Clinical significance of microRNA-130b in osteosarcoma and its role in cell growth and invasion
2015-10-28LieDaoYuRiLongJinPengChengGuZhiHengLingXiangJinLinJingYuDu
Lie-Dao Yu, Ri-Long Jin, Peng-Cheng Gu, Zhi-Heng Ling, Xiang-Jin Lin, Jing-Yu Du
Department of Orthopedics, the First Affiliated Hospital of Zhejiang University, No. 79 Qingchun Road, Hangzhou 310006, China
Clinical significance of microRNA-130b in osteosarcoma and its role in cell growth and invasion
Lie-Dao Yu, Ri-Long Jin, Peng-Cheng Gu, Zhi-Heng Ling, Xiang-Jin Lin, Jing-Yu Du*
Department of Orthopedics, the First Affiliated Hospital of Zhejiang University, No. 79 Qingchun Road, Hangzhou 310006, China
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
microRNA-130b
Osteosarcoma
Tumor growth
Invasion
Clinical significance
Objective: To investigate clinical significance of microRNA-130b (miR-130b) in osteosarcoma and its role in cell growth and invasion. Methods: miR-130b expression was detected in 68 samples of surgically resected osteosarcoma and matched normal tumor-adjacent tissues by qRT-PCR. The expression of miR-130b was altered by corresponding vectors in osteosarcoma cells, and then Western blot was used to detect the expression of PPARγ. BrdU cell proliferation and Transwell assays were performed to determine cell proliferation and invasion. Results: The expression of miR-130b in osteosarcoma tissues was significantly higher than that in normal tumor-adjacent tissues. Its expression in patients with metastasis was significantly higher than that in those without metastases. miR-130b expression in tumor tissues was significantly associated with tumor size, clinical stage and distant metastasis. And its expression was significantly correlated with overall survival and disease free survival. miR-130b overexpression obviously repressed the expression of PPARγ, and resulted in significant increase of Saos-2 cell proliferation and invasion. On the contrast, repressing miR-130b expression with its inhibitor significantly increased PPARγ expression, and inhibited MG-63 cell proliferation and invasion. Conclusions: The high-expression of miR-130b is correlated with the adverse clinicopathological features and poor prognosis in osteosarcoma. miR-130b may regulate proliferation and invasion of osteosarcoma cells by targeting PPARγ, suggesting miR-130b may play a key role in the progression of osteosarcoma.
1. Introduction
Osteosarcoma is the most common type of primary malignant tumor of bone, accounting for 60% of all malignant bone tumors in teenage[1]. With the advancement of multiple therapeutic strategies for osteosarcoma including surgical resection, adjuvant chemotherapy and radiotherapy, the 5 year survival of the nonmetastatic patients has improved to approximately 60%-70%[2,3]. However, for patients with metastasis or recurrence, the efficacyof chemotherapy is significantly decreased, and the long-term prognosis remains poor with a survival rate of 5%-20%[3]. Therefore, identifying novel biomarkers and clarifying the molecular mechanisms of the metastasis and recurrence of osteosarcoma may greatly improve the prognosis of osteosarcoma patients.
microRNAs (miRNAs) are a large group of non-coding RNAs containing 18-25 nucleotides and post-transcriptionally regulate the expression of multiple genes by interacting with their 3'-untranslated regions. Numerous deregulated miRNAs have been found to play fundamental roles in the development and progression of malignant diseases[4-10] including cell growth, differentiation,invasion, metastasis, and angiogenesis. Studies of melanoma[11],gastric carcinoma[12,13], bladder cancer[14] and colorectal cancer[15]have shown that aberrantly elevated miR-130b expression in these malignancies was correlated with poor prognosis of patients,indicating an oncogenic role of miR-130b in human cancers. In colorectal cancer, miR-130b has been found to promote the migration and invasion of cancer cells by targeting PPARγ[15]. However, the clinical significance of miR-130b expression in osteosarcoma tissues and its functional role on the migration and invasion of osteosarcoma cells have not been examined.
In this study, we evaluated the expression of miR-130b in osteosarcoma tissues by quantitative polymerase chain reaction. Our results confirmed that miR-130b expression was significantly increased in osteosarcoma tissues. And its elevated expression was closely associated with poor clinical features of osteosarcoma patients including large tumor size, advanced tumor node metastasis (TNM) stage, and distal metastasis. Furthermore, in vitro experiments showed that miR-130b could promote the progression of osteosarcoma by promoting cell proliferation and invasion. This study proposes that miR-130b may be a promising biomarker and attractive therapeutic target of osteosarcoma.
2. Material and methods
2.1. Patients and specimens
Cancerous tissues and adjacent non-tumor tissues (>3 cm distance to the resection margin) were collected from 68 patients who underwent curative resection of osteoscarcoma. The clinicopathological data of all enrolled patients were demonstrated in Table 1. All clinical samples were used after obtaining informed consent. All patients did not receive any blood transfusion, radiation treatment, or chemotherapy. The protocols of this study have been approved by the Ethics Committee of University.
2.2. Real-time quantitative PCR
Total RNA was isolated from clinical tissues by TRIZOL®reagent(Life Technologies, Carlsbad, CA, USA) under the guidance of operation instructions. The first strand cDNA was synthesized by the Revertid™First Strand cDNA Synthesis Kit (TaKaRa, Shiga,Japan). Two μL of cDNA obtained from each sample was amplified and quantified by real-time PCR of TaqMan Human MiRNA Assay(Applied Biosystems). miR-130b expression relative to U6 was calculated by the method of 2-ΔΔCt.
2.3. Cell culture
Human osteoscarcoma cell lines, Saos-2 and MG-63, were purchased from American Type Culture Collection, and was cultured in Dulbecco's modified Eagle medium (DMEM, Gibco, USA),containing 10% fetal bovine serum (Gibco) in a humidified 5% CO2incubator at 37 °C. Cells at logarithmic growth phase were collected for further experiments.
2.4. Transfection of miRNA mimics and inhibitors
miRNA vectors including miR-130b expression vector(miR10004680-1-5), the control vector for miR-130b (miR01201-1-2), miR-130b inhibitor (miR20004680-1-5) and the negative control for the miR-130b inhibitor (miR02201-1-2) bought from RiboBio (RiboBio, Guangzhou, China). Cells were seeded at a 1×105cells per well in a six-well plate and transfected with miRNA vectors at a final concentration of 50 nM by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) under the guidance of operation instructions. Total RNA and protein were collected 3 d posttransfection for experimental analyses.
2.5. Western blot
RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% TritonX-100, 5 mM ethylenediaminetetraacetic acid) was used to extract the proteins of cell lysates. Protein concentration was determined using the BCA Kit (Pierce, IL, USA). Protein samples (20 μg)were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blots were then probed with antibodies against PPARγ-(1:1 000;Cell Signaling Technology, MA, USA) and-actin (1:1 500, Boster,Wuhan, China). Blots were then incubated with the appropriate fluorescent secondary antibody (1:5 000; Boster). Images were acquired by the Bio-Rad Gel imaging system and analyzed by the software program as specified by Bio-Rad.
2.6. Cell proliferation assay
Saos-2 or MG-63 cells were seeded at 5×103cells per well in a 96-well plate, and was transfected with miRNAs vectors. Twenty-four h after transfection, cell proliferation ability was assessed with Proliferation ELISA, BrdU (5-bromodeoxyuridine)(chemiluminescent) (Roche, USA).
2.7. Transwell assay
The invasion assay was performed in 24-well Transwell units (BD Biosciences) with 8 um porosity polycarbonate filters. Each filter was coated with 70 μL matrigel (BD Biosciences, Franklin Lakes,NJ, USA) at 1 mg/mL on the inner layer. 1.5×105Saos-2 or MG-63 cells suspended in 200 μL reduced serum DMEM medium were added into the upper chamber, and 800 μL DMEM medium containing 20% FBS was added into the lower chamber. After incubating for 24 h, cells were fixed in 4% paraformaldehyde for 3 min, and then permeabilized in methanol for 20 min. Cells on the inner layer were removed with a cotton swab, and the adherent cells on undersurface of the insert were stained with 0.3% crystal violet dye for 10 min. The filters were washed with PBS and images were taken. Invaded cells on undersurface were counted under a lightmicroscope.
2.8. Statistical analysis
All data are presented as mean±SEM. The GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA) was used for Pearson chi-square test and Two-tailed Student's t test. Kaplan-Meier method was employed to plot overall survival and diseasefree survival curves, and the prognostic effect of miR-130b was evaluated using the log-rank test. P<0.05 was considered statistically significant.
3. Results
3.1. Elevated expression of miR-130b expression in osteosarcoma tissues
Compared with that in matched non-tumor tissues, miR-130b expression in tumor tissues was significantly elevated (P<0.05,Figure 1A). The level of miR-130b in metastases group was significantly higher than that in non-metastases group (P<0.05,Figure 1B). These results suggest that miR-130b plays an oncogenic role in osteosarcoma and is involved in the metastasis of osteosarcoma.
3.2. Correlation between clinicopathological characteristics and expression of miR-130b
As shown in Table 1, clinical association analysis by the Pearson chi-square test demonstrated that increased expression of miR-130b was significantly correlated with large tumor size (P=0.020),advanced tumor stage P<0.001 and distal metastasis (P=0.020). These results indicate increased expression of miR-130b is correlated with poor clincopathological features in osteosarcoma. Patients with high miR-130b level had significantly decreased overall survival(P<0.05, Figure 2A) and disease free survival (P<0.05, Figure 2B).
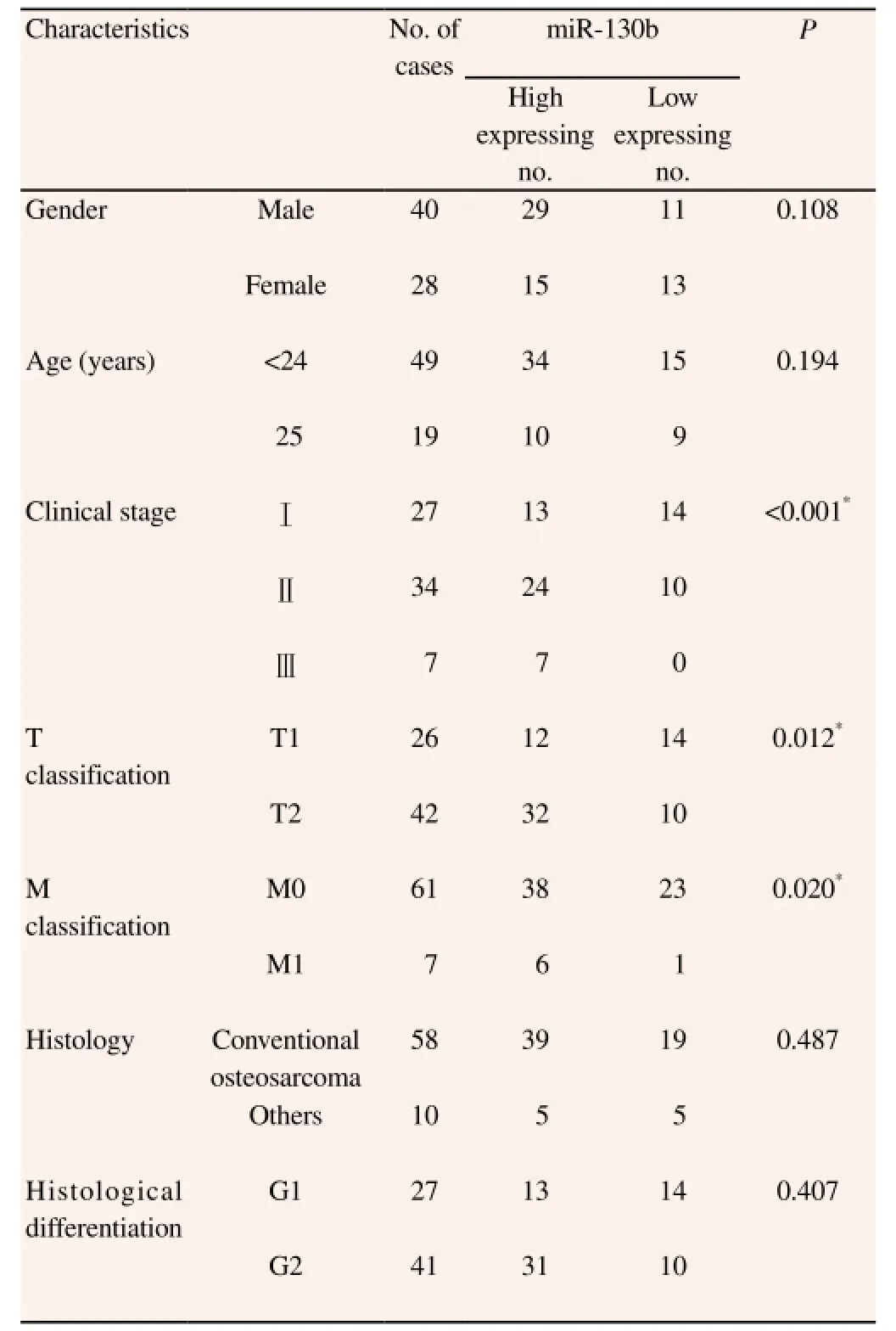
Table 1 Correlation between the clinicopathological characteristics and expression of miR-130b in the osteosarcoma patients (n=68).
3.3. Overexpression of miR-130b promotes proliferation and invasion of osteosarcoma cells
As shown in Figure 2, transfection of miR-130b expressing plasmid resulted in significantly increased level of miR-130b in Saos-2 cells (Figure 3A, P<0.05). Moreover, PPARγ, a well-defined downstream target of miR-130b in colorectal cancer cells,was significantly down-regulated accordingly (Figure 3B, P<0.05). And BrdU incorporation assay and transwell assay demonstrated overexpression of miR-130b in Saos-2 cells resulted in significantly increased proliferation (Figure 3C, P<0.05) and increased invasion(Figure 3D, P<0.05) respectively.
3.4. Down-regulation of miR-130b inhibits proliferation and invasion of osteosarcoma cells
The expression of miR-130b was significantly reduced after transfecting its inhibitor into MG-63 cells (Figure 4A, P<0.05). And the expression of PPARγ was significantly increased after down-regulating miR-130b (Figure 4B, P<0.05). Functionally, after repressing the expression of miR-130b, decreased proliferation(Figure 4C, P<0.05) and invasion (Figure 4D, P<0.05) of MG-63 cells were observed.
4. Discussion
miRNAs can regulate gene expression at post-transcriptional level by binding to 3'-untranslated regions of target messenger RNAs,and thus participate in many critical biological processes including cell differentiation, morphogenesis and tumorigenesis[16,17]. The critical role of miRNAs in the development and progression of human cancers have been widely accepted. miRNAs can play either oncogenic role or tumor suppressive role in human malignancies[18-20]. Moreover, miRNAs have been regarded as promising biomarkers and attractive therapeutic targets for human cancers[21].
miR-130b is being actively investigated in many kinds of human cancers[11-15,22-25]. It has been found to be down-regulated in papillary thyroid carcinoma[22], endometrial cancer[23], pituitary adenomas[24] and pancreatic cancer[25]. However, in melanoma[11],gastric carcinoma[12,13], bladder cancer[14], and colorectal cancer[15],miR-130b expression was found to be aberrantly elevated. Increased level of miR-130b in the serum of colon cancer patients was associated with resistance to chemotherapy[26]. And miR-130b has been regarded as an effective biomarker for HCC patients[27,28]. In this study, we evaluated the expression status of miR-130b in 68 pairs of osteosarcoma tissues and adjacent non-tumor tissues using qRT-PCR. The result revealed a significant increase of miR-130b expression in osteosarcoma tissues. Furthermore, we investigated the clinical significance of miR-130b in osteosarcoma. Clinical association analysis proved that miR-130b was closely associated with large tumor size, advanced TNM stage and distal metastasis. Moreover, increased level of miR-130b was correlated with poorer prognosis of osteosarcoma patients. These results indicate that miR-130b serves as an oncogenic miRNA in osteosarcoma and can potentially act as a novel biomarker of the prognosis of osteosarcoma patients.
Previous study demonstrated that miR-130b could down-regulate PTEN, E-cadherin, Snail and VEGF by inhibiting PPARγ, and thus resulted in increased proliferation, EMT and angiogenesis of colorectal cancer[15]. In endometrial cancer, miR-130b could contribute to EMT of cancer cells by targeting DICER1[29]. This study demonstrated that overexpression of miR-130b in MG-63 cells could inhibit the expression of PPARγ which was found to be down-regulated in osteosarcoma and could regulate proliferation,invasion and resistance to chemotherapy[30,31]. Accordingly, our functional experiments confirmed that overexpressing miR-130b led to increased proliferation and invasion of Saos-2 cells. On the other way, down-regulating miR-130b resulted in elevation of PPARγ expression, and decreased proliferation and invasion in MG-63 cells. These results indicate that miR-130b may promote the growth and metastasis of osteosarcoma by repressing PPARγ.
In summary, our results demonstrate that miR-130b is up-regulated in osteosarcoma, and its elevated expression is associated with large tumor size, distal metastasis and advanced TNM stage. Patients with increased level of miR-130b have a significantly poorer prognosis. Functionally, miR-130b may promote the proliferation and invasion of osteosarcoma cells by targeting PPARγ, and thus contributes to the development and progression of osteosarcoma. This studyindicates that miR-130b may be a promising biomarker and therapeutic target in osteosarcoma.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Rabinowicz R, Barchana M, Liphshiz I, Linn S, Futerman B, Ben-Arush MW. Cancer incidence and survival among infants in Israel, 1998-2007. Pediatric Hematol Oncol 2013; 30(7): 646-654.
[2] Letourneau PA, Xiao L, Harting MT, Lally KP, Cox CS Jr, Andrassy RJ, et al. Location of pulmonary metastasis in pediatric osteosarcoma is predictive of outcome. J Pediatric Surg 2011; 46(7): 1333-1337.
[3] Pierz KA, Womer RB and Dormans JP. Pediatric bone tumors:osteosarcoma ewing's sarcoma, and chondrosarcoma associated with multiple hereditary osteochondromatosis. J Pediatric Orthoped 2001;21(3): 412-418.
[4] Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol 2012; 13(6): e249-e258.
[5] Osman A. MicroRNAs in health and disease--basic science and clinical applications. Clin Lab 2012; 58(5-6): 393-402.
[6] Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116(2): 281-297.
[7] Mishra PJ. MicroRNAs as promising biomarkers in cancer diagnostics. Biomarker Res 2014; 2: 19.
[8] Gurtan AM and Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol 2013; 425(19): 3582-3600.
[9] Wu X, Weng L, Li X, Guo C, Pal SK, Jin JM, et al. Identification of a 4-microRNA signature for clear cell renal cell carcinoma metastasis and prognosis. PloS One 2012; 7(5): e35661.
[10] Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma:diagnostic and therapeutic aspects. Tumour Biol: J Int Society Oncodevelopmental Biol Med 2013; 34(4): 2093-2098.
[11] Sand M, Skrygan M, Sand D, Georgas D, Gambichler T, Hahn SA, et al. Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res 2013; 351(1):85-98.
[12] Kim BH, Hong SW, Kim A, Choi SH, Yoon SO. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol 2013; 107(5): 505-510.
[13] Lai KW, Koh KX, Loh M, Tada K, Subramaniam MM, Lim XY, et al.. MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer 2010; 46(8): 1456-1463.
[14] Scheffer AR, Holdenrieder S, Kristiansen G, von Ruecker A, Muller SC, Ellinger J. Circulating microRNAs in serum: novel biomarkers for patients with bladder cancer? World J Urol 2014; 32(2): 353-358.
[15] Colangelo T, Fucci A, Votino C, Sabatino L, Pancione M, Laudanna C, et al. MicroRNA-130b promotes tumor development and is associated with poor prognosis in colorectal cancer. Neoplasia 2013; 15(10): 1218-1231.
[16] Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136(2): 215-233.
[17] Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Ann Rev Med 2009; 60: 167-179.
[18] Garzon R, Marcucci G. Potential of microRNAs for cancer diagnostics,prognostication and therapy. Curr Opin Oncol 2012; 24(6): 655-659.
[19] Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer:rationale, strategies and challenges. Nat Rev Drug Dis 2010; 9(10): 775-789.
[20] Sandhu S, Garzon R. Potential applications of microRNAs in cancer diagnosis, prognosis, and treatment. Semin Oncol 2011; 38(6):781-787.
[21] Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122(1): 6-7.
[22] Yip L, Kelly L, Shuai Y, Armstrong MJ, Nikiforov YE, Carty SE, et al. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol 2011; 18(7): 2035-2041.
[23] Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, et al. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 2013; 32(27):3286-3295.
[24] Leone V, Langella C, D'Angelo D, Mussnich P, Wierinckx A, Terracciano L, et al. Mir-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cellular Endocrinol 2014; 390(1-2): 1-7.
[25] Zhao G, Zhang JG, Shi Y, Qin Q, Liu Y, Wang B, et al. MiR-130b is a prognostic marker and inhibits cell proliferation and invasion in pancreatic cancer through targeting STAT3. PloS One 2013; 8(9): e73803.
[26] Kjersem JB, Ikdahl T, Lingjaerde OC, Guren T, Tveit KM, Kure EH. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol Oncol 2014; 8(1): 59-67.
[27] Liu AM, Yao TJ, Wang W, Wong KF, Lee NP, Fan ST, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open 2012;2(2): e000825.
[28] Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and selfrenewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010; 7(6): 694-707.
[29] Li BL, Lu C, Lu W, Yang TT, Qu J, Hong X, et al. miR-130b is an EMT-related microRNA that targets DICER1 for aggression in endometrial cancer. Med Oncol 2013; 30(1): 484.
[30] He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, et al. Synergistic antitumor effect of the activated PPARγ gamma and retinoid receptors on human osteosarcoma. Clin Cancer Res: Official J Am Association Cancer Res 2010; 16(8): 2235-2245.
[31] Fu L, Xiong X, Wang L, Gao X, Ye Y, Jia J, et al. Antitumor efficacy of two novel non-thiazolidinedione compounds as peroxisome proliferatoractivated receptor-gamma agonists in human osteosarcoma cells in vitro. Chemotherapy 2009; 55(6): 468-476.
15 June 2015
Jing-Yu Du, Department of Orthopedics, the First Affiliated Hospital of Zhejiang University, No. 79 Qingchun Road, Hangzhou 310006, Zhejiang,China.
Tel: 13682178701
E-mail: dujingyu24324@163.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
