孤独症谱系障碍动物模型的研究进展
2015-05-04胡纯纯刘春雪
胡纯纯 刘春雪 徐 秀
·综述·
孤独症谱系障碍动物模型的研究进展
胡纯纯 刘春雪 徐 秀
孤独症谱系障碍(ASD)是一种神经发育障碍性疾病[1],其核心症状为社交和(或)沟通障碍,狭隘兴趣与刻板行为。目前,ASD的病因尚不明确。多数研究认为,遗传[2]与发育的早期环境因素[3]共同作用,在ASD的病因中发挥了关键作用。
世界大范围的流行病学调查中,ASD在人群中的发病率约为1%,男性是女性的2~3倍[4,5]。美国近期流行病学调查ASD的发病率达到1/68[6]。因此,深入了解ASD的病因和机制,寻求治疗显得尤为重要。目前,神经生物学研究已经明确了ASD的脑血流灌注模式和神经生化特点。此外,在ASD的神经系统连接特点、神经解剖和细胞与分子水平上也取得了一些进展[7]。然而,以ASD患儿为样本的机制研究仍旧存在很多的局限性,①通常不能获得高质量的尸检脑组织资源,②神经影像学所能提供的信息非常有限,③各种基因型与表型的临床资料非常零散等。因此,构筑动物模型可以更好地了解ASD的神经分子生物特性与脑解剖特点,以及明确各类不同的认知与行为亚型。
1 ASD动物模型
动物模型作为研究ASD的一个工具,其优势在于:①取得在活体中难以取得的神经组织标本;②便于控制变量,研究基因功能;③观察生化或基因在ASD神经病理学中的作用;④观察社会行为;⑤获得干预疗效等。动物模型也存在局限性:①无法完全模拟人类的病理生理机制,②动物社交行为与人存在差异等。
一个理想的动物模型需具备3种效度,①表面效度:需包含疾病的典型表现;②构建效度:需与疾病的生物学机制类似;③预测效度:对疾病的有效干预能在模型中产生预期结果[8,9]。现已用于ASD研究的动物模型有果蝇、斑马鱼、鼠和灵长类动物等,本文就生化作用诱导、基因分子缺陷和以生化作用为基础的基因型建立的ASD动物模型进行文献复习和归纳。
2 生化诱导ASD动物模型
近年来的研究表明,环境因素在ASD病因学中起着重要作用[10~12]。而胎儿发育关键期(如胎儿期或围生期)暴露于环境致病因素,致孕期感染或炎症反应而导致ASD,也是其病因研究的重点[13,14]。通过动物模型,能够得到生化因素诱导后其脑解剖与行为异常的结果,为研究神经系统功能、筛选新药和探索治疗方法提供新的思路。
VPA鼠模型是经典的ASD动物模型,而近期实验研究则更注重VPA剂量及时间差异效应下的不同损害。
VPA的剂量研究实验发现[18~21],孕期第12.5 d单次腹腔注射VPA 600 mg·kg-1可以诱发子鼠典型的ASD表现。此动物模型除有ASD的核心症状外,对疼痛敏感性降低而对非疼痛刺激敏感性增高。Banerjee等[22]通过对12.5 d的孕期母鼠注射不同剂量的VPA(500, 600 mg·kg-1),并运用条件恐惧装置和社交互动装置等测试子鼠,发现虽然剂量相似,但注射600 mg子鼠模型有更明显的焦虑行为,在社交互动区域花费的时间更少,并且在社交情绪学习方面存在异常,这为ASD社交情绪学习的研究提供了方向。
Reynolds等[23]在VPA时间差异效应研究中发现,在幼鼠出生后6~12 d注射VPA 150 mg·kg-1·d-1,此ASD幼鼠的听觉敏感性低,与既往孕期母鼠注射VPA的ASD子鼠模型听觉敏感性高不同,说明在大脑发育的不同阶段暴露于VPA可能造成的不同损害。
而VPA对其他动物模型的影响,Lee等[24]也做过有趣的实验。将受精5 d后的斑马仔鱼放置于含VPA 2 mmol·L-1的鱼缸中急性处理3 h,与对照组比较,发现斑马鱼在发育过程暴露于VPA后出现短暂的部分神经细胞增殖减少,但未在实验组成鱼后观察到与其他动物类似的行为缺陷。这种情况应与斑马鱼在发育过程中神经修复有关。Jacob
等[25]将斑马鱼的受精胚胎去卵膜后,分别在3~24 h、~48 h和~72 h用VPA 0.625 mmol·L-1处理,发现斑马鱼5-HT神经元分化受损,使其产生ASD样症状,并且在~48 h的处理效果最为明显,而在~72 h后则对5-HT的表达无影响。这些神经分子机制的研究将对未来分子靶向治疗提供重要的线索。
2.2 丙酸(PPA)动物模型 PPA是一种短链的脂肪酸,作为肠道细菌的代谢终产物,常用作食品防腐剂。文献报道存在胃肠紊乱的ASD儿童其短链脂肪酸等生物标志物存在异常[26],并伴免疫异常[27,28]。
PPA鼠模型分别从免疫生化及行为学方面的改变证明了PPA与ASD的相关性。Shultz等[29]报道鼠脑室内注射PPA可导致解剖和行为方面的异常,其脑室出现反应性角质化与活化的小胶质细胞,并通过行为学实验,发现PPA鼠存在学习记忆障碍,刻板重复行为及感觉运动异常,类似于人类ASD。MacFabe等[30]也进一步证实了前者的实验,连续3 d将PPA 250 mg·kg-1注射入雄性成鼠中,并对其脑组织做免疫组化分析,发现脑组织中胶质化反应性星形细胞和被激活的小胶质细胞,揭示了其最初的神经免疫性反应。El-Ansary等[31]将引起神经毒性的PPA剂量(250 mg·kg-1)注入成年白化鼠中,发现社交行为障碍、典型的认知功能损害和重复行为,并且伴随氧化应激标志物的上升和谷胱甘肽的下降,其谷胱甘肽过氧化物酶和过氧化氢酶的活性也随之降低。Foley等[27]对孕12~16 d的胎鼠每日注射PPA 500 mg·kg-1,与对照组比较,其寻巢反应能力减弱,提示在嗅觉诱导的社交认知中存在障碍,并且青春期的雄鼠相比于对照组有更多的探索行为,提示社交记忆的缺陷。因此,有研究者提出,在胎儿期PPA暴露可对新生鼠、青春期鼠和成年鼠社交行为产生一定的影响。PPA导致的神经毒性也许能作为一个重要的环境因素成为ASD的病因。
2.3 双酚基丙烷(BPA)动物模型 BPA是重要的有机化工原料,是苯酚和丙酮的重要衍生物。被广泛用于罐头食品和饮料的包装等。作为内分泌干扰物,胎儿期及围生期的暴露将导致儿童神经发育的异常[32,33]及行为的改变[34~36]。很多研究都揭示了内分泌干扰素与ASD及注意力缺陷多动障碍的关系[37]。
Wolstenholme等[34]对孕期暴露BPA后的连续3代鼠行社会认知测试,与对照组比较,表现出更多的探索行为,不仅如此,3代鼠缺乏对新刺激(陌生鼠)的认知,说明在社交记忆中存在缺陷;而在旷场试验中则表现过度活跃。证实了BPA对鼠的社会认知及行为等方面具有长期的影响。
而BPA对其它动物模型也存在影响。Kaurr等[38]运用黑腹果蝇作为BPA动物模型。给果蝇喂食不同剂量的BPA(0、0.1、0.5、1 mmol·L-1)食物,观察果蝇在自主运动方面的差异,发现其较对照组有更多的重复行为(如梳理行为),且有非正常的社交互动(与周围果蝇距离缩短),而喂食0.5 mmol·L-1的果蝇症状最具典型性。这些结论也证明了果蝇作为神经发育研究动物模型的可行性,均存在ASD相关行为。
2.4 其它生化模型 经过数十年的研究,各个学者均提出过不同的生化模型假说。比如抗体模型[39,40],通过诱导鼠模型的研究,证实了母体产生的特异性抗体(IgE13-IgE18及IgG),能够影响胎儿的神经发育而产生ASD样行为[41];博尔纳病毒(BDV)模型[42],BDV感染后的成鼠表现出更多的社交障碍及刻板行为,类似于ASD患儿[43];氯蜱硫磷模型,虽未能证明对人类产生ASD样症状[44],但在斑马鱼暴露于大剂量氯蜱硫毒药的研究中[45],则证实能诱导其产生ASD样行为;过量高频射线模型[46],将恒河短尾猿在孕早期暴露于过度的X射线中,发现成年猿相较于对照组有重复刻板行为,重复语言及认知行为损害,而临床研究证实,高频射线能导致ASD发病率升高[47]。在人和动物模型的研究中,发现环境因素均占有非常重要的作用,因此,不同的生化模型对ASD的研究均具有非常重要的意义。表1总结生化诱导剂致鼠ASD表型及神经生化改变。
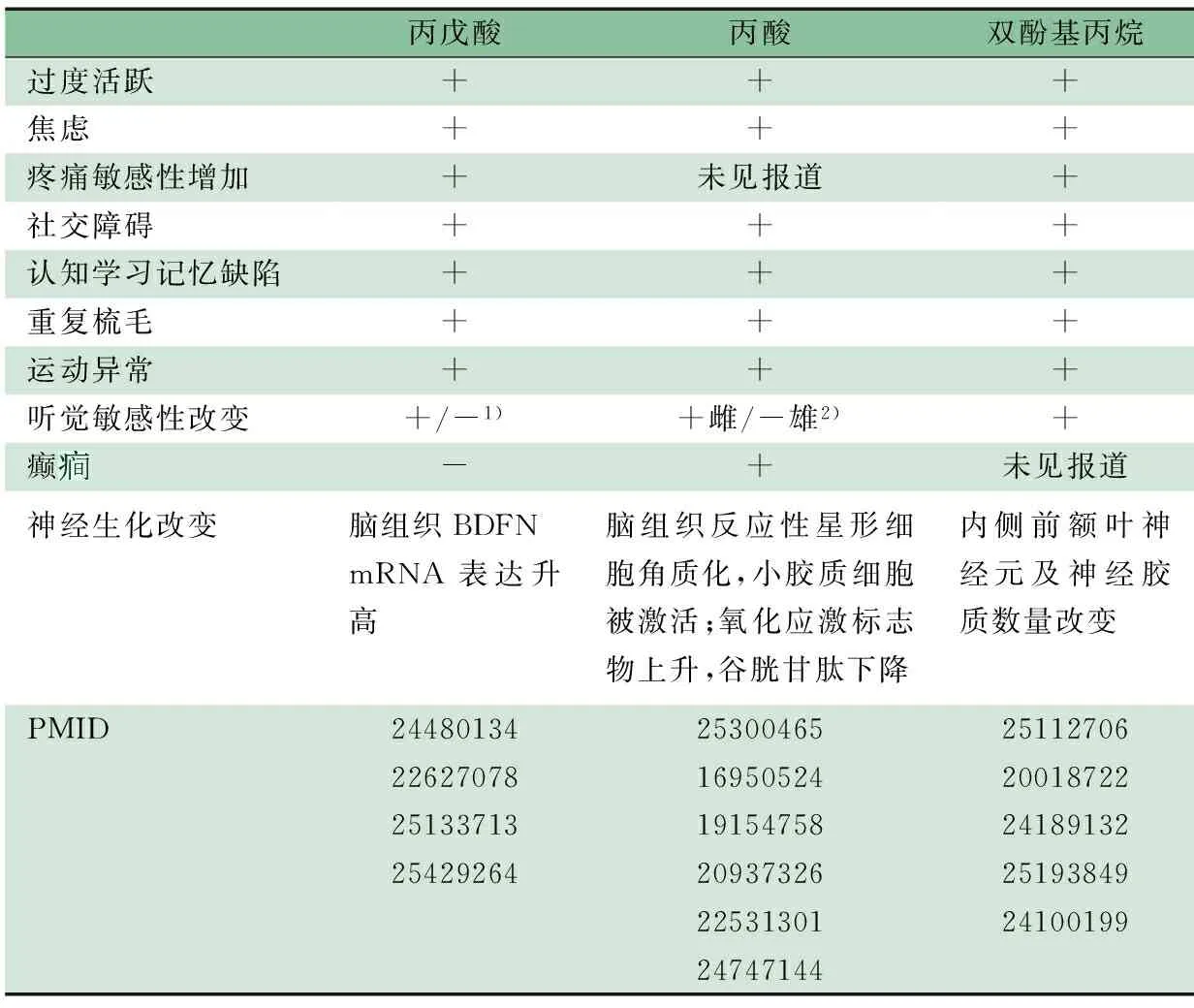
表1 生化诱导剂致鼠ASD表型及神经生化改变
注 PMID 为PubMed indexed唯一标识码;+: 阳性,-: 阴性;1)注射VPA的时间差异性,其鼠的听觉敏感性出现升高和降低;2)PPA效应存在性别差异,母鼠听觉敏感性升高而雄鼠降低
3 基因诱导ASD动物模型
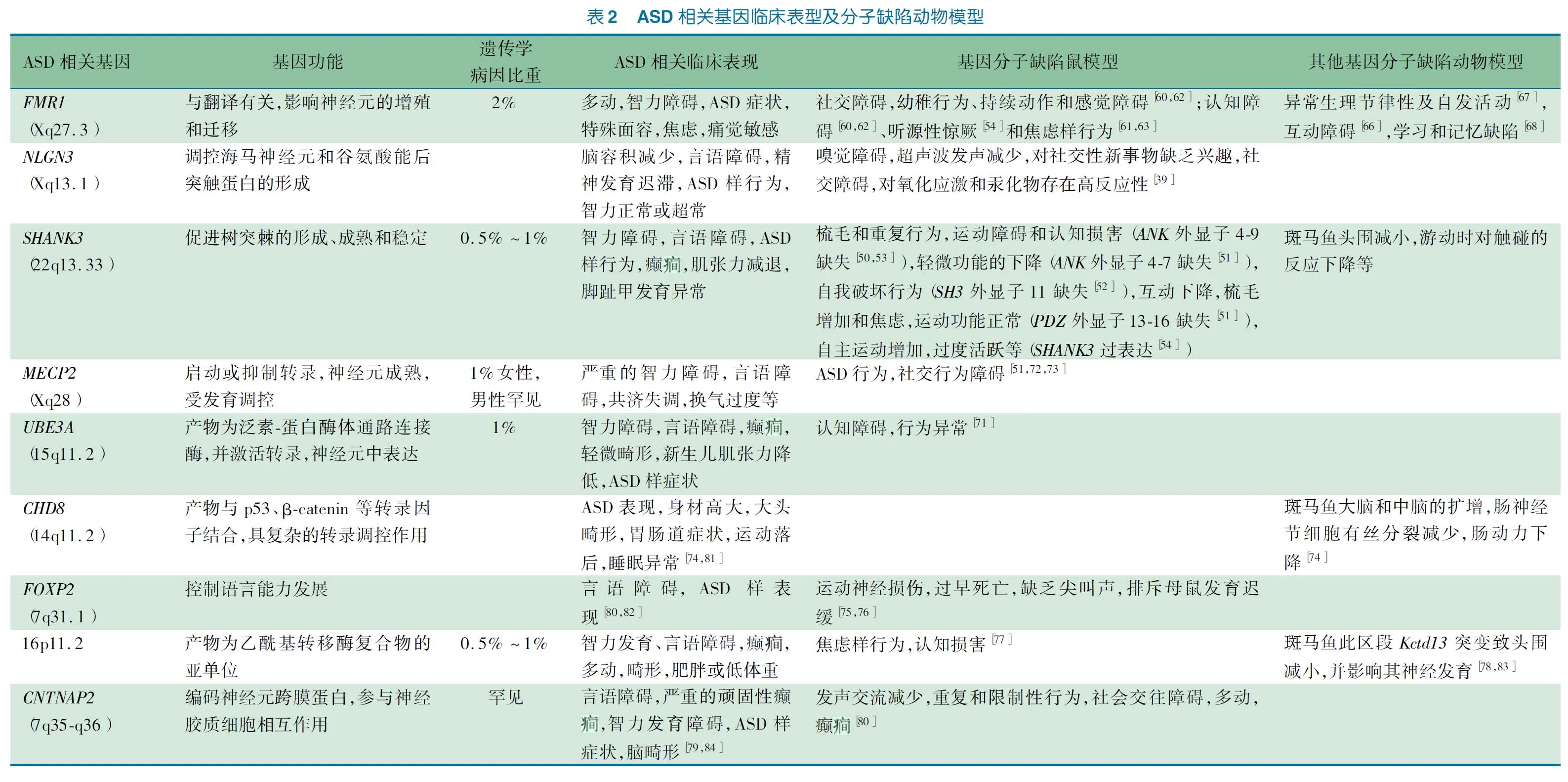
近年来依靠基因组学研究的发展,ASD的遗传学研究取得了巨大进展。通过不同的基因测序技术,鉴定了大批ASD的易感及致病基因位点。建立于基因突变之上,能够获得大量的ASD动物模型。目前热门的动物模型突变基因包括Fmr1、Nlgn3、Shank3、Mecp2、Ube3a、Chd8、Foxp2、Kctd13和Cntnap2等。然而,ASD的遗传异质性是研究机制及治疗的主要瓶颈,这也为动物模型的研究提供了一个重点的方向。
3.1SHANK3模型 人类基因组中有三组SHANK基因,分别为SHANK1、SHANK2和SHANK3,表达在大脑的不同区域,并在兴奋性突触后膜致密区中编码突触支架蛋白。在部分ASD患者中,已证实检测到SHANK基因的突变[48]。最近,SHANK3基因成为研究ASD基因的热点。
Bangash等[49]构建的ASD鼠模型类似人类的SHANK3微缺失,即缺失羧基段。Shank3微缺失鼠表现为NMDA受体功能下降,使其神经发育受损及影响突触的可塑性,使其学习记忆受损。Wang等[50]构建的Shank3缺陷鼠模型,通过分类Shank3相关的各个亚型,来评估Shank3的功能。不同外显子缺失的鼠可导致不同程度的功能缺陷,比如自我伤害、重复梳毛和社交障碍等[50~54]。通过分类Shank3的不同基因缺失对小鼠表型的影响,可以阐述其ASD发生发展的分子机制,以期为分子靶向治疗提供新思路。
除了鼠模型外,Gauthier等[55]在下调Shank3同源基因的斑马鱼模型中发现,与对照组相比,表现为头围的减小、游动时对触碰的反应下降等。由此证明了斑马鱼的Shank3基因在其中枢神经系统功能中所起的作用。
3.2FMR1模型 FMRP(Fragile X mental retardation protein)是脆性X相关蛋白(FXRP)的成员之一。其脆性X综合征的发病被证明是由X染色体上的FMR1基因缺陷导致蛋白合成减少所致。部分破坏其分子通路的儿童有典型的ASD表现[56]。脆性X综合征儿童的其他表现有认知障碍、特殊面容、多动和焦虑等[57~59]。
对于Fmr1缺陷的鼠模型,Hamilton等[60]将敲除Fmr1的鼠进行了认知、社交、学习能力和自主运动测试,发现Fmr1敲除鼠存在与人类ASD患者相似的行为学表现,而其它研究也证明了此结果[61~63]。
果蝇Fmr1基因与人在其蛋白功能区域的同源性为35%,相似性为56%[64,65]。研究发现[66,67],Fmr1突变果蝇存在行为学异常。Kanellopoulos等[68]发现果蝇dFmr下降后其突触的mGluR水平上升,而使cAMP减少,可导致其联合性的学习和记忆缺陷,从而阐述了Fmr1基因缺陷导致ASD样反应的分子机制。
3.3NLGN3模型NLGN基因编码的蛋白是后突触的细胞黏附蛋白成员之一。它的功能是调控海马神经元的形成,以及谷氨酸能后突触蛋白的形成,并与ASD相关[69]。
Nlgn突变鼠模型在感官行为和感觉加工方面存在缺陷,并且行为方面的缺陷与ASD患者的特征有着惊人的一致性[39]。Radyushkin等[70]报道敲除Nlgn3的鼠模型在脑的不同结构上存在差异:在灰质结构,如海马,纹状体,丘脑较正常个体为小。这种突变体的表型在人类的ASD中也有发现。

基因诱导ASD动物模型总结见表2。
4 以生化作用为基础的基因型ASD动物模型
基因与环境的相互作用已成为ASD研究的热点[85,86]。部分研究认为,环境造成基因表达差异的差异,是最终导致动物模型产生ASD表型的原因。Jacob等[25]发现由于VPA导致斑马鱼模型ascl1b/Ascl1基因表达的下降,从而导致血清胺能分化的下降。同样,Kolozsi等[87]的实验将VPA与Nlgn3相结合,发现胎儿期暴露VPA后的小鼠Nlgn3 mRNA的表达明显低于对照组,并认为VPA小鼠的ASD样行为亚型可能由于Nlgn3的低表达导致。这些实验均证明了环境与基因相互作用下的表观遗传学机制在疾病发生发展中的作用。
5 结论
动物模型对于研究ASD至关重要。生化诱导ASD动物模型,能够控制变量,探索不同的环境毒素(生化因素)在ASD发病中的作用。通过生化作用诱导动物模型,还可研究环境影响下神经发育特点和环境生化因素影响下的表观遗传机制。当动物模型在去除环境毒素(生化)的条件后出现症状的可逆性,为研究ASD的治疗提供了更多的方向。基因分子缺陷ASD动物模型,其优势在于目标明确,可通过诱导单一基因分子缺陷来研究ASD。由于ASD基因分子缺陷的临床资料较为零散,通过复制大量动物样本,能够提高可信度。通过动物模型,能将ASD基因型与不同表型关联性进行总结,以探究不同基因型的最佳干预疗效。
目前,ASD运用的动物模型种类多种多样 。 不同种类动物模型也存在其各自的优缺点 。 果蝇模型, 其繁殖周期短、多产、经济环保和基因较简单,但它与人类亲缘关系较远[38]。斑马鱼模型,作为新兴模型,其具有繁殖快、交配行为受光周期控制、产卵量多、受精卵在体外受精、前期胚胎整体透明、便于使用药物和易于饲养等优势[84]。鼠模型是ASD研究的经典模型,其基因型与人同源性较高,较为经济,而对其行为观察及学习记忆的技术条件也较成熟[88]。灵长类动物,与人同源性最高,并在情绪、社交等方面与人类最为类似,但存在研究周期长、耗费较高和动物伦理等问题。总之,鼠模型经济成本、时间成本、技术条件、同源性和行为学观察总体评估最优。可根据不同实验需要,选择最优的动物模型。
6 展望
未来动物模型研究的重点应建立在其ASD基因型与表型关联性的基础上,从而能为更有效地研发和选择治疗方案打下基础。目前ASD的干预疗法多建立在改善行为的基础上,未来的治疗方向应更倾向于分子疗法。目前,针对Mecp2突变鼠,运用基因修复技术能使其神经损伤得到修复[88~90],并且运用病毒转染Ube3a能使Angelman综合征鼠模型的异常行为得到改善[91]。随着越来越多ASD临床资料的收集,并对ASD症状规范性的总结,辅之更深入地了解神经系统的机制,再具备更理想的动物模型,ASD将会被更加透彻的研究。
[1]Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th edn (DSM-5). Arlington, VA: American Psychiatric Publishing, 2013:55-59
[2]Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics, 2004,113(5):472-486
[3]Rai D, Lee BK, Dalman C, et al. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ, 2013,346:2059
[4]Wingate M, Kirby RS, Pettygrove S, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. MMWR Surveillance Summaries, 2014,63:2
[5]Mattila ML, Kielinen M, Linna SL, et al. Autism Spectrum Disorders According to DSM-IV-TR and Comparison With DSM-5 Draft Criteria: An Epidemiological Study. J Am Acad Child Psy, 2011,50(6):583-592
[6]Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of Autism Spectrum Disorders in a Total Population Sample. Am J Psychiatr, 2011,168(9):904-912
[7]Baruth JM, Wall CA, Patterson MC, et al. Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): A Review. Autism Res, 2013,6(2):119-133
[8]Tania M, Khan MA, Xia K. Recent advances in animal model experimentation in autism research. Acta Neuropsychiatr, 2014,26(5):264-271
[9]Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev, 2004,10(4):248-258
[10]Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiat, 2014,4
[11]Berg R. Autism-an environmental health issue after all? J Environ Health, 2009,71(10):14-18
[12]Deth R, Muratore C, Benzecry J, et al. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology, 2008,29(1):190-201
[13]Meldrum SJ, Strunk T, Currie A, et al. Autism spectrum disorder in children born preterm-role of exposure to perinatal inflammation. Front Neurosci, 2013,7,123
[14]Zerbo O, Iosif AM, Walker C, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord, 2013,43(1):25-33
[15]Gentile S. Drug treatment for mood disorders in pregnancy. Curr Opin Psychiatr, 2011,24(1):34-40
[16]Rasalam AD, Hailey H, Williams J, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol, 2005,47(8):551-555
[17]Gentile S. Risks of neurobehavioral teratogenicity associated with prenatal exposure to valproate monotherapy: a systematic review with regulatory repercussions. CNS Spectr, 2014,19(4):305-315
[18]Kuwagata M, Ogawa T, Shioda S, et al. Observation of fetal brain in a rat valproate-induced autism model: a developmental neurotoxicity study. Int J Dev Neurosci, 2009,27(4):399-405
[19]Schneider T, Roman A, Basta-Kaim A, et al. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrino, 2008,33(6):728-740
[20]Stanton ME, Peloso E, Brown KL, et al. Discrimination learning and reversal of the conditioned eyeblink reflex in a rodent model of autism. Behav Brain Res, 2007,176(1):133-140
[21]Schneider T, Turczak J, Przewlocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacol, 2006,31(1):36-46
[22]Banerjee A, Engineer CT, Sauls BL, et al. Abnormal emotional learning in a rat model of autism exposed to valproic acid in utero. Front Behav Neurosci, 2014,8:378
[23]Reynolds S, Millette A, Devine DP. Sensory and Motor Characterization in the Postnatal Valproate Rat Model of Autism. Dev Neurosci-Basel, 2012,34(2-3):258-267
[24]Lee Y, Kim Y, Yun J, et al. Valproic acid decreases the proliferation of telencephalic cells in zebrafish larvae. Neurotoxicol Teratol, 2013,39:91-99
[25]Jacob J, Ribes V, Moore S, et al. Valproic acid silencing of ascl1b/Ascl1 results in the failure of serotonergic differentiation in a zebrafish model of fetal valproate syndrome. Dis Model Mech, 2014,7(1):107-117
[26]Wang L, Conlon MA, Christophersen CT, et al. Gastrointestinal microbiota and metabolite biomarkers in children with autism spectrum disorders. Biomark Med, 2014,8(3):331-344
[27]Foley KA, MacFabe DF, Vaz A, et al. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: Implications for autism spectrum disorders. Int J Dev Neurosci, 2014,39(S1):68-78
[28]Al-Owain M, Kaya N, Al-Shamrani H, et al. Autism spectrum disorder in a child with propionic acidemia. JIMD Rep, 2013,7:63-66
[29]Shultz SR, MacFabe DF, Martin S, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: Further development of a rodent model of autism. Behav Brain Res, 2009,200(1):33-41
[30]MacFabe DF, Cain NE, Boon F, et al. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res, 2011,217(1):47-54
[31]El-Ansary AK, Ben Bacha A, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflamm, 2012,9(74)
[32]Elsworth JD, Jentsch JD, VandeVoort CA, et al. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology, 2013,35:113-120
[33]Golub MS, Wu KL, Kaufman FL, et al. Bisphenol A: Developmental Toxicity from Early Prenatal Exposure. Birth Defects Res B, 2010,89(6):441-466
[34]Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav, 2013,64(5):833-839
[35]Itoh K, Yaoi T, Fushiki S. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology, 2012,32(4):447-457
[36]Nakamura K, Itoh K, Dai HM, et al. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev-Jpn, 2012,34(1):57-63
[37]de Cock M, Maas Y, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr, 2012,101(8):811-818
[38]Kaur K, Simon AF, Chauhan V, et al. Effect of bisphenol A on Drosophila melanogaster behavior - A new model for the studies on neurodevelopmental disorders. Behav Brain Res, 2015,284:77-84
[39]Camacho J, Jones K, Miller E, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res, 2014,266:46-51
[40]Singer HS, Morris C, Gause C, et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol, 2009,211(1-2):39-48
[41]Croonenberghs J, Wauters A, Devreese K, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med, 2002,32(8):1457-1463
[42]Lancaster K, Dietz DA, Moran TH, et al. Abnormal social behaviors in young and adult rats neonatally infected with Borna disease virus. Behav Brain Res, 2007,176(1):141-148
[43]Hornig M, Lipkin WI. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: epidemiology, hypotheses, and animal models. Ment Retard Dev Disabil Res Rev, 2001,7(3):200-210
[44]Williams AL, DeSesso JM. Gestational/perinatal chlorpyrifos exposure is not associated with autistic-like behaviors in rodents. Crit Rev Toxicol, 2014,44(6):523-534
[45]Richendrfer H, Pelkowski SD, Colwill RM, et al. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol Teratol, 2012,34(4):458-465
[46]Selemon LD, Friedman HR.Motor stereotypies and cognitive perseveration in non-human primates exposed to early gestational irradiation. Neuroscience, 2013,248:213-224
[47]Kane RC. A possible association between fetal/neonatal exposure to radiofrequency electromagnetic radiation and the increased incidence of autism spectrum disorders (ASD). Med Hypotheses, 2004,62(2):195-197
[48]Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron, 2013,78(1):8-27
[49]Bangash MA, Park JM, Melnikova T, et al. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell, 2011,145(5):758-772
[50]Wang XM, McCoy PA, Rodriguiz RM, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet, 2011,20(15):3093-3108
[51]Johnson RA, Lam M, Punzo AM, et al. 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J Appl Physiol, 2012,112(5):704-710
[52]Schmeisser MJ, Ey E, Wegener S, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature, 2012,486(7402):256-260
[53]Bozdagi O, Sakurai T, Papapetrou D, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism, 2010,1(15)
[54]Han K, Holder JJ, Schaaf CP, et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature, 2013,503(7474):72-77
[55]Gauthier J, Champagne N, Lafreniere RG, et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. P Natl Acad Sci USA, 2010,107(17):7863-7868
[56]Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron, 2012,74(2):285-299
[57]Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Dev Neurosci-Basel, 2011,33(5):379-394
[58]Bailey DB, Raspa M, Olmsted M, et al. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am J Med Genet A, 2008,146A(16):2060-2069
[59]Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. Scientific World Journal, 2006,6:1164-1176
[60]Hamilton SM, Green JR, Veeraragavan S, et al. Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci, 2014,128(2):103-109
[61]Udagawa T, Farny NG, Jakovcevski M, et al. Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat Med, 2013,19(11):1473
[62]Krueger DD, Osterweil EK, Chen SP, et al. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. P Natl Acad Sci USA, 2011,108(6):2587-2592
[63]Rogoz Z, Skuza G. Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol Rep, 2011,63(6):1547-1552
[64]Gao FB. Understanding fragile X syndrome: Insights from retarded flies. Neuron, 2002,34(6):859-862
[65]Zhang YQ, Bailey AM, Matthies H, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell, 2001,107(5):591-603
[66]Bolduc FV, Valente D, Nguyen AT, et al. An assay for social interaction in Drosophila fragile X mutants. Fly, 2010,4(3):216-225
[67]Dockendorff TC, Su HS, McBride S, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron, 2002,34(6):973-984
[68]Kanellopoulos AK, Semelidou O, Kotini AG, et al. Learning and memory deficits consequent to reduction of the fragile X mental retardation protein result from metabotropic glutamate receptor-mediated inhibition of cAMP signaling in drosophila. J Neurosci, 2012,32(38):13111-13124
[69]Chadman KK, Gong SC, Scattoni ML, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res, 2008,1(3):147-158
[70]Radyushkin K, Hammerschmidt K, Boretius S, et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav, 2009,8(4):416-425
[71]Jana NR. Understanding the pathogenesis of angelman syndrome through animal models. Neural Plast, 2012,7,10943
[72]Derecki NC, Cronk JC, Lu ZJ, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature, 2012,484(7392):105
[73]Pearson BL, Defensor EB, Pobbe R, et al. Mecp2 truncation in male mice promotes affiliative social behavior. Behav Genet, 2012,42(2):299-312
[74]Bernier R, Golzio C, Xiong B, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 2014,158(2):263-276
[75]Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav, 2011,10(1):17-27
[76]Shu WG, Cho JY, Jiang YH, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. P Natl Acad Sci USA, 2005,102(27):9643-9648
[77]Pucilowska J, Vithayathil J, Tavares EJ, et al. The 16p11.2 Deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked tothe ERK MAPK pathway. J Neurosci, 2015,35(7):3190-3200
[78]Golzio C, Willer J, Talkowski ME, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature, 2012,485(7398):111-363
[79]Rodenas-Cuadrado P, Ho J, Vernes SC. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet, 2014,22(2):171-178
[80]Penagarikano O, Geschwind DH. What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol Med, 2012,18(3):156-163
[81]O′Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature, 2012,485(7397):136-246
[82]Bowers JM, Konopka G. The role of the FOXP family of transcription factors in ASD. Dis Markers, 2012,33(5):251-260
[83]Blaker-Lee A, Gupta S, McCammon JM, et al. Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes. Dis Model Mech, 2012,5(6):834-851
[84]Bruneel B, Matha M, Paesen R, et al. Imaging the zebrafish dentition: from traditional approaches to emerging technologies. Zebrafish, 2015,12(1):1-10
[85]Kim YS, Leventhal BL. Genetic Epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiat, 2015,77(1):66-74
[86]Meek SE, Lemery-Chalfant K, Jahromi LB, et al. A review of gene environment correlations and their implications for autism: a conceptual model. Psychol Rev, 2013,120(3):497-521
[87]Kolozsi E, Mackenzie RN, Roullet FI, et al. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3in mice. Neuroscience, 2009,163(4):1201-1210
[88]Guy J, Gan J, Selfridge J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science, 2007,315(5815):1143-1147
[89]Kerr B, Soto C J, Saez M, et al. Transgenic complementation of MeCP2 deficiency: phenotypic rescue of Mecp2-null mice by isoform-specific transgenes. Eur J Hum Genet, 2012,20(1):69-76
[90]Giacometti E, Luikenhuis S, Beard C, et al. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. P Natl Acad Sci USA, 2007,104(6):1931-1936
[91]Daily JL, Nash K, Jinwal U, et al. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for angelman syndrome. Plos One, 2011,6(e2722112)
(本文编辑:张崇凡)
10.3969/j.issn.1673-5501.2015.06.013
复旦大学附属儿科医院 上海,201102
徐秀,E-mail:xuxiu@shmu.edu.cn
2015-07-15
2015-11-20)
