Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma
2015-10-28ShaoXinYaoGuiSongZhangHongXiaCaoGuangSongZangTuoLiWeiTaoZhang
Shao-Xin Yao, Gui-Song Zhang, Hong-Xia Cao, Guang Song*, Zang-Tuo Li, Wei-Tao Zhang
1Department of Interventional Therapy, Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan, Hebei, 063000
2Department of Interventional Therapy, Luanxian People's Hospital, Tangshan, Hebei, 063700
Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma
Shao-Xin Yao1, Gui-Song Zhang2, Hong-Xia Cao1, Guang Song1*, Zang-Tuo Li1, Wei-Tao Zhang1
1Department of Interventional Therapy, Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan, Hebei, 063000
2Department of Interventional Therapy, Luanxian People's Hospital, Tangshan, Hebei, 063700
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September
Hepatocellular carcinoma
Th17 Cells
Treg Cells
miR-21
Correlation analysis
Objective: To study the correlation between miR-21 and Treg/Th17 ratio in the microenvironment of rats with hepatocellular carcinoma. Methods: Diethylnitrosamine was used to build the hepatocellular carcinoma model of rats; the content of Treg cells and Th17 cells and the expression of miR-21 in the peripheral blood of rats with hepatocellular carcinoma were detected. The statistical analysis was performed on the correlation between miR-21 expression and Treg/ Th17 ratio. Results: Hepatocellular carcinoma model of rats was successfully constructed. The proportion of Th17 cells among all CD4+T cells in the peripheral blood of rats with hepatocellular carcinoma was 5.319%, which was higher than the control group; while the proportion of Treg cells was 9.472%, which was higher than the control group. Treg/Th17 ratio in the model group was 1.781, compared with 1.478 in the control group. The expression of miR-21 was increased in the peripheral blood of rats with hepatocellular carcinoma and it showed a positive correlation with the ratio of Treg/Th17. Conclusions: There is a positive correlation between the expression level of miR-21 and the ratio of Treg/Th17.
1. Introduction
The primary hepatocellular carcinoma is some kind of common malignant tumor and its pathogenesis is related to various factors[1]. It is still difficult to diagnose and treat such disease. The current findings suggested that the tumor cells could evade the immune surveillance and thus result in the occurrence and development of tumor[2]. Two new subtypes of CD4+T cells, Treg cell and Th17 cell, played the important regulatory role in many tumors[3]. In recent years, many researches indicated that the microRNA was closely related to the occurrence, development and metastasis of hepatocellular carcinoma[4]. But it has been still unknown whethermicroRNA is related to the immune balance in which Treg/ Th17 join in and its specific mechanism of molecular biology. In this study, relying on the constructed hepatocellular carcinoma model of rats, we studied the correlation between the microRNA and Treg/Th17 cell subsets in the microenvironment of rats with hepatocellular carcinoma.
2. Materials and methods
2.1.Construction of hepatocellular carcinoma model of rats
The diethylnitrosamine was purchased from Sigma Corporation. The normal saline was used to prepare diethylnitrosamine as the solution with the concentration of 4 mg/mL, which was kept in a dark place.
50 purchased male SD rats with the weight of 180-220 g wererandomly divided into two groups, namely the control group (n=10)and model group (n=40).
The intraperitoneal injection of diethylnitrosamine was given to rats in the model group (with the dosage of 20 mg/kg, while the same dosage of normal saline to the control group), 3 times a week and lasted for 12 wk. The dietetic condition, mental state, and changes in the weight and clothing hair of experimental animals were observed every day during the administration. In 16th week,the venous blood was drawn from the experimental animals and then they were executed after anesthesia. After the observation of laparotomy on the gross morphology of liver, the liver was taken out and fixed by formalin to prepare the paraffin-embedded sections. The pathological examination was performed on the liver after HE staining.
2.2. Detection of content of Th17 cell and Treg cell
Cells were collected in the EP tube for 5 min of centrifugation at 2 000 rpm. Then the supernate was decanted. It was washed by PBS once. The process of centrifugation was repeated to remove the supernate. Then the fluorescent-labeled CD4 antibody was added and it was kept in a dark place at the room temperature for 30 min. It was washed by the proper PBS and then centrifuged at 2 000 rpm for 5 min afterwards. The supernate was decanted. Th17 cell and Treg cell were labeled respectively. For Th17 cell, 100 μL Th17 permeabilization wash buffer A was added and kept in a dark place at the room temperature for 10 min. Then the proper content of PBS was added for 5 min of centrifugation at 2 000 rpm. The supernate was decanted. Then B solution was added by 100 μL. The fluorescent-labeled anti-IL-17 antibody was added and it was kept in a dark place at the room temperature for 30 min of incubation. It was washed by PBS once again. Then the proper content of PBS was used to resuspend the cells. It was transferred to the flow tube and analyzed on the machine. For Treg cell, 500 μL Foxp3 permeabilization wash buffer was added and kept in a dark place at 4 for 30 min of incubation. Then it was washed by the special buffer and centrifuged at 2 000 rpm for 5 min. The supernate was decanted. Five μL fluorescent-labeled anti-Foxp3 antibody was added and it was kept in a dark place at the room temperature for 30 min of incubation. It was washed by PBS. Then PBS was used to resuspend the cells and it was transferred to the flow tube and analyzed by the machine.
2.3. Real-time and quantitative PCR detection
Trizol method was adopted to extract and separate the total RNA from the obtained PBMC cells. All test kits were purchased from Promega. The reverse transcription was performed on extracted RNA according to the operation procedure of reverse transcription kit. Then the real-time and quantitative PCR detection was carried out, where PCR instrument was ABI 7300 and the used reagent was SYBR Green purchased from TOYOBO. The matching primer of each gene was as follows: IL-17A F: 5'-TCCCACGAAATCCAGGATGC-3',R:5'-G G AT G T T C AG G T T G AC C AT C AC-3';I L-1 7 F F: 5'- G C G T T T C C AT G T C AC G TA AC A-3',R:5'-C AG C C C A AG T T C C TAC AC T G G-3';F o x p 3 F:5'-G T G G C C C G G AT G T G A G A A G-3',R:5'-GGAGCCCTTGTCGGATGATG-3'. The specific primer for miR-21 was purchased from RiboBio.
3. Results
3.1. Construction and evaluation of hepatocellular carcinoma model of rats
As the administration time became longer, compared with the control group, rats in the model group showed a significantly poorer mental state, decreased dietetic condition, slowly increased weight, sluggish reaction and messy and dull clothing fair. According to the pathological examination in 16th week, it could be seen that hepatocytes possessed the typical characteristics of hepatocellular carcinoma such as the big nucleus and deep staining. It also showed the significant atypical hyperplasia to be arranged in the block or stripe pattern, as well as the infiltrative growth to the normal hepatocytes around, as shown in Figure 1. In this study,the mortality was controlled at 7.5% (3/40). In the 16th week, the cancer rate was up to 81.1% (30/37).
3.2. Treg/Th17 ratio in the peripheral blood of hepatocellular carcinoma model of rats
For the rats with hepatocellular carcinoma in the model group,compared with the normal rats in the control group, the flow cytometer was adopted to analyze the content of Th17 cells (CD4+,IL17+) and Treg cells (CD4+, Foxp3+) in the peripheral blood. As shown in Table 1, the proportion of Th17 cells (CD4+, IL17+)among all CD4+T cells in the peripheral blood of 30 rats withhepatocellular carcinoma was 5.319%, while the one in the normal rats was 3.721%, with the statistical difference (P<0.001); the proportion of Treg cells (CD4+, Foxp3+) among all CD4+T cells in the peripheral blood of 30 rats with hepatocellular carcinoma was 9.472%, while the one in the normal rats was 5.498%, with the statistical difference (P<0.001). According to the analysis on Treg and Th17 cells in the model group and control one, the ratio of Treg and Th17 for the rats in the model group was 1.781, while the one in the control group was 1.478, with the statistical difference(P<0.001).
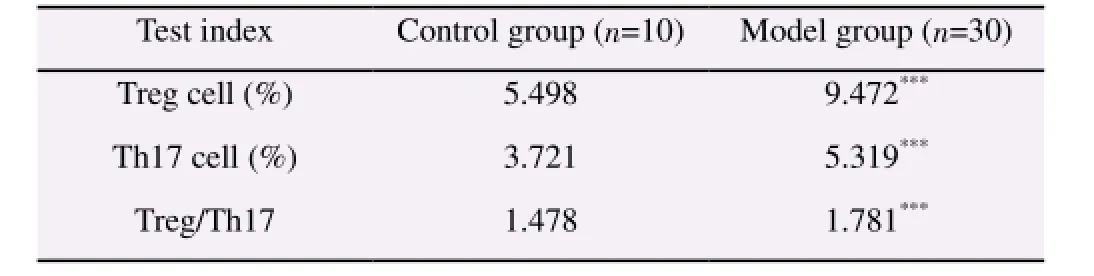
Table 1 Treg/Th17 ratio in the peripheral blood of hepatocellular carcinoma model of rats.
To further validate the experimental data, real-time and quantitative PCR was employed to compare the mRNA level of IL-17 and Foxp3 between the rats with hepatocellular carcinoma in the model group and normal rats in the control group. As shown in Figure 2, the expression of IL-17A, IL-17F and Foxp3 in the model group was significantly higher than the one in the control group,with the statistical difference (P<0.05).
3.3. Correlation between expression of miR-21 and Treg/ Th17 ratio in the peripheral blood of hepatocellular carcinoma model of rats
Among rats with cancer, there was the very specific expression profile of microRNA in the peripheral blood, such as miR-21, which was some kind of up-regulated microRNA in many tumors. Besides, the content of miR-21 in the peripheral blood of rats with hepatocellular carcinoma was also increased. In this study, realtime and quantiative PCR was employed to detect the expression of miR-21 in two groups. Results showed that the content of miR-21 in the peripheral blood of rats in the model group was significantly higher than the one in the control group, as shown in Figure 3.
Based on the ratio of Treg and Th17 and the content of miR-21 in the peripheral blood of rats in the control group and model one, the correlation between miR-21 and ratio of Treg/Th17 was constructed. As shown in Figure 4, there was a positive correlation between the content of miR-21 and ratio of Treg/Th17. Such result indicated that the increased expression of miR-21 might contribute to the imbalance between Treg cells and Th17 ones in rats with hepatocellular carcinoma.
4. Discussion
Th17 cells and Treg cells all belong to CD4+T lymphocytes, but different from Th1 cells and Th2 cells. Th17 cells can secrete the interleukin 17, which play the critical role in the pathogenesis of allergic diseases and autoimmune diseases. CD4+CD25+Treg cellscan resist the inflammation and maintain the immune tolerance and thus keep the immune system in the state of balance. Th17 cells and Treg cells restrain each other functionally. For the autoimmune diseases, there is the immune imbalance of Th17/Treg. The state of balance between them plays an important role in maintaining the steady state of immune system[5,6].
In recent years, findings showed that microRNA was of critical importance in the occurrence and development of tumor and its wide application could be expected in the future. The abnormal expression of a series of microRNA in hepatocellular carcinoma cells could play the role of ‘cancer gene' or ‘tumor suppressor gene'[7]. MiR-21 is a microRNA that has been studied much. Hu et al reported that miR-21 could regulate the target gene HEPN1 to promote the proliferation of hepatocellular carcinoma cells[8];Wang et al found that the expression level of miR-21 could predict the prognosis of hepatocellular carcinoma[9]; Bao et al found that miR-21 could inhibit the expression of target gene PTEN and hSulf-1 and thus promote the occurrence and development of hepatocellular carcinoma through AKT/ERK signal pathway[10]. However, it still has been unknown whether there is the correlation between miR-21 and the imbalance of Th17 and Treg cells in the microenvironment of tumor.
At present, there are limited researches on the correlation between microRNA and the imbalance of Th17 and Treg cells in the microenvironment of tumor. Lv et al reported that miR141-CXCL1-CXCR2 signal-induced Treg recruitment could regulate the metastasis and survival rate of non-small cell lung cancer[11];Dong et al found that, in patients with rheumatoid arthritis, there was the correlation between the reduced expression of miR-21 and the imbalance of Th17 and Treg cells[12]. Though this study confirmed the positive correlation between the up-regulated expression of miR-21 and the ratio of Th17/Treg, it is still essential to study the specific regulatory mechanism of molecular biology,in order to provide the new target spots for the diagnosis and treatment of diseases.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Yeo Y, Gwack J, Kang S, Koo B, Jung SJ, Dhamala P, et al. Viral hepatitis and liver cancer in Korea: an epidemiological perspective. Asian Pac J Cancer Prev 2013; 14(11): 6227-6231.
[2] Nault JC, Villanueva A. Intratumor molecular and phenotypic diversity in hepatocellular carcinoma. Clin Cancer Res 2015; 21(8): 1786-1788.
[ 3] Raj N, Attardi LD. Tumor suppression: p53 alters immune surveillance to restrain liver cancer. Curr Biol 2013; 23(12): R527-R530.
[4] Yevsa T, Kang TW, Zender L. Immune surveillance of pre-cancerous senescent hepatocytes limits hepatocellular carcinoma development. Oncoimmunology 2012; 1(3): 398-399.
[5] Martinez-Bosch N, Navarro P. Targeting Galectin-1 in pancreatic cancer:immune surveillance on guard. Oncoimmunology 2014; 3(8): e952201.
[ 6] Li L, Yang C, Zhao Z, Xu B, Zheng M, Zhang C, et al. Skewed T-helper(Th)1/2- and Th17/T regulatorycell balances in patients with renal cell carcinoma. MolMed Reports 2015; 11(2): 947-953.
[7] Lad DP, Varma S, Varma N, Sachdeva MU, Bose P, Malhotra P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leuk Lymphoma 2015;1-5.
[ 8] Nguyen AH, Berim IG, Agrawal DK. Cellular and molecular immunology of lung cancer: therapeutic implications. Expert Rev Clin Iimmunol 2014; 10(12): 1711-1730.
[9] Gong J, He XX, Tian A. Emerging role of microRNA in hepatocellular carcinoma (Review). Oncology Lett 2015; 9(3): 1027-1033.
[10]Yang J, Han S, Huang W, Chen T, Liu Y, Pan S, et al. A meta-analysis of microRNA expression in liver cancer. PLoS One 2014; 9(12): e114533.
[1 1]Moshiri F, Callegari E, D'Abundo L, Corra F, Lupini L, Sabbioni S,et al. Inhibiting the oncogenic mir-221 by microRNA sponge: toward microRNA-based therapeutics for hepatocellular carcinoma. Gastroenterol Hepatol Bed Bench 2014; 7(1): 43-54.
[1 2]Yu S, Liu C, Li L, Tian T, Wang M, Hu Y, et al. Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest 2015; 95(2): 157-167.
15 June 2015
Song Guang, Department of Interventional Therapy,Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan, Hebei 063000, China.
Tel: 13832851196
E-mail: jaymaomaocs@163.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
