一氧化碳释放分子3通过抑制肺泡上皮细胞凋亡减轻脂多糖诱导的新生鼠急性肺损伤
2015-05-04蔡康兴
蔡康兴 汪 丽 王 婷 罗 莉 陈 龙 王 楠 史 源
·论著·
一氧化碳释放分子3通过抑制肺泡上皮细胞凋亡减轻脂多糖诱导的新生鼠急性肺损伤
蔡康兴1汪 丽1王 婷2罗 莉2陈 龙1王 楠1史 源1
目的 探讨一氧化碳释放分子3(CORM3)对脂多糖(LPS)诱导的新生鼠急性肺损伤(ALI)肺泡上皮细胞凋亡的影响。方法 32只新生SD大鼠均分为对照组、LPS组、CORM3组和失活CORM3(iCORM3)组;LPS组、CORM3组和iCORM3组采用LPS气管内滴注建立新生鼠ALI模型,分别腹腔注射生理盐水、CORM3和iCORM3;对照组不建立ALI模型,腹腔注射生理盐水。于建模后12 h取肺组织,苏木素-伊红染色观察肺组织病理改变,湿干(W/D)比值测定,肺泡灌洗液(BALF)细胞计数及蛋白含量测定。体外培养A549细胞,LPS诱导细胞凋亡。MTT检测细胞活性,Tunel染色观察组织、细胞凋亡变化。结果 ①与对照组相比,LPS组肺组织形态结构明显紊乱,肺泡压缩,肺间质大量炎性细胞浸润;CORM3组肺组织形态结构基本正常,间质炎性细胞浸润少;iCORM3组见肺组织水肿及大量炎性细胞浸润。②与对照组相比,LPS组肺组织W/D比值、BALF细胞数及蛋白含量明显升高,肺泡上皮细胞凋亡率明显增多[(37.3±4.5)%vs(3.0±1.0)%];A549细胞活力下降,凋亡率明显升高[(29.6±4.1)%vs(3.6±1.0)%],差异均有统计学意义 (P<0.05)。CORM3组肺组织W/D比值、BALF细胞数和蛋白含量较LPS组显著下降,肺泡上皮细胞凋亡率减少[(19.3±4.6)%];A549细胞活力回升,凋亡率[(15.3±4.5)%]明显降低,差异均有统计学意义 (P<0.05)。LPS组与iCORM3组间上述指标差异均无统计学意义。结论 CO通过抑制肺泡上皮细胞凋亡减轻新生鼠ALI。
一氧化碳释放分子3; 脂多糖; 肺损伤; 凋亡; 肺泡上皮细胞
感染是引起新生儿急性肺损伤(ALI)的常见原因之一[1,2],可表现为免疫系统激活,各种炎症因子和趋化因子表达增加,引起中性粒细胞广泛浸润,导致肺血气交换屏障破坏[3]。肺泡上皮细胞在稳定肺泡结构,维持新生儿正常呼吸功能和应对外源刺激等方面均发挥重要作用[4]。在ALI过程中外来因素的直接损害和过度炎症反应均可破坏肺泡上皮细胞[5.6],而抑制ALI过程中肺泡上皮细胞凋亡能明显缓解病程进展[7],提示在ALI病程中,肺泡上皮细胞可能发挥了重要的作用。一氧化碳(CO)在体内微量存在,主要由血红素氧合酶催化血红素生成,低剂量CO具有抗炎、抗凋亡、抗增殖作用,也有舒张血管的作用[8]。在多种ALI的动物模型中,CO均有良好的肺保护效应[8,9],在败血症新生儿也发现CO的水平显著高于正常新生儿[10]。本研究建立新生鼠ALI模型,以CO释放分子3(CORM3)作为干预药物,探讨CO的保护作用及其相关机制。
1 方法
1.1 试剂 LPS(0111:B4)购于Sigma公司,CORM3购于ApexBIO公司;Tunel试剂盒购于Roche公司;MTT购于Biosharp公司;Dapi购于碧云天生物公司;失活CORM3(iCORM3)为去除CO的羰基骨架结构,采用Stec等[11]方法制备,CORM3室温下放置24 h,使用前用移液枪反复吸吹液体,排出释放的CO气体分子。
1.2 实验动物 清洁级7日龄新生SD大鼠32只,雌雄不限,体重13~18 g,购自第三军医大学附属大坪医院实验动物中心[动物许可证号:SGCK(渝)2012-0005]。
1.3 分组和处理 32只新生大鼠均分为4组: 对照组、LPS组、CORM3组和iCORM3组。新生鼠予10%水合氯醛麻醉,沿颈正中线剪开皮肤,钝性分离肌肉及筋膜,暴露气管。LPS组、CORM3组和iCORM3组予气管内滴注LPS (10 mg·kg-1,生理盐水溶解)20 μL制备新生鼠ALI模型[12];分别腹腔注射100 μL生理盐水、CORM3 (10 mg·kg-1L,生理盐水溶解) 和iCORM3 (10 mg·kg-1L,生理盐水溶解);对照组气管内滴注和腹腔注射100 μL生理盐水。12 h后处死大鼠取肺组织备检。实验动物处置符合动物伦理学要求。
1.4 细胞培养及LPS诱导细胞凋亡 人肺腺癌细胞株A549细胞(第三军医大学野战外科研究所惠赠)以含10%胎牛血清的DMEM培养液,37℃、5%CO2饱和湿度传代培养,0.25%胰酶消化液传代。所有实验均采用对数生长期细胞。LPS和CORM3溶解于DMEM基础培养基中, -20℃保存备用,LPS溶液加药前震荡涡旋,二者同时加药。96孔板细胞接种浓度为每孔10×103,反应体系200 μL;6孔板细胞接种浓度为每孔4×105,反应体系2 mL。
1.5 肺组织湿干(W/D)比值测定 取右肺上叶生理盐水漂洗,吸水纸吸去肺组织表面液体,电子天平称重为肺组织湿重;70℃恒温箱烘烤72 h[13],待肺组织称重不再发生变化,为肺组织干重。
1.6 肺组织病理观察 取右肺中下叶用4%多聚甲醛固定,脱水,包埋,制备组织石蜡切片(4 μm),脱蜡后苏木素-伊红染色,光镜下观察[14]。
1.7 肺泡灌洗液(BALF)蛋白含量及细胞计数测定 取左肺叶以24G留置针沿气管两环状软骨间插入,拔出针芯,1 mL注射器取200 μL冰冻生理盐水缓慢灌洗肺组织3次,每次回收率达>80%。收集BALF,500g、4℃离心10 min,上清用于蛋白含量测定,细胞团块重悬计数[12]。
1.8 细胞凋亡Tunel分析 按照罗氏公司Tunel试剂盒操作说明行染色[15]。将切好的石蜡切片常规脱蜡至水化(细胞标本采用细胞涂片于玻片上制备),3%H2O2甲醇阻断内源性过氧化物酶15 min,PBS漂洗5 min×3次,加入20 mg·mL-1蛋白酶K工作液常温下反应20 min(细胞标本采用0.3% Triton-X-100作用10 min),PBS漂洗5 min×3次,加入Tunel反应液(临用前配置,避光包装置于冰上)于37℃避光反应60 min,同时设置阳性对照和阴性对照,阳性对照在加入Tunel反应液前加入脱氧核糖核酸酶反应10 min,后续步骤同实验组;阴性对照加入不含TdT酶,只含荧光素的液体反应,其余步骤同实验组。PBS漂洗5 min×3次,加入Dapi染核,常温下避光反应20 min;PBS漂洗5 min×3次,DAB显色,当背景开始变深时停止反应,双蒸水漂洗后甲基绿复染核,常规脱水、透明、中性树胶封片。
细胞Tunel实验不进行DAB显色,其余步骤同组织Tunel染色。绿色通道(505~535 nm)可见凋亡细胞显示绿色信号,蓝色通道(475~490 nm)可见细胞核(Dapi染核)为蓝色信号;以细胞胞核Dapi蓝色和Tunel绿色荧光信号重叠为细胞凋亡阳性信号,任意选取3个视野,分别计数A549凋亡细胞和正常细胞。
1.9 MTT法检测细胞活力 取对数生长期细胞,0.25%胰酶消化,将细胞悬液浓度调成5×104·mL-1接种至96孔板,每孔200 μL,细胞培养过夜后分别加入浓度0.000 8、0.004、0.02、0.1、0.5、2.5和12.5 mmol·L-1的CORM3, LPS 200 μg·L-1, 阴性和阳性对照分别加入等量培养液和LPS,每组设5个复孔,培养24 h后加入MTT溶液(5 mg·mL-1)20 μL,4 h后加入150 μL DMSO,摇床上振荡溶解。酶标仪于490 nm波长测定吸光值[16]。
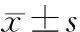
2 结果
2.1 新生鼠肺组织病理变化 对照组肺组织形态结构基本正常,肺间质炎性细胞浸润现象不明显(图1A);LPS组肺组织形态结构明显紊乱、水肿,肺泡压缩,肺间质大量炎性细胞浸润(图1B);CORM3组肺组织水肿较LPS组明显轻微,炎性细胞浸润明显少(图1C);iCORM3组肺组织水肿及炎性细胞浸润同LPS组(图1D)。
2.2 肺组织W/D比值和BALF蛋白含量、细胞计数比较 表1显示,肺组织W/D比值、BALF细胞数目和蛋白含量4组间差异总体上有统计学意义(P均<0.001),组间两两比较,上述3个指标LPS组均显著高于对照组(P<0.05);CORM3组与LPS组比较,W/D比值、BALF细胞计数和蛋白含量显著下降,差异有统计学意义(P<0.05),iCORM3组与LPS组3个指标差异均无统计学意义。
2.3 肺泡细胞凋亡变化 4组肺泡细胞凋亡见图2。对照组、LPS组、CORM3组和iCORM3组肺泡细胞凋亡率分别为(3.0±1.0)%、(37.3±4.5)%、(19.3±4.6)%和(36.7±0.9)%,其中LPS组高于对照组,CORM3组低于LPS组,差异有统计学意义(P<0.05);iCORM3组和LPS组差异无统计学意义。



GroupW/DratioBALF/×104BALFprotein/mg·mL-1Control5.33±0.2113±30.048±0.026LPS6.00±0.1863±90.103±0.028CORM35.59±0.2929±60.056±0.029iCORM35.93±0.3261±100.102±0.029F11.66385.68531.071P<0.001<0.001<0.001
Notes W/D ratio, BALF and BALF protein of LPS group siginificantly differed from control group, allPvalues were less than 0.001; whereas the observations of CORM3 group were siginificantly lower than LPS groups, the correspondingPvalues were 0.005, <0.001 and 0.001, respectively.

图1 4组新生鼠肺组织病理变化(苏木精-伊红染色,20 ×)
Fig 1 The lung tissue pathology of four groups (HE staining, 20×)
Notes A: The control group. The lung tissue kept normal morphological structure, and inflammatory cells infiltrated in the pulmonary interstitium were not obvious. B: LPS group. The morphological structure of lung tissue disordered, alveoli were compressed, and a lot of inflammatory cells infiltrated in pulmonary interstitium. C: The CORM3 group. Compared with the LPS group, the edema of lung tissue was relieved significantly in CORM3 group, and inflammatory cells infiltrated in alveolar interstitium were less. D: The iCORM3 group. The condition of edema of lung tissue and infiltration of inflammatory cells had not obviously improved

图2 4组Tunel检测肺泡细胞凋亡(40×)
Fig 2 The index of AECs apoptosis by Tunel assay(40×)
Notes A: control group, B:LPS group, C: CORM3 group, D: iCORM3 group. Transfer Tunel stain to DAB signal, brown signal of Tunel positive cells could be seen, and normal cells kept pale green signal. The red arrows shown in figure were apoptotic AECs
2.4 LPS刺激对A549细胞活力影响及CORM3的保护作用 图3显示,阳性对照细胞活性显著低于阴性对照,加入CORM3干预后细胞活力逐渐回升,当CORM3浓度>0.1 mmol·L-1时,MTT结果显示OD值不再发生明显变化。
2.5 4组A549细胞凋亡率比较 4组A549细胞凋亡情况如图4所示,对照组细胞凋亡率为(3.6±1.0)%,LPS组细胞凋亡率[(29.6±4.1)%]较对照组增加,差异有统计学意义(P<0.05);CORM3组细胞凋亡率为(15.3±4.5)%,与LPS组差异有统计学意义(P<0.05);iCORM3组细胞凋亡率为(27.6±8.7)%,与LPS组差异无统计学意义。

图3 MTT检测A549细胞活力变化
Fig 3 Detection of A549 cell vitality by MTT
Notes The protective role of CORM3 which directed against the reduction of activity of A549 cells induced by LPS. 1)P<0.05,vsnegative control group; 2)P<0.05,vspositive control (LPS) group

图4 4组Tunel检测A549细胞凋亡(20×)
Fig 4 Detection of A549 cell apoptosis by Tunel assay (20×) in 4 groups
Notes To define the apoptotic A549 cells by Tunel (upper panel) and Dapi (middle panel) double dying. The apoptotic A549 cells appeared green signal after Tunel dying, and the nucleus of A549 cells were dyed blue. They were affirmed as apoptotic cells when Tunel dying and Dapi dying overlapped (lower panel)
3 讨论
新生鼠的免疫系统未完全成熟,在应对外源性刺激时所表现出的反应与成年鼠存在较大差异[17],不同给药方式所造成的组织损伤也有明显区别,在本研究前期的预实验中已得到了证实。因此,构建较理想的新生鼠ALI模型尤为重要。Martin团队研究发现,采用气管直接给予LPS刺激新生鼠和成年鼠,新生鼠BALF中炎性细胞的浸润远低于成年鼠[18]。LPS常用于构建各种炎症模型[19,20],其刺激诱发ALI的方式有多种,包括腹腔给药[21]、尾静脉给药[22]、雾化吸入给药[11]和气管直接给药[12]。在预实验发现,腹腔给药方式建立ALI模型,其损伤程度较轻,个体差异较大;而采用气管给药方式直接作用于肺泡,造成局部的ALI更为稳定,且对其他脏器功能的影响较小,从肺组织病理学来看,LPS刺激12 h后,肺正常组织结构明显紊乱,并伴随大量炎性细胞浸入肺间质和肺泡,肺间质水肿、增宽,肺泡压缩,并伴随有大量肺泡细胞的凋亡,与文献报道的结果相一致[23]。
目前,CO对ALI的保护作用已得到广泛证实,但CO在新生鼠ALI的研究尚少。本研究发现,CORM3组较LPS组其肺组织结构紊乱明显好转,中性粒细胞的浸润明显减少,BALF蛋白含量、细胞数目显著下降;而iCORM3干预未起到治疗作用,提示CORM3复合物中真正发挥作用的是其携带的CO分子,这与CO在成年鼠中的保护效应相一致[24]。既往认为CO的保护与其抗炎效应有关[24,25],过度的炎症反应被认为是造成ALI的主要损伤因素,低浓度CO具有良好的抗炎效果,其机制与多条信号通路均有密切的联系。近年发现, CO还能抑制血管内皮细胞的凋亡,起到保护ALI的作用[26]。本研究肺组织Tunel染色结果证实CORM3抑制了ALI导致的肺泡细胞凋亡。A549具有肺泡上皮细胞的生物学特征,广泛运用于ALI体外实验[27],本研究MTT结果显示,LPS作用24 h后,细胞活力明显下降,而予CORM3干预,细胞活力呈增强趋势。细胞Tunel染色同样证实,CORM3能明显抑制LPS刺激诱导的A549细胞凋亡。
既往研究显示,CO对ALI的保护主要是抑制肺泡巨噬细胞相关炎症因子和趋化因子的释放,减轻炎症反应,同时保护肺血管内皮功能[8]。本研究证实了CO可通过抑制肺泡上皮细胞凋亡,从而发挥其保护肺损伤的作用,这为探讨CO在ALI中的保护机制提供了一个新的研究方向。
本研究的不足之处:由于新生鼠取材受限,没能取得血气变化的结果以及监测血液CO浓度的变化。
[1]Rubenfeld, DG. Epidemiology of acute lung injury. Crit Care Med, 2003, 31(S4): 276-284
[2]胡亚美,江载芳,主编.实用儿科学.第7版.北京:人民卫生出版社,2002:2548
[3]Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol, 2007, 7(5): 379-390
[4] Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med, 2001, 163(4): 1021-1029
[5] Martin TR, Hagimoto N, Nakamura M, et al. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc, 2005, 2(3):214-220
[6] Bem RA, Bos AP, Matute-Bello G, et al. Lung epithelial cell apoptosis during acute lung injury in infancy. Pediatr Crit Care Med, 2007, 8(2):132-137
[7]Ma X, Xu D, Ai Y, et al. Fas inhibition attenuates lipopolysaccharide-induced apoptosis and cytokine release of rat type II alveolar epithelial cells. Mol Biol Rep, 2010, 37(7): 3051-3056
[8]Ryter SW, Choi AM. Carbon monoxide: present and future indications for a medical gas. Korean J Intern Med, 2013, 28(2): 123-140
[9] Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs, 2005, 14(11):1305-1318
[10] Shi Y, Pan F, Li H, et al. Plasma carbon monoxide levels in term newborn infants with sepsis. Biol Neonate, 2000,78(3):230-232
[11] Stec DE, Bishop C, Rimoldi JM, et al. Carbon monoxide (CO) protects renal tubular epithelial cells against cold-rewarm apoptosis. Ren Fail, 2007, 29(5): 543-548
[12] Martin TR, Ruzinski JT, Wilson CB, et al. Skerrett. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response . J Infect Dis, 1995 ,171(1):134-144
[13]Xu M, Cao FL, Zhang YF, et al. Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice. Acta Pharmacol Sin, 2015, 36(2): 179-187
[14]Zhou ZH, Sun B, Lin K, et al. Prevention of rabbit acute lung injury by surfactant, inhaled nitric oxide, and pressure support ventilation. Am J Respir Crit Care Med, 2000, 161(2): 581-588
[15]Li Z, Choo-Wing R, Sun H, et al. A potential role of the JNK pathway in hyperoxia-induced cell death, myofibroblast transdifferentiation and TGF-β1-mediated injury in the developing murine lung. BMC Cell Biol, 2011, 12: 54
[16]Cherng YG, Chang HC, Lin YL, et al. Apoptotic insults to human chondrocytes induced by Sodium nitroprusside are involved in sequential events, including cytoskeletal remodeling, phosphorylation of mitogen-activated protein kinase kinase kinase-1/c-Jun N-terminal kinase, and Bax-mitochondria-mediated caspase activation. J Orthop Res, 2008, 26(7): 1018-1026
[17]Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol, 2004, 4(7): 553-564
[18]Martin TR, Ruzinski JT, Wilson CB, et al. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response . J Infect Dis, 1995,171(1):134-144
[19] McKallip RJ, Ban H, Uchakina ON. Treatment with the hyaluronic Acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation, 2015, 38(3): 1250-1259
[20] Chung KW, Lee EK, Kim DH, et al. Age-related sensitivity to endotoxin-induced liver inflammation: Implication of inflammasome/IL-1for steatohepatitis. Aging Cell , 2015, 14(4):524-533
[21] Bhargava R, Altmann CJ, Andres-Hernando A, et al. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre,but not post,sepsis administration of TNF-α antibodies. PLoS One, 2013, 8(11): e79037
[22] Kimura T, Nojiri T, Hosoda H, et al. C-type natriuretic peptide attenuates lipopolysaccharide-induced acute lung injury in mice. J Surg Res, 2015, 194(2): 631-637
[23]Kitamura Y, Hashimoto S, Mizuta N, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med, 2001, 163(3 Pt 1): 762-769
[24]Ghosh S, Wilson MR, Choudhury S, et al. Effcts of inhaled Carbon monoxide on acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol, 2005, 288(6): L1003-L1009
[25]Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol, 1999, 276(4 Pt 1): L688-L694
[26] Wang X, Wang Y, Kim HP, et al. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem, 2007 ,282(3):1718-1726
[27] Liu X, Shao K, Sun T. SIRT1 regulates the human alveolar epithelial A549 cell apoptosis induced by Pseudomonas aeruginosa lipopolysaccharide. Cell Physiol Biochem, 2013, 31(1):92-101
(本文编辑:丁俊杰)
CORM3 attenuates acute lung injury induced by LPS via inhibiting AECs apoptosis in neonatal rats
CAIKang-xing1,WANGLi1,WANGTing2,LUOLI2,CHENLong1,WANGNan1,SHIYuan1
(1DepartmentofPediatrics,DapingHospital,ThirdMilitaryMedicalUniversity,Chongqing400042; 2Department1,InstituteofSurgeryResearch,DapingHospital,ThirdMilitaryMedicalUniversity,StateKeyLaboratoryofTrauma,BurnsandCombinedInjury,Chongqing400042,China)
SHI Yuan,E-mail:petshi530@vip.163.com
ObjectiveTo study the protective effects of CORM3 treatment on AECs apoptosis after LPS inducing acute lung injury in neonatal rats. MethodsThirty two SD neonate rats were divided equally into four groups, the control group, LPS group,CORM3 group and iCORM3 group. Neonate rat acute lung injury was induced by LPS intratracheal administration in LPS group, CORM3 group and iCORM3 group received treatment of itraperitoneal injection of saline, CORM3 and iCORM3 respectively. The control group received intraperitoneal injection of saline. Animals in each group were sacrificed after 12h modeling, the histopathologic changes were observed by H-E staining, and lung tissue was seperated and weighed, wet and dry ratio of lung tissue was calculated, lung tissue damage was detected by BALF cell counting and protein quantitative analysis. Cultivated A549 cell apoptosis was induced by LPS in vitro, cell activity was determined by MTT test, cell apoptosis was watched by Tunel dyeing.ResultsFirstly, compared with the control group, the morphological structure of lung tissue was disordered in model group, alveoli were compressed, and a lot of inflammatory cells in filtrated in pulmonary interstitium. CORM3 group kept basic normal morphological structure, and inflammatory cells infiltrated in alveolar interstitium were less, the iCORM3 group was consistent with the LPS group in lung morphological structure and inflammatory cells infiltration. Secondly, compared with the control group, the W/D ratio, BALF cell number and protein content increased significantly in LPS group, and the number of AECs apoptosis was increased obviously[(37.3±4.5)%vs(3.0±1.0)%]. The A549 cells activity was decreased, and percentage of apoptosis cells increased significantly[(29.6±4.1)%vs(3.6±1.0)%,P<0.05],with a statistically significant difference. In CORM3 group compared with the LPS group, the W/D ratio, BALF cell number and protein content decreased obviously, and the number of AECs apoptosis was decreased[(19.3±4.6)%]. The A549 cells activity rebounded, and the rate of cells apoptosis[(15.3±4.5)%] decreased significantly, the difference was statistically significant(P < 0.05).There were no significant changes between the iCORM3 group and the CORM3 group in these indexes.ConclusionCORM3 attenuates neonate rat acute lung injury induced by LPS via inhibiting AECs apoptosis.
Carbon monoxide-releasing molecule 3; Lipopolysaccharid; Lung injury; Apoptosis; Alveolar epithelial cell
重庆市科技计划项目:cstc2013yykfA0193, 国家自然科学基金:81100458
1 第三军医大学附属大坪医院儿科 重庆,400042;2 第三军医大学附属大坪医院野战外科研究所一室 重庆,400042
史源,E-mail:petshi530@vip.163.com
10.3969/j.issn.1673-5501.2015.06.012
2015-07-20
2015-11-23)
