Anti-proliferation and radiosensitization effects of chitooligosaccharides on human lung cancer line HepG2
2015-10-28FuShiHanBoHanCuiXiaoFangYouYanFenXingXiWenSun
Fu-Shi Han, Bo-Han Cui, Xiao-Fang You, Yan-Fen Xing, Xi-Wen Sun
1Medical Imaging Division, Pulmonary Hospital Affiliated to Tongji University, No. 507, Zhengmin Road, Yangpu District, Shanghai, 200433, China
Anti-proliferation and radiosensitization effects of chitooligosaccharides on human lung cancer line HepG2
Fu-Shi Han, Bo-Han Cui, Xiao-Fang You, Yan-Fen Xing, Xi-Wen Sun*
1Medical Imaging Division, Pulmonary Hospital Affiliated to Tongji University, No. 507, Zhengmin Road, Yangpu District, Shanghai, 200433, China
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
Chitooligosaccharides
Lung cancer
Radiotherapy
Radiosensitization
Cell cycle
Apoptosis
Objective: To observe the anti-proliferation and radiosensitization effect of chitooligosaccharides (COS) on human lung cancer cell line HepG2. Methods: CCK-8 assay was employed to obtain the inhibition ratio of COS on HepG2 cells at 24 h after treatment. The clonogenic assay was used to analyze the cell viability of RAY group and RAY+COS group with X-ray of 0, 1, 2, 4, 6 and 8 Gy, and the cell survival curve was used to analyze the sensitization ratio of COS. Flow cytometry was employed to detect cell cycle and apoptosis rate in control group, RAY group and RAY+COS group after 24 h treatment. Results: COS inhibited the proliferation of HepG2 cells, and the inhibition rate positively correlated with the concentration of COS. The cell viability decreased with increasing exposure dose in RAY group and RAY+COS group. The cell viabilities of RAY+COS group were lower than those of RAY group at the dose of 4, 6 and 8 Gy (P<0.05), and the sensitization ratio of COS was 1.19. There were higher percentage at G2/M phase and apoptosis rate, and lower percentage at S phase in RAY+COS group versus the other two groups (P<0.01). Conclusions: COS can inhibit the proliferation of HepG2 cells, and enhance the radiosensitization of HepG2 cells,induce apoptosis and G2/M phase arrest.
1. Introduction
Despite of the rapid development of modern medical technology,cancer is still the biggest challenge in medical field, posing immense threat on human's life and health. Bad habits like smoking , together with worsening air pollution and industrial fumes directly cause the surging population with lung cancer[1,2]. Lung cancer has topped the list of cancer rate in many developed countries and morbidity in major cities of China, which urges the tough fight against lung cancer[3-5]. Radiotherapy is one of the three weapons against lung cancer and plays an immeasurably important role in the treatment of lung cancer with over 70% patients[6-8]. However, attacking wrongobjects happens due to low capacity of radioactive rays to indentify the pathological cells, thus, the top priority for researchers is to efficiently eliminate the ‘invaders' and meanwhile protect the ‘peers'. Chitooligosaccharides (COS), produced from chitin, becomes the focus because its characteristics like easy solubility and high absorptivity are apparently better than polymer sugar. Currently,few COS reports focused on its application in radiosensitization of cancer cell[9,10]. This research aims to investigate the elevating sensibility of human lung cancer line HepG2 treated with COS to radiotherapy, and to verify the auxiliary synergism of COS on radiotherapy in order to explore the therapy for cancer with better efficacy and lower damage.
2. Materials and methods
2.1. Cell line and experimental materials
Human lung cancer line HepG2 was purchased from Instituteof Cell Biology, Chinese Academy of Sciences. Electron linear accelerator was purchased from Nanjing Chuang Rui Ying Biotechnology Co., LTD. CO2incubator was purchased from Shanghai GemTop Scientific Instrument Co., LTD. COS was purchased from Shanghai Hui Cheng Biological Technology Co., LTD. CCK-8 kit was purchased from Shanghai Li Rui Biotechnology Co., LTD.
2.2. Experimental methods
Cell lines HepG2 of seven concentration levels were created and HepG2 of each concentration was divided into 3 wells at the same time to make parallel samples. The cell lines HepG2 were cultured until the logarithmic phase. After digestion and dilution,the concentration was made into 4×104/mL and according to 0.1 mL/well, HepG2 was put into 96-well board for attachment culture in suitable environment. Twenty four hours later, the fresh culture medium of 0.11 mL/well diluted with COS was replaced and COS was added into each well with quantity of 0, 0.055, 0.11, 0.22, 0.33,0.44, 0.55 mg. Twenty four hours after COS infiltration, CCK-8 reagent was added along the wall at 0.01 mL/well. A little tapping was for the good mixture of reagent and culture solution. Four hours after the full reaction, optical density (OD) in each group was determined at the wave length of 450 nm. The procedure was repeated 3 times to investigate the anti-proliferation effect of COS on HepG2 and hereby COS with concentration of 1.0 mg/mL was taken for the further research.
X ray of six exposure dose levels was created with RAY group and RAY+COS group in each dose level and each group was divided into 3 wells at the same time to make parallel samples. According to 200/well for exposure dose of 0, 1, 2 Gy, 400/well for 4, 6 Gy and 800/well for 8 Gy, X ray was inoculated on 6-well culture plate and single-cell suspension of different concentrations was added to it for attachment culture in the incubator in suitable condition. Six hours later, COS of certain quantity was added to each well in RAY+COS group to make the concentration of 1.0 mg/mL. Culture medium of the equivalent quantity was added to each well in RAY group for 24 h infiltration. The tissue analogue of about 1 cm was capped on the culture plates in both groups and exposed to 6MvX ray, at the distance of 100 cm and dose rate of 2 Gy/min. The culture was continued for 10 d. After rinsing, fastening and staining, the number of cell cluster combined of more than 50 units was counted. The procedure was repeated 3 times to collect data and draw up the cell survival curve so that the sensitization enhancement ratio can be calculated by formula sensitization enhancement ratio= Do(RAY)/ Do(RAY+COS), where Dowas the final slope.
Three groups were created and each group was divided into 3 wells to make parallel samples. Cell suspension of 1×105/mL was inoculated into 6-well culture plate for attachment culture in the suitable environment for 24 h. Certain COS was added to RAY+COS group to make the concentration of 1.0 mg/mL. Culture medium of equivalent quantity was added to control group and RAY group. RAY group and RAY+COS group were exposed to 4 Gy X ray after 24 h infiltration. The culture was continued for 24 h after changing the fresh culture medium. After digestion, rinsing, dilution and other operation, the cell cycle and apoptosis rate were determined.
2.3. Statistical methods
SPSS17.0 software was used for statistical analysis. Measurement data were expressed by mean value±standard deviation (mean±SD)and t test was applied. If P<0.05, the differences were considered statistically significant.
3. Results
3.1. Anti-proliferation effect of COS on HepG2
Compared with the OD value without addition of COS, the other OD values were decreased with the increasing COS concentrations and the differences were statistically significant (P<0.01), which suggested that the amount of living cells was decreased with increasing COS quantity, the proliferation of HepG2 infiltrated with COS was inhibited and that the inhibition ratio and COS concentration was positively correlated. Detailed information was in Table 1.
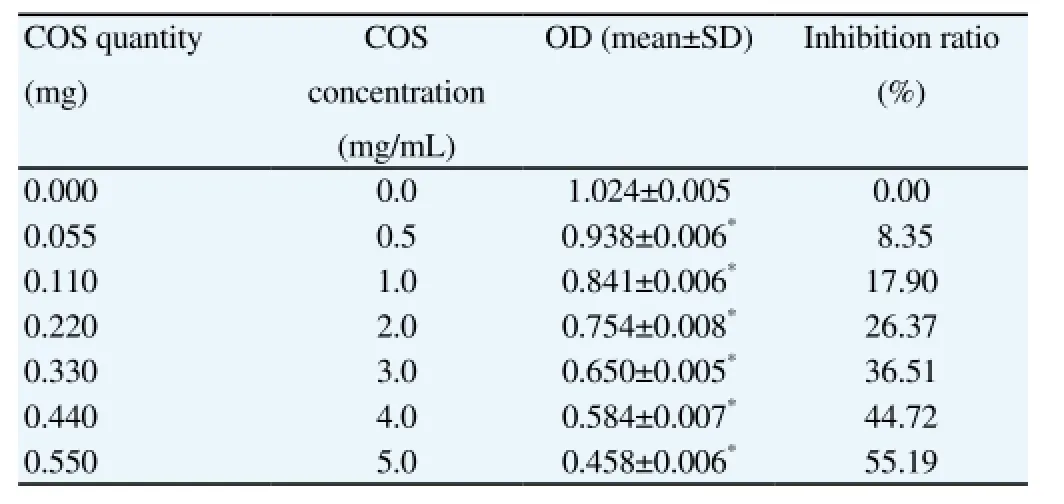
Table 1 Anti-proliferation effect of COS on HepG2.
3.2. Comparison of cell survival rates
Compared with the exposure dose of 0, the survival rates in RAY group and RAY+COS group were gradually decreased with increasing exposure dose and the decreasing range in RAY+COS group was bigger. The survival rates in RAY+COS group were slightly lower than in RAY group at the exposure dose of 1, 2 Gy but the differences were not statistically significant (P>0.05). When atthe exposure dose of 4, 6, and 8 Gy, the survival rates in information were lower than in RAY group and the differences were statistically significant (P<0.05). Detailed information was in Table 2. The sensitization enhancement ratio of COS to HepG2 was 1.19.

Table 2 Comparison of cell survival rates in groups at different exposure doses.
3.3. Comparison of cell cycle distributions
Compared with control group, the percentages of cells at S phase in RAY group and RAY+COS group were significantly lower while cells at G2/M phase were higher; and both differences were statistically significant (P<0.01). Cells at Go/G1phase in RAY+COS group were relatively more and the difference was statistically significant (P<0.05). In contrast with RAY group, cells at S phase in RAY+COS group took up smaller percentage and cells at G2/M phase higher percentage, with statistically significant differences(P<0.01). The differences in duration ratio at Go/G1phase in both groups were not statistically significant (P>0.05). Detailed information was in Table 3.
The apoptosis rates in RAY group and RAY+COS group were much higher than in control group and the differences were statistically significant (P<0.01). The apoptosis rate in RAY+COS group was higher than in RAY group, with statistically significant differences (P<0.05). Detailed information was in Table 4 and Figure 1.
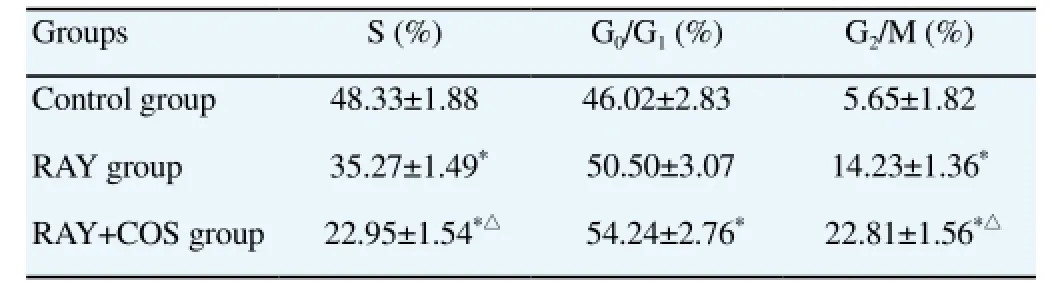
Table 3 Comparison of cell cycle distribution.

Table 4 Comparison of cell apoptosis rate.
4. Discussion
Heavy haze rised lung cancer incidence and the close connection between PM 2.5 and lung cancer has been confirmed by researchers all over the world[11-13]. The annual growth rate of lung cancer incidence in China is astoundingly over 25% and population that dies from lung cancer each year has increased to 4.5 times that of 1982[14]. The present research aims to create a combined medication of COS and radiotherapy by taking the human lung cancer cell line HepG2 as the experimental object, to enhance the radiotherapeutic effect and reduce adverse effect so that to improve the life quality of cancer patients.
4.1.Direct anti-proliferation effect
The successful application of enzymolysis approach in the 1990s achieved the industrial production of COS[15]. That natural low molecular sugar polymer is compatible with human bodyand without adverse effect, has drawn the attention of medical investigators for its various physiological regulation functions[16-18]. The present research verifies that COS has the direct antiproliferation effect on human lung cancer cell line HepG2 and the inhibitory rate is synchronously increased with the increasing COS quantity, which also verifies that COS expresses tumor-suppression activity against various cell lines. Kim and Karagozlu, et al. reported the inhibitory and killing effects of COS on leukemia HL-60 cell and gastric cancer AGS cell respectively[19,20]. Fernandes also established models to verify that COS inhibited the development of bladder tumor[21].
4.2. Promoting apoptosis of cancer cells
Ever since X ray and radium were discovered and applied to radiotherapy, radiotherapy has made great achievements in less than 100 years and become one of the major methods against cancer. Data revealed that the cure rate of cancers including oropharyngeal,tonsillar, maxillary cancer by radiotherapy was over 35% on average and early-stage cervical cancer even reached 86%-94%; radiotherapy was widely used before and after resection to shrink the tumor and eliminate the residue[22-24]. However, the collateral damage of radioactive rays could cause normal tissue, which is a huge burden and ordeal for patients. The present research testifies that RAY group and RAY+COS group could ecourage mass apoptosis of cancer cells and RAY+COS group with stronger lethality is more efficient at the same exposure dose, which supports the theory that COS can regulate the natural apoptosis of cancer cells from multiaspects of gene and protein expression. Apoptosis is inevitable for normal cells, which is beneficial for the organism to keep alive and adapt to surroundings while cancer cells enter the abnormal growth model due to the loss of apoptosis regulation. Researches have demonstrated that COS can lower the expression of B-cell lymphoma 2 to open multiple pathways to stimulate apoptosis like reducing the intracellular glutathione level and animating oxidation,accelerating transmembrane transport of Ga2+, initiating inducing apoptosis of relevant inhibitor TG, activating cytochrome complex to shift from mitochondria to cytoplasm and producing superoxide anion[25-27]. In addition, COS can also elevate the Bax level, release Caspase and restrain synthesis of ATP to stimulate apoptosis[28].
4.3. Changing cancer cell cycle distribution
The testing results of flow cytometry indicated that compared with control group, the duration at each stage of cell cycle in RAY group and RAY+COS group was changing. And RAY+COS group under the effect of COS had more significant chang in contrast with RAY group; S phase was relatively shortened; Go/G1and G2/M phases were relatively prolonged. Cell cycle kinetics provides theoretical guidance for radiotherapy; cells at M phase are the weakest with the lowest threshold value of being killed; cells at G2phase are the most seriously injured after exposure to rays with retarding speed of cell division; cells at S phase are the least affected by radiation[29]. The combined therapy of COS infiltration and radiotherapy can make cancer cells stay at G0/G1and G2/M phases to a larger extent and reduce the number of cells entering into S phase so that to improve the therapeutic effect of radiotherapy.
In conclusion, COS not only plays the direct anti-proliferation effect on HepG2 growth, but also promotes cells apoptosis, leads to the changing of proliferation process and efficiently improves the sensitivity of HepG2 to radioactive rays. When COS is combined with radiotherapy, it can greatly improve the therapeutic effect and meanwhile reduce the adverse damage on patients caused by radiotherapy. As the ‘Jack of all trades' in medical field, COS possesses tremendous potential in clinical treatment.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology,etiology, and prevention. Clin Chest Med 2011; 32(4): 605-644.
[2] Field JK, Oudkerk M, Pedersen JH, Duffy SW. Prospects for population screening and diagnosis of lung cancer. Lancet 2013; 382(9893): 732-741.
[3] Bosetti C, Malvezzi M, Rosso T, Bertuccio P, Gallus S, Chatenoud L,et al. Lung cancer mortality in European women: trends and predictions. Lung Cancer 2012; 78(3): 171-178.
[4] Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69-90.
[5] Malvezzi M, Bosetti C, Rosso T, Bertuccio P, Chatenoud L, Levi F, et al. Lung cancer mortality in European men: trends and predictions. Lung Cancer 2013; 80(2): 138-145.
[6] Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH,Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012; 82(1): 457-462.
[7] Stessin AM, Sison C, Schwartz A, Ng J, Chao CK, Li B. Does adjuvant radiotherapy benefit patients with diffuse-type gastric cancer? Results from the surveillance, epidemiology, and end results database. Cancer 2014; 120(22): 3562-3568.
[8] Mauguen A, Le Péchoux C, Saunders MI, Schild SE, Turrisi AT,Baumann M, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012;30(22): 2788-2797.
[9] Luo Z, Dong X, Ke Q, Duan Q, Shen L. Downregulation of CD147 by chitooligosaccharide inhibits MMP-2 expression and suppresses the metastatic potential of human gastric cancer. Oncol Lett 2014; 8(1): 361-366.
[10] Wu H, Aam BB, Wang W, Norberg AL, Sørlie M, Eijsink VG, et al. Inhibition of angiogenesis by chitooligosaccharides with specific degrees of acetylation and polymerization. Carbohydr Polym 2012; 89(2): 511-518.
[11] Fajersztajn L, Veras M, Barrozo LV, Saldiva P. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer 2013; 13(9): 674-678.
[12] Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 2013; 14(9):813-822.
[13] Turner MC, Krewski D, Pope CA III, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med 2011;184(12): 1374-1381.
[14] She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest 2013; 143(4): 1117-1126.
[15] Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 2015; 13(3):1133-1174.
[16] Termsarasab U, Cho HJ, Kim DH, Chong S, Chung SJ, Shim CK, et al. Chitosan oligosaccharide-arachidic acid-based nanoparticles for anticancer drug delivery. Int J Pharm 2013; 441(1-2): 373-380.
[17] Yousef M, Pichyangkura R, Soodvilai S, Chatsudthipong V, Muanprasat C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action. Pharmacol Res 2012; 66(1): 66-79.
[18] Zhang P, Liu W, Peng Y, Han B, Yang Y. Toll like receptor 4 (TLR4)mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int Immunopharmacol 2014; 23(1): 254-261.
[19] Karagozlu MZ, Karadeniz F, Kong CS, Kim SK. Aminoethylated chitooligomers and their apoptotic activity on AGS human cancer cells. Carbohydr Polym 2012; 87(2): 1383-1389.
[20] Kim EK, Je JY, Lee SJ, Kim YS, Hwang JW, Sung SH, et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorg Med Chem Lett 2012; 22(19): 6136-6138.
[21] Fernandes JC, Sereno J, Garrido P, Parada B, Cunha MF, Reis F, et al. Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Mar Drugs 2012; 10(12): 2661-2675.
[22] Folkert MR, Shih KK, Abu-Rustum NR, Jewell E, Kollmeier MA,Makker V, et al. Postoperative pelvic intensity-modulated radiotherapy and concurrent chemotherapy in intermediate and high-risk cervical cancer. Gynecol Oncol 2013; 128(2): 288-293.
[23] Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013; 85(4): 941-947.
[24] Liu C, Yan G, Helmig R, Lebron S, Kahler D. SU-E-J-47: development of a high-precision, image-guided radiotherapy, multi-purpose radiation isocenter quality-assurance calibration and checking system. Med Phys 2014; 41(6): 165-166.
[25] Matés JM, Segura JA, Alonso FJ, Márquez J. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol 2012; 86(11): 1649-1665.
[26] Hammadi M, Oulidi A, Gackière F, Katsogiannou M, Slomianny C, Roudbaraki M, et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J 2013; 27(4): 1600-1609.
[27] Tan DX, Hardeland R, Manchester LC, Galano A, Reiter RJ. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr Med Chem 2014; 21(13):1557-1565.
[28] Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P,Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12(12): 860-875.
[29] Wu TY, Saw CL, Khor TO, Pung D, Boyanapalli SS, Kong AN. In vivo pharmacodynamics of indole-3-carbinol in the inhibition of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice:involvement of Nrf2 and cell cycle/apoptosis signaling pathways. Mol Carcinog 2012; 51(10): 761-770.
15 June 2015
Xi-Wen Sun, Medical Imaging Division, Pulmonary Hospital Affiliated to Tongji University, No. 507, Zhengmin Road, Yangpu District, Shanghai,200433, China.
Tel: 021-65115006
Mobile: 13816593938
E-mail: xwsun40@smmail.cn
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
