs were SSEA-4-positive by flow cytometry. Zemel'ko et al[9] showed high expression of SSEA-4 by immunofluorescence.
MSCs from different tissues also display significant differences in proliferative potential. Barlow et al[10] suggested that the proliferation rate of adipose-derived mesenchymal stem cells(ADSCs) was much higher than that of BMSCs or placenta-derived MSCs. However, placenta-derived MSCs were able to maintain a longer proliferative phase and be continuously cultured for up to 160 d, with up to 64 population doublings. In contrast, BMSCs can only be continuously cultured for 60 d, with up to 12 population doublings[10]. Another study reported that the population-doubling time in umbilical cord-derived mesenchymal stem cells (UCMSCs)was much lower than that of ADSCs, but UCMSCs showed earlier morphological changes and a more rapid decline in amplification ability[9-11]. Some studies found that amniotic fluid-derived MSCs and ADSCs exhibited different levels of telomerase activity[12],suggesting different amplification characteristics.
Studies on the differentiation potentials of MSCs from various sources have confirmed the differentiation potential of mesoderm three-line, but their capacity to differentiate into cells of other lineages remains controversial. Panepucci et al[13] suggested that the functional difference between MSCs from different sources may be related to the origin of the cells. BMSCs are prone to differentiate into osteoblasts, while UCMSCs are prone to angiogenesis, confirming the 'imprint' differentiation potential theory of MSC lineage proposed by Satomura et al[14]. However, Liu et al[15] believed that the strong potential of BMSCs to differentiate into osteoblasts was due to the presence of more osteogenic and chondrogenic progenitor cells in BMSC cultures, rather than in the inherent characteristics of BMSCs. Numerous studies have investigated the differentiation potential of MSCs. Some researchers reported that sex differences affected the osteoblast-cytogenesis efficiency of MSCs, with ADSCs from male donors being more likely to differentiate than those from female donors[16]. Researchers also found that the osteogenic, but not adipogenic potential of ADSCs declined with age[17].
Low immunogenicity and immune regulation are important features of MSCs, making them suitable for allotransplantation. Melief et al showed that, although both BMSCs and ADSCs had immunomodulatory functions, differences in cytokine secretion led to ADSCs having a stronger immunomodulatory function, equal to that of BMSCs[18]. Another similar study suggested that the immunomodulatory ability of placenta-derived MSCs was superior to that of BMSCs and ADSCs [19].
Biological differences among MSCs derived from different tissues determine their different clinical applications. In the current study,we isolated MSCs from adipose tissues, umbilical cord tissues and menstrual blood using different separation methods, and compared their biological characteristics, genetic stabilities, and gene expression patterns, to provide reference information to aid future clinical cell therapies.
2. Materials and methods
2.1. Sample sources
Adipose tissues (n=6), umbilical cord tissues (n=6), bone marrow samples (n=1) and menstrual blood samples (n=6) were obtained from 17 healthy donors recruited from the First Affiliated Hospital of Zhejiang University, Hangzhou, China. The donors were informed about the preparation and application of the specimens before collection, and signed informed consent was obtained. The study was approved by the ethical committee of the First Affiliated Hospital of Zhejiang University.
2.2. Cell isolation and culture
2.2.1. ADSC isolation and culture
Liposuction was performed in healthy donors to aspirate lipid, as described by Zuk et al[20]. The lipid was cut into pieces, digested with 0.1% type Ⅰ collagenase (Roche, Penzberg, Germany) at 37 ℃for 30 min, neutralized with medium, and washed. The cells were then resuspended in DMEM containing 10% fetal bovine serum(Hyclone, Logan, UT), seeded into a culture flask, and placed in a 5% CO2incubator with saturated humidity at 37 ℃. When the cells reached 80%-90% confluence, they were digested with 0.25% trypsin-EDTA (Gibco, Carlsbad, CA), seeded in a flask at a density of 5×103/cm2, and successively passaged until cell senescence (cells were unable to be amplified).
2.2.2. UCMSC isolation and culture
Umbilical cord tissue (5-10 cm) was stripped from healthy women during labor, treated with Watertown's glue, and cut into pieces about 1 mm3. DMEM medium (Hyclone) containing 10% fetal bovine serum was added and the cells were seeded into a culture flask, and placed in a 5% CO2incubator with saturated humidity at 37 ℃ for primary adherent culturing. Passaging was carried out as above.
2.2.3. MenSC isolation and culture
Menstrual blood was collected from healthy women on the second day of menstruation using a menstruation cup (E-vans Biotech,Hangzhou, China), mixed thoroughly, and filtered through 150-μm mesh. Mononuclear cells were isolated by density-gradient centrifugation. Menstrual stem cell culture medium (E-vans Biotech)was then added, the cells were seeded in a culture flask and cultured in a 5% CO2incubator with saturated humidity at 37 ℃. Cells were passaged as described above.
2.2.4. BMSC isolation and culture
Human bone marrow was collected from healthy adult donors. BMSCs were harvested according to the methods described by Barlow et al[10]. Mononuclear cells were isolated and plated in culture flasks.
2.3. Analysis of cell proliferation characteristics
2.3.1. Growth curve
The three kinds of cells of the same generation (P5) were selected by MTT assay to determine the growth curve. The cells were seeded in 96-well culture plates at a density of 1 000 cells per well. The cells were measured every 24 h, at six parallel points. Another group served as a blank control. The culture was maintained for 7 d, after which 20 μL of MTT solution (Sigma-Aldrich, St. Louis, MO)was added into each test well. The cells were further cultured in an incubator at 37 ℃ for 4 h, and the cultures were then terminated. The supernatant was carefully aspirated and discarded from each well, and 150 μL of dimethylsulfoxide was added into each well,followed by shaking for 10 min. The optical density of each well was measured at 490 nm. The results were recorded and a cell growth curve was constructed.
2.3.2. Population doublings
Detailed data on cell passaging were recorded as described in section 2.2, and the population doublings for each group of cells were calculated[21].
2.3.3. Determination of cell-colony formation
Cell cloning experiments were performed with P5 cells in logarithmic growth phase[21]. One hundred, 200, or 300 cells were added to each well of a six-well plate in a density gradient, and 3 mL of culture medium was added per well. The plates were placed in a 5% CO2incubator with saturated humidity at 37 ℃ for 2-3 wk, with the medium replaced every 3 d. The cultures were terminated when the clones became visible in the culture plates. The supernatant was discarded and the cells were rinsed twice with phosphate-buffered saline and fixed with 5 mL methanol for 15 min. The fixation fluid was discarded and Giemsa staining solution was added for 10-30 min. The staining solution was slowly washed off with water and the cells were air dried. Clones with more than 10 cells were counted under a microscope, and the colony-forming rate was calculated.
2.4. Flow cytometric analysis
P5 cells at 80%-90% confluence were collected, washed and used to prepare a 1.0×105/mL cell suspension. Mouse anti-human monoclonal antibodies against CD29, CD34, CD45, CD73, CD90,CD105, and HLA-DR, CD117, SSEA-4 were added, respectively, at 20 μL each. The solution was mixed and incubated at 4˚C or on ice for 30 min away from light. The solution was then centrifuged and the supernatant was discarded. Phosphate-buffered saline was added to the cell pellet, centrifuged, and washed twice. Cells were then detected using a flow cytometer (FC500MCL, Beckman Coulter,Fullerton, CA) after resuspension.
2.5. Gene expression analysis
Affymetrix gene chips (Human U133 Genome Array) were used for mRNA expression profiling. Experimental protocols for the gene chips were performed according to the manufacturer's technical instructions. RNA was isolated from 1.0×106P5 cells using Trizol reagent (Invitrogen, Grand Island, NY). Total RNA was purified using a Qiagen RNeasy mini kit (Qiagen, Valencia, CA). Single and double-stranded cDNAs were synthesized from total RNA samples using SuperScript Ⅱ (Invitrogen, CA). Biotin-labeled cRNA was also synthesized. Microarray hybridization was performed, after which the chips were washed, scanned, and image acquisition was carried out. GCOS 1.4 was used to analyze the image data. After RNA normalization, differences in expression levels between two conditions were analyzed by Student's t-tests. A P value <0.01 and fold change >2 were considered to indicate a significant difference. An intersection of differential expression for MenSC4 compared with UCMSC4, and for MenSC5 compared with BMSC5 was used to identify genes differentially expressed in MenSCs. Differentiallyexpressed genes were analyzed using the Database for Annotation,Visualization and Integrated Discovery (DAVID, available at http:// david.abcc.ncifcrf.gov/).
2.6. Statistical analysis
The data were analyzed by one-way analysis of variance using SPSS 16.0 software. The data were expressed as mean ± standard deviation. P<0.05 was considered significant.
3. Results
3.1. Morphological observations of MSCs derived from different tissues
We isolated MSCs from human adipose tissues, umbilical cord tissues and menstrual blood, with a success rate of 100%. During primary culture, most spindle cells were arranged radially, presenting colony-like growth. However, some oval cells were observed besides the long spindle cells in primary adherent blood cell cultures. Cells gradually formed single, spindle communities with increasing passage numbers. Cells derived from umbilical cord and menstrual blood could reach 80% confluence after 2-3 wk, while the growth rate of primary adipocytes was slower, ie. 3-4 wk.
As shown in Figure 1, MenSCs had the largest single-cell volume and UCMSCs the smallest. Cell-cell contact inhibition was remarkable in ADSCs and MenSCs, but insignificant in UCMSCs,with overlap of cells. The volume of the MSCs increased gradually with increasing passage number and the cells became flattened,making it difficult for them to form a single monolayer. UCMSCs and ADSCs gradually differentiated into polygonal cells with long protrusions, while severe granulation appeared on the surface of MenSCs.
3.2. Cell proliferation
According to the growth curve of P5 MSCs, UCMSCs showed the strongest proliferative capacity, with a doubling time of about 21 h, compared with doubling times for MenSCs and ADSCs of approximately 26 and 30 h, respectively (Figure 2B). UCMSCs had the fastest proliferation rate, followed by MenSCs, while the doubling rate of ADSCs remained similar throughout the passages. MenSCs initially proliferated slowly, reached a peak at P8, and then gradually decreased, allowing them to maintain a high proliferative activity. UCMSCs sustained rapid multiplication until P16, and then followed the same trend as ADSCs and MenSCs. Population doublings reduced with increasing passage (Figure 2A). The clonality of the different MSC types declined in the order MenSCs>ADSCs> UCMSCs (Figure 2C).
3.3. Determination of cell immunophenotypes
Stromal cell markers (CD29, CD73, CD90, CD105) were expressed in ADSCs, MenSCs and UCMSCs, with a high positivity rate. However, hematopoietic cell markers (such as CD34 and CD45), the human embryo developmental stage-specific phenotype SSEA-4,and the immune marker HLA-DR were not expressed, suggesting low immunogenicity (Table 1).
3.4. Detection of cell stability
Karyotype analysis was performed in MSCs at P10 and P20 to verify the genetic stability of the cells after cell passage. The chromosome structure remained stable after 20 generations of serial passages of MSCs from all three origins (Figure 3).
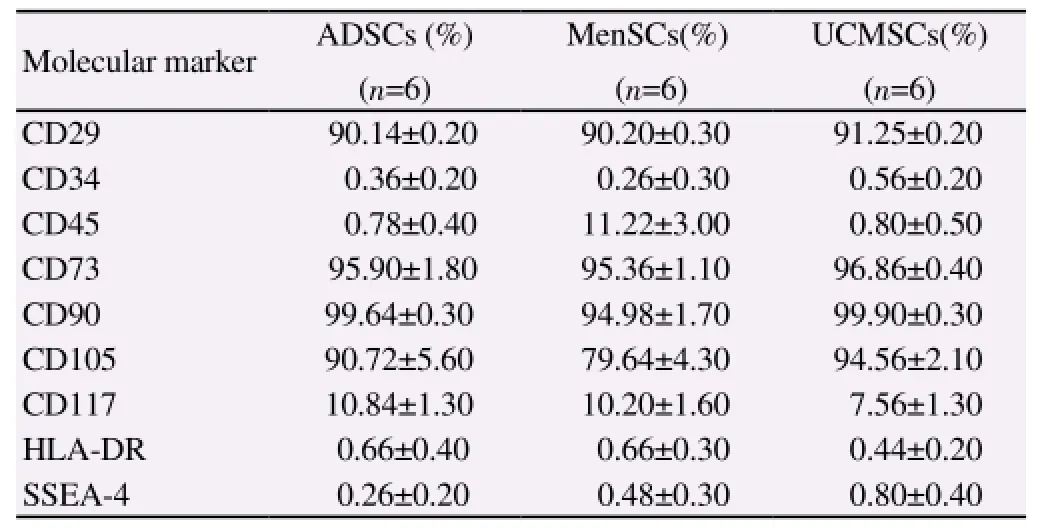
Table 1 Immunophenotypic analysis of ADSCs, UMSCs and MenSCs at passage 5.
3.5. Gene expression analysis
Samples of menstrual blood and umbilical cord, and samples of menstrual blood and bone marrow were collected from the same donor. Differences in gene expression caused by different genetic backgrounds were investigated by comparing the genome-wide expression spectra of the samples using an Affymetrix microarray. A total of 768 genes specifically upregulated or downregulated in MenSCs were found (Figure 4A). The upregulated genes included CD44, MAP3K5, IFITM1, and NES, and the downregulated genes included POSTN, OSTM1, and IGFBP3. The main pathways showing significant enrichment of differentially-expressed genes were the transforming growth factor (TGF)-β, mitogen-activated protein kinase (MAPK), p53, type II diabetes, cytokines and their receptors, insulin signaling and other cell function-related pathways. The main pathways enriched for downregulated genes were the systemic lupus erythematosus, O-glycan biosynthesis, and TGF-β signaling pathways. The MAPK signaling pathway, complement and coagulation cascade pathway, and type Ⅱ diabetes pathway showed more enrichment of upregulated genes (Table 2).
4. Discussion
BMSCs are widely-used adult stem cells that provide an ideal resource for cell therapy. However, there remain many unresolved issues regarding the clinical application of BMSCs, including the fact that cell numbers and their multi-directional differentiation potential are influenced by age[22]. Researchers are therefore investigating suitable alternative cell sources. Umbilical cord-derived UCMSCs and menstrual blood-derived MenSCs have attracted much attention. This study aimed to demonstrate the differences between ADSCs,UCMSCs and MenSCs with respect to their in vitro and molecular characteristics.
MSCs derived from all three sources produced long, spindle cells in in vitro culture. However, differences among the three cell types became evident during cell culture. 1) There were obvious differences in cell-cell contact inhibition; ADSCs and MenSCs were significantly inhibited, while UCMSCs were not. 2) MenSCs had the largest cell volume, and UCMSCs the smallest. 3) Different numbers of MSCs were obtained from different sources[23]. In this study, although MSCs could be isolated successfully from the three different tissues, the resulting cells showed noticeable differences in cultivation rates. Compared with ADSCs and UCMSCs, MenSCs had a higher isolation rate. Previous studies showed that ADSCs accounted for 2% of karyocytes[24], while UCMSCs accounted for 0.08%[25].
MSCs from different tissues demonstrated different capabilities in terms of cell proliferation and passage. Growth curve analysis and passage experiments indicated that UCMSCs had the strongestamplification potential, followed by MenSCs and ADSCs. In contrast, MenSCs showed significantly higher passage ability than UCMSCs and ADSCs.
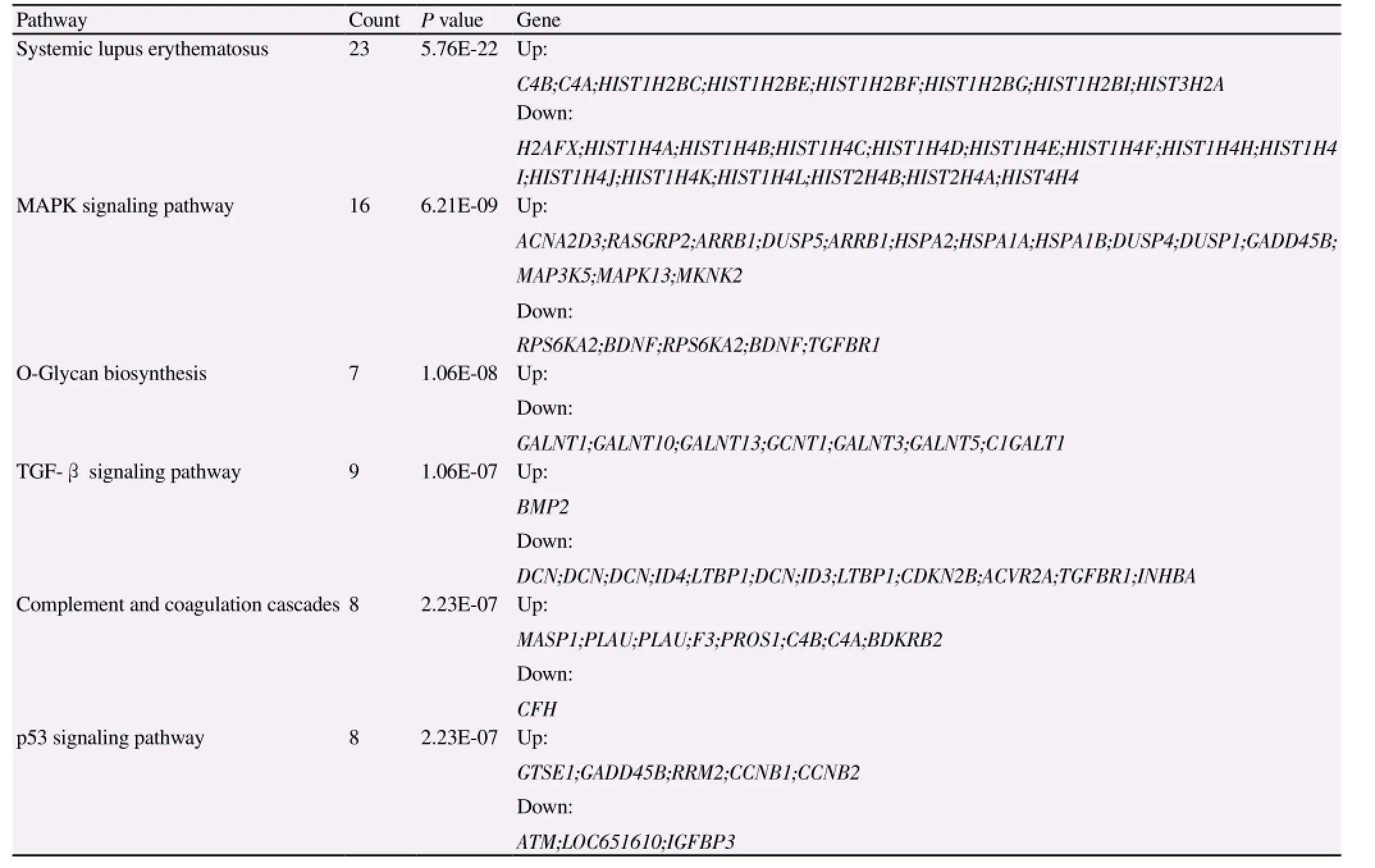
Table 2 Pathways significantly enriched for differentially-expressed genes.
Our study showed that all three kinds of MSCs had colony-forming abilities, consistent with previous reports[26]. MenSCs had greater clonality than ADSCs and UCMSCs. Regarding the colony-forming ability of MenSCs, it was reported that single-cell suspensions derived from uterine tissue formed two types of colonies after 15 d, including endometrial epithelial cells [(0.22±0.07)%] and endometrial stromal cells [(1.25±0.18)%][27].
Karyotype analysis is used to analyze the morphological characteristics of the chromosomes in the nucleus, which forms the fundamental basis for species classification. Previous studies reported a lower probability of chromosomal variations in longterm MSC cultures[28-30]. In this study, we found no chromosomal abnormalities in any of the three kinds of MSCs at P10 or P20,suggesting chromosomal stability at least until P20.
Regarding immunophenotyping, we analyzed the expression of CD29, CD34, CD45, CD73, CD90, CD105, CD117, SSEA-4 and HLA-DR by flow cytometry and found no significant differences among the three kinds of MSCs. All three kinds of MSCs were negative for the hematopoietic stem cells markers CD34 and CD45,but positive for the typical MSC markers CD29, CD73, CD90 and CD105. SSEA-4 is an early embryonic carbohydrate antigen usually used to label human microdifferential pluripotent embryonic stem cells and embryo decomposition products at the blastocyst stage. Our cells were negative for SSEA-4, consistent with previous reports[31-34]. HLA-DR was also negative, which confirmed the immunogenicity of MSCs.
Gene expression is regulated by a variety of factors, and minimizing these influences presents a challenge for analyzing gene expression in MSCs. We therefore compared gene expression between MenSCs and UCMSCs from the same donor, and between MenSCs and BMSCs from the same donor. We then screened 768 genes in MenSCs which were specifically upregulated or downregulated under the same genetic background.
CD44 is an important adhesion molecule that supports and promotes the migration of MSCs[34]. CD44 expression was 10-fold higher in MenSCs compared with UCMSCs and BMSCs, suggesting that MenSCs had stronger adhesion ability in culture.
The insulin-like growth factor-binding protein IGFBP5 can inhibit binding of insulin-like growth factor (IGF)-1 to its receptor, further affecting metabolic activities related to cell growth and survival. IGFBP5 has been shown to inhibit osteoblast differentiation[33],and we therefore concluded that BMSCs had lower IGFBP5 activity than MenSCs, leading to strong osteogenic or chondrogenic differentiation potential. POSTN and OSTM1 are important osteogenic and chondrogenic genes that were largely downregulated in MenSCs compared with UCMSCs and BMSCs, which also confirmed the inferior osteogenic and chondrogenic differentiation potentials of MenSCs.
IGFBP3 can inhibit proliferation and promote apoptosis[32]. IGFBP3 tended to be downregulated in MenSCs, indicating that these cells were less prone to senescence and apoptosis. This supported our conclusion that MenSCs had a stronger passage ability, consistent with the results of in vitro proliferation of MenSCs in previous studies[31].
MAP3K5 is mainly involved in signal transduction of cell apoptosis. It also plays an important role in the innate immune response, and regulates signal transduction of a variety of stressors and receptor-mediated inflammatory signals. As an interferoninduced transmembrane protein, IFITM1 is associated with high resistance to various viral infections. High expression levels of MAP3K5 and IFITM1 implied that MenSCs had stronger abilities to modulate the inflammatory environment and generate defense reactions.
The neuroectodermal marker NES is a key factor in brain development, and plays an important role in the survival, renewal and proliferation of neural progenitor cells. Compared with UCMSCs and BMSCs, NES expression was upregulated 8-14-fold in MenSCs, suggesting a greater differentiation potential in the nervous system.
In summary, the results of this study demonstrated that MSCs from adipose and umbilical cord tissues and menstrual blood all displayed the basic characteristics of MSCs, but with significant differences. MenSCs had higher extraction efficiency and stronger colonyforming and long-term passage abilities, but weaker amplification capability compared with UCMSCs. The coexistence of several cells with different morphologies suggested the existence of more stem cell types in menstrual blood. Information on gene expression profiles of MSCs from different tissues provides reference information to improve our understanding of cell function, and to aid the selection of appropriate cell types for clinical applications. Analysis of differentially-expressed genes revealed that MenSCs have advantages in terms of immune inflammation and stress response and neural differentiation potential, but limited osteogenic and chondrogenic differentiation abilities, compared with UCMSCs and BMSCs. This study identified MenSCs as a prospective source of cells for the clinical study of regenerative medicine, which may in turn lay the foundation for screening for specific molecular markers of MenSCs.
Conflict of interest statement
We all declare that we have no conflict of interest.
Acknowledgements
This work was supported by the National High-tech R&D Program(863 program, No. 2012AA020905), the Key Technologies R&D Program of Zhejiang Province (No. 2012C13015-2), the Hangzhou Key Technologies R&D Program (No. 20122513A49), and the National Natural Science Foundation of China (No. 81201783 and 81372463).
[1] Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411):143-147.
[2] Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418(6893): 41-49.
[3] Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004; 103(5): 1669-1675.
[4] Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 2007; 16(6): 931-952.
[5] Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med 2007; 5: 57.
[6] Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, et al. Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med 2013; 11: 56.
[7] Khanjani S, Khanmohammadi M, Zarnani AH, Talebi S, Edalatkhah H,Eghtesad S, et al. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J Tissue Eng Regen Med 2013.
[8] Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013; 2013: 901821.
[9] Zemel'ko VI, Kozhukharova IB, Alekseenko LL, Domnina AP,Reshetnikova GF, Puzanov MV, et al. Neurogenic potential of human mesenchymal stem cells isolated from bone marrow, adipose tissue and endometrium: a comparative study. Tsitologiia 2013; 55(2): 101-110.
[10] Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev 2008; 17(6): 1095-1107.
[11] Stanko P, Kaiserova K, Altanerova V, Altaner C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(3): 373-377.
[12] Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006; 99(5): 1285-1297.
[13] Panepucci RA, Siufi JL, Silva WA, Jr., Proto-Siquiera R, Neder L,Orellana M, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004;22(7): 1263-1278.
[14] Satomura K, Krebsbach P, Bianco P, Gehron Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J Cell Biochem 2000; 78(3): 391-403.
[15] Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem cells 2007; 25(3): 750-760.
[16] Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adiposederived stem cells. Ann Plast Surg 2008; 60(3): 306-322.
[17] Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Engineering Regenerative Med 2009;3(4): 290-301.
[18] Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med 2013; 2(6): 455-463.
[19] Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, Hwang SG, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol 2012; 13(2): 219-224.
[20] Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13(12): 4279-4295.
[21] Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 1999; 145(4): 769-782.
[22] Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cellular Biochem 2001; 82(4): 583-590.
[23] Behrens P, Bosch U, Bruns J, Erggelet C, Esenwein SA, Gaissmaier C, et al. Indications and implementation of recommendations of the working group 'Tissue Regeneration and Tissue Substitutes' for autologous chondrocyte transplantation (ACT). Z Orthop Ihre Grenzgeb 2004; 142(5):529-539.
[24] Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 2010; 38(6):1110-1116.
[25] Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc 2009; 17(6): 561-577.
[26] Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 2005; 23(2): 220-229.
[27] Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004; 70(6): 1738-1750.
[28] Buyanovskaya OA, Kuleshov NP, Nikitina VA, Voronina ES, Katosova LD, Bochkov NP. Spontaneous aneuploidy and clone formation in adipose tissue stem cells during different periods of culturing. Bull Exp Biol Med 2009; 148(1): 109-112.
[29] Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H,et al. Clinical-grade production of human mesenchymal stromal cells:occurrence of aneuploidy without transformation. Blood 2010; 115(8):1549-1553.
[30] Dominina AP, Fridliandskaia, II, Zemel'ko VI, Pugovkina NA, Kovaleva ZV, Zenin VV, et al. Mesenchymal stem cells of human endometrium do not undergo spontaneous transformation during long-term cultivation. Tsitologiia 2013; 55(1): 69-74.
[31] Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue:isolation, characterization, and differentiation potentialities. Bull Exp Biol Med 2005; 140(1): 138-143.
[32] Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 2009; 259(2): 150-156.
[33] Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton's jellyderived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res 2011; 346(1): 53-64.
[34] Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 2013; 15(3): 330-343.
15 June 2015
Dr. Charlie Xiang, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, 79 Qingchun Road, Hangzhou 310003, China.
Tel.: +86-571-87236436
Fax: +86-571-86971817
E-mail: cxiang@zju.edu.cn
Foundation project: This work was supported by the National High-tech R&D Program (863 program, No. 2012AA020905), the Key Technologies R&D Program of Zhejiang Province (No. 2012C13015-2), the Hangzhou Key Technologies R&D Program (No. 20122513A49), and the National Natural Science Foundation of China(No. 81201783 and 81372463).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
