肾素-血管紧张素系统基因多态性与儿童青少年高血压易感关联的系统评价和Meta分析
2015-05-04叶冰冰苏丹艳刘冬立覃素元劳金泉庞玉生
叶冰冰 苏丹艳 刘冬立 覃素元 劳金泉 庞玉生
·论著·
肾素-血管紧张素系统基因多态性与儿童青少年高血压易感关联的系统评价和Meta分析
叶冰冰 苏丹艳 刘冬立 覃素元 劳金泉 庞玉生
目的 评价肾素-血管紧张素系统中的血管紧张素转换酶(ACE)基因插入/缺失(I/D)、血管紧张素原(ANG)基因M235T和血管紧张素Ⅱ受体-1(AT1R)基因A1166C多态性与儿童青少年高血压易感的关联。方法 计算机检索EMBASE、PubMed、HUGE、中国知网、维普数据库、万方数据库和中国生物医学文献数据库,检索时间均为建库至2015年11月27日,纳入3个基因多态性与儿童青少年高血压关联的病例对照研究。提取高血压组和对照组基因型和等位基因频率,行文献偏倚风险评价。采用Stata 12.0软件,分别以隐性、显性、共显性、相加和等位基因模型对高血压组和对照组上述3个基因多态性与高血压的关联行Meta分析。根据对照组人群和种族行亚组分析。结果 7篇文献进入Meta分析,病例组824例,对照组1 731例。7篇文献存在中等偏倚风险。①纳入6篇文献的ACE基因I/D多态性在隐性模型下与儿童青少年高血压的发病风险关联(OR=1.405, 95%CI:1.073~1.840,P=0.013)。②上述5种分析模型,ANG基因M235T和AT1R基因A1166C多态性(分别纳入3和2篇文献)与儿童青少年高血压发病风险均无关联。③亚组分析结果显示,2篇文献肥胖人群的ACR基因I/D多态性在隐性、相加和等位基因模型下显著增加儿童青少年高血压的发病风险(隐性模型:OR=1.564 , 95%CI:1.054~2.321,P=0.026;相加模型:OR=2.017, 95%CI:1.137~3.576,P=0.016; 等位基因模型:OR=1.406, 95%CI: 1.076~1.838,P=0.013)。;对一般儿童青少年人群的高血压发病风险无影响,且与种族和高血压分类方法无关。结论ANG基因M235T、AT1R基因A1166C多态性与儿童青少年高血压发病风险无关联,ACE基因I/D多态性可能与肥胖儿童青少年高血压的发病风险关联。
基因多态性; 肾素-血管紧张素系统; 高血压; 儿童青少年; Meta分析; 系统评价
近年来,高血压在儿童青少年中的患病率呈逐年增高趋势[1, 2]。多种族流行病学研究证实儿童青少年高血压的发病率为2%~3%[3, 4]。儿童期血压水平可影响其成年后的血压水平[5]。有研究表明,在总体人群的血压变异原因中,遗传因素占30%~50%[6],高血压的易感基因是近年来关注的热点问题。肾素-血管紧张素系统 (RAS)通过其效应激素血管紧张素原Ⅱ(AⅡ)在调节血压的稳态中起重要作用[7],其中血管紧张素转换酶(ACE)、血管紧张素原(ANG)、血管紧张素Ⅱ受体-1(AT1R)以及肾素作为RAS的重要组成部分,参与了原发性高血压的发生[8, 9]。血浆中ACE和ANG的升高可引起AⅡ的升高,提示RAS的基因多态性在高血压的发生和发展中起重要作用。关于RAS基因多态性和儿童青少年高血压发病风险的研究不多且样本量不大,结果也不一致,因此有必要对RAS基因多态性与儿童青少年高血压的关联行Meta分析,以明确和丰富高血压的易感基因。
1 方法
1.1 高血压定义 按照2004年美国高血压教育项目工作组制定的儿童青少年高血压标准[1],以SBP和(或)DBP≥同性别、同年龄儿童血压P95诊断为高血压;以SBP和(或)DBP在同性别、同年龄儿童血压P90~P95为高血压前期。
1.2 文献纳入标准 ①病例对照研究,高血压和(或)高血压前期为病例组,血压正常为对照组;②研究对象为<18岁的儿童青少年[10];③研究的目的基因为ACE(I/D),ANG(M235T)和AT1R(A1166C)多态性;④能够直接或间接提供不同基因型、等位基因频率的数据。
1.3 文献排除标准 重复发表的文献。
1.4 文献检索策略 数据库:EMBASE、PubMed、哈特福德用户组交流(HUGE)数据库、中国知网,万方数据库和中国生物医学文献数据库,并回溯纳入文献的参考文献,检索起止时间均为建库至2015年11月27日。
英文检索词:angiotensin-converting enzyme,angiotensinogen,angiotensinogen Ⅱ type-1 receptor,renin-angiotensin system, ACE, ANG, AT1R, RAS, gene polymorphism, hypertension,high blood pressure。
以PubMed数据库为例检索式为:(angiotensin-converting enzyme OR angiotensinogen OR angiotensinogen Ⅱ type-1 receptor OR Renin-Angiotensin System OR ACE OR ANG OR AT1R OR RAS)AND (gene polymorphism)AND (hypertension OR high blood pressure) AND (children OR adolescence)。
中文检索词:血管紧张素转换酶、血管紧张素原、血管紧张素Ⅱ受体-1、肾素-血管紧张素系统、基因多态性、高血压、儿童青少年。
以中国知网为例检索式:(血管紧张素转换酶 OR 血管紧张素原 OR 血管紧张素Ⅱ受体-1 OR 肾素-血管紧张素系统) AND 基因多态性 AND 高血压 AND 儿童青少年。
1.5 文献筛选、资料提取和偏倚风险评估 均由叶冰冰和苏丹艳完成,如遇分歧与庞玉生讨论决定。
1.5.1 文献筛选和资料提取 首先阅读文题和摘要排除明显不相关的文献,对可能符合纳入标准的文献阅读全文。提取文献中第一作者、发表时间、国家、语种、种族、样本量、样本来源、实验方法和各基因型在病例组和对照组的例数,并对纳入文献行Hardy-Weinberg平衡检验。

1.6 统计学分析 采用Stata 12.0软件行Meta分析,采用OR作为效应量行Meta分析。异质性检验P<0.10采用随机效应模型,P≥0.10采用固定效应模型合并结果。分别采用隐性、显性、共显性、相加和等位基因模型分析基因多态性与高血压发病风险的关联。对于存在显著统计学异质性的文献,采用亚组分析异质性原因。P<0.05为差异有统计学意义。
2 结果
2.1 文献检索一般情况 初步检索到228篇文献,7篇文献[12~18]符合本文纳入标准进入Meta分析(图1),英文5篇,中文1篇,韩文1篇。高血压组824例,对照组1 731例。6篇文献[13~18]研究ACE基因I/D多态性,3篇文献[12,15,17]研究AT1R基因A1166C多态性,2篇文献[15,17]研究ANG基因M235T多态性。各基因型和等位基因分布情况如表2所示。3篇文献病例组为原发性高血压[15,17,18];余4篇文献中未严格限定为原发性高血压。3篇文献[12,17,18]研究对象为亚洲人群;3篇为欧洲人群[14~16];文献[13]为混合种族背景。6篇研究ACE基因I/D多态性的文献中,文献[13, 16]的病例组和对照组均为肥胖的儿童青少年人群;余4篇[14,15,17,18]为一般儿童青少年人群。纳入7篇文献的一般情况见表1。

图1 文献筛选流程图
Fig 1 Flow chart of aricle screening and selection process
2.2 文献偏倚风险评价 图2显示,纳入的7篇文献均未行连锁不平衡检测、多态性鉴定、计算错误率、多重检验校正及功能验证性实验;7篇文献均未描述是否采用盲法;6篇文献的病例组和对照组均来自同一人群;均描述了病例组人口学及临床信息;对各研究基因型分布的Hardy-Weinberg遗传平衡检验发现,6篇文献的对照组符合Hardy-Weinberg平衡,文献[15]对照组不符合Hardy-Weinberg平衡(P<0.05)。
2.3ACE基因I/D多态性与儿童青少年高血压关联Meta分析 6篇文献报道了ACE基因I/D多态性与儿童青少年高血压的关联,病例组539例,对照组829例,分别采用5种模型分析。表2所示,ACE基因I/D多态性隐性模下与儿童青少年高血压的发病风险关联有统计学意义(OR=1.405, 95%CI:1.073~1.840,P=0.013)。异质性存在于显性模型,相加模型和等位基因模型中(显性模型:I2=51.6%;相加模型:I2=60.8%;等位基因模型:I2=61.6%)
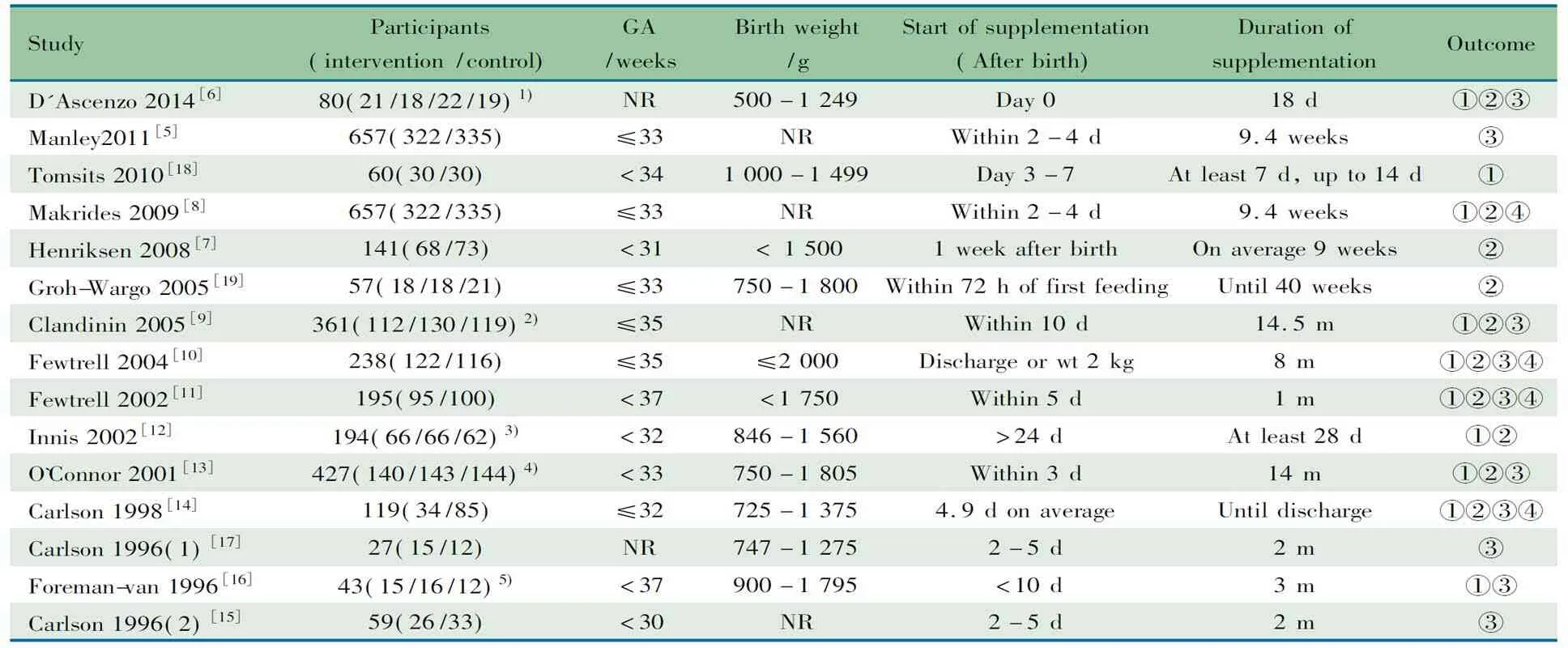
表1 7篇文献的基本情况(n)
Notes HWE: Hardy-Weinberg equilibrium;ACED/I: angiotensin converting enzyme gene I/D polymorphism;AT1RA1166C: angiotensin II type 1 receptor gene A1166C polymorphism;ANGM235T: angiotensinogen gene M235T polymorphi
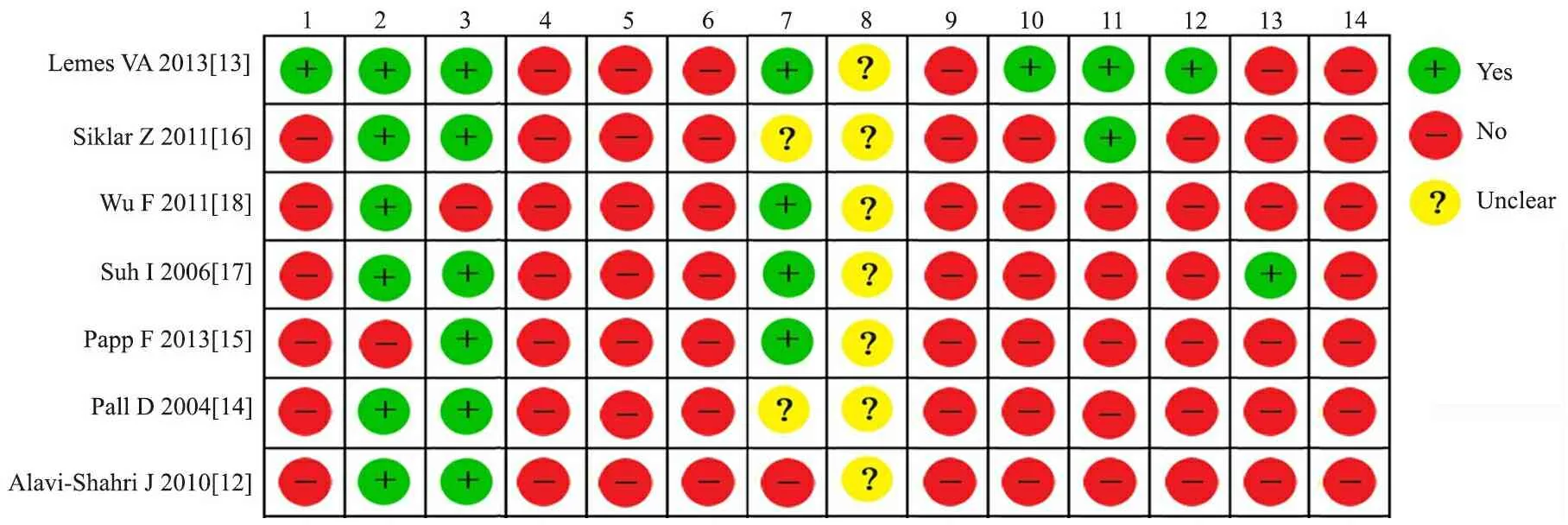
图2 7篇文献的偏倚风险评价结果
Fig 2 Quality of 7 included studies
Notes 1:power;2:controls characterization;3:case characterization;4: LD exploration;5:polymorphism identification;6:genotyping error check;7:Hardy-Weinberg equilibrium;8: blinding;9:multiple testing ;10:covariate adjusment;11:risks;12:population stratification adjustment;13:replication;14:functional study
2.4AT1R基因A1166C多态性与儿童青少年高血压关联的Meta分析 3篇文献病例组149例,对照组578例,如表2所示,无论何种模型,AT1R基因A1166C多态性与儿童青少年高血压的发病风险均关联无统计学意义,隐性模型结果见图3。在所有模型中,各研究间异质性均无统计学意义。
2.5ANG基因M235T多态性与儿童青少年高血压关联的Meta分析 2篇文献病例组136例,对照组324例。如表2所示,合并效应量结果显示所有模型ANG基因M235T多态性与儿童青少年高血压的发病风险关联均无统计学意义,隐性模型结果见图3。异质性存在显性模型和共显性模型中(显性模型:I2=78.9%;共显模型:I2=89.3%)。
表2ACED/I,AT1RA1166C和ANGM235T多态性与儿童青少年高血压发病风险的Meta分析结果
Tab2ResultsofthepooleddataofACED/I, AT1RA1166C, ANGM235Tpolymorphismandhypertensioninpediatricpatientsinmeta-analysis
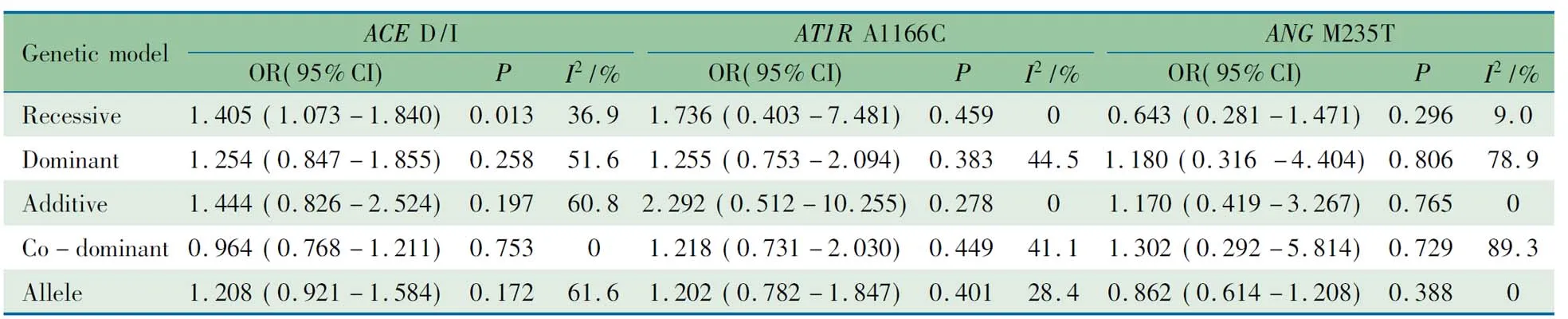
Geneticmod-elACED/IOR(95%CI)PI2/%AT1RA1166COR(95%CI)PI2/%ANGM235TOR(95%CI)PI2/%Recessive1.405(1.073-1.840)0.01336.91.736(0.403-7.481)0.45900.643(0.281-1.471)0.2969.0Dominant1.254(0.847-1.855)0.25851.61.255(0.753-2.094)0.38344.51.180(0.316-4.404)0.80678.9Additive1.444(0.826-2.524)0.19760.82.292(0.512-10.255)0.27801.170(0.419-3.267)0.7650Co-domi-nant0.964(0.768-1.211)0.75301.218(0.731-2.030)0.44941.11.302(0.292-5.814)0.72989.3Allele1.208(0.921-1.584)0.17261.61.202(0.782-1.847)0.40128.40.862(0.614-1.208)0.3880
Notes VV: variant homozygous; WV: heterozygous; WW: wild homozygous. Recessive model: VVvsWV+WW; dominant model: VV+WVvsWW; additive model: VVvsWW; co-dominant model: WVvsVV+WW; allele model: VvsW

图3 隐性模型下ACEI/D,ANGM235T和AT1RA1166C多态性与儿童青少年高血压易感关联的Meta分析
Fig3ForestplotsoftherelationshipbetweenACE (I/D), ANG (M235T), AT1R(A1166C) polymorphisms and hypertension in overall pediatric patients
2.6 亚组分析 报道ACE基因I/D多态性与儿童青少年高血压关联的6篇文献中,有2篇文献的病例组和对照组均为肥胖的儿童青少年人群[13, 16],行亚组分析。如表3所示,在肥胖人群中,ACE基因I/D多态性在隐性、相加和等位基因模型下显著增加儿童青少年高血压的发病风险(隐性模型:OR=1.564 , 95%CI:1.054~2.321,P=0.026;相加模型:OR=2.017, 95%CI:1.137~3.576,P=0.016; 等位基因模型:OR=1.406, 95%CI: 1.076~1.838,P=0.013)。在一般人群中,5种模型ACE基因I/D多态性与儿童青少年高血压的发病风险关联均无统计学意义。
以种族、高血压分类在一般人群中行亚组分析显示(表4),不同种族、高血压分类ACE基因I/D多态性与儿童青少年高血压的发病风险均无关联。
3 讨论
本文纳入7篇病例对照设计的遗传关联性研究,文献偏倚风险的评价结果提示:7篇文献在研究设计、病例组及对照组来源、一般信息、实验方法、统计学方法等条目的符合率较低。6/7篇文献对照组符合Hardy-Weinberg平衡。由于纳入文献数量少,未行发表偏倚检验。本文Meta分析的证据强度为中等。
RAS是复杂的内分泌和旁分泌系统,其组成成分介导了血压稳态的调节。因此,RAAs基因编码的组件是高血压遗传研究的重要候选基因。ACE基因定位于17q23,其第16个内含子第287个碱基的插入和(或)缺失多态性与高血压有关。ANG基因位于1q42-43,当编码ANG的外显2区域核苷酸704处胸腺嘧啶T被胞嘧啶C替代, 导致基因编码产物第235号氨基酸由蛋氨酸M变异为苏氨酸T,即M235T分子变异[19]。同时发现AT1R基因位于1166处的胸腺嘧啶A变异为胞嘧啶C,会出现3种基因型,AA, AC和CC,并与高血压的发生有关[8]。ACE基因I/D 和ANG基因M235T 多态性被证实与血浆中ACE和ANG的水平有关[20,21]。而ACEⅡ则是通过AT1R发挥作用。ACE基因作为RAS的重要组成部分,其多态性将会导致血管收缩反应,打破循环稳态。有研究报道在成人中ACE基因 DD 基因型和高水平的ACE有关[22,23]。Ajala等[24]在巴西儿童中发现携带DD 基因型儿童有更高ACE水平及活性。这种效应在韩国儿童中也有同样的发现[25]。提示,ACE基因多态性通过影响ACE的水平及活性,最终影响血压水平。
表3ACED/I 多态性和儿童青少年高血压发病风险关联的人群分层Meta分析结果
Tab 3 Subgroup analysis of association betweenACED/I polymorphism and risk of hypertension in pediatric patients stratified by population in meta-analysis
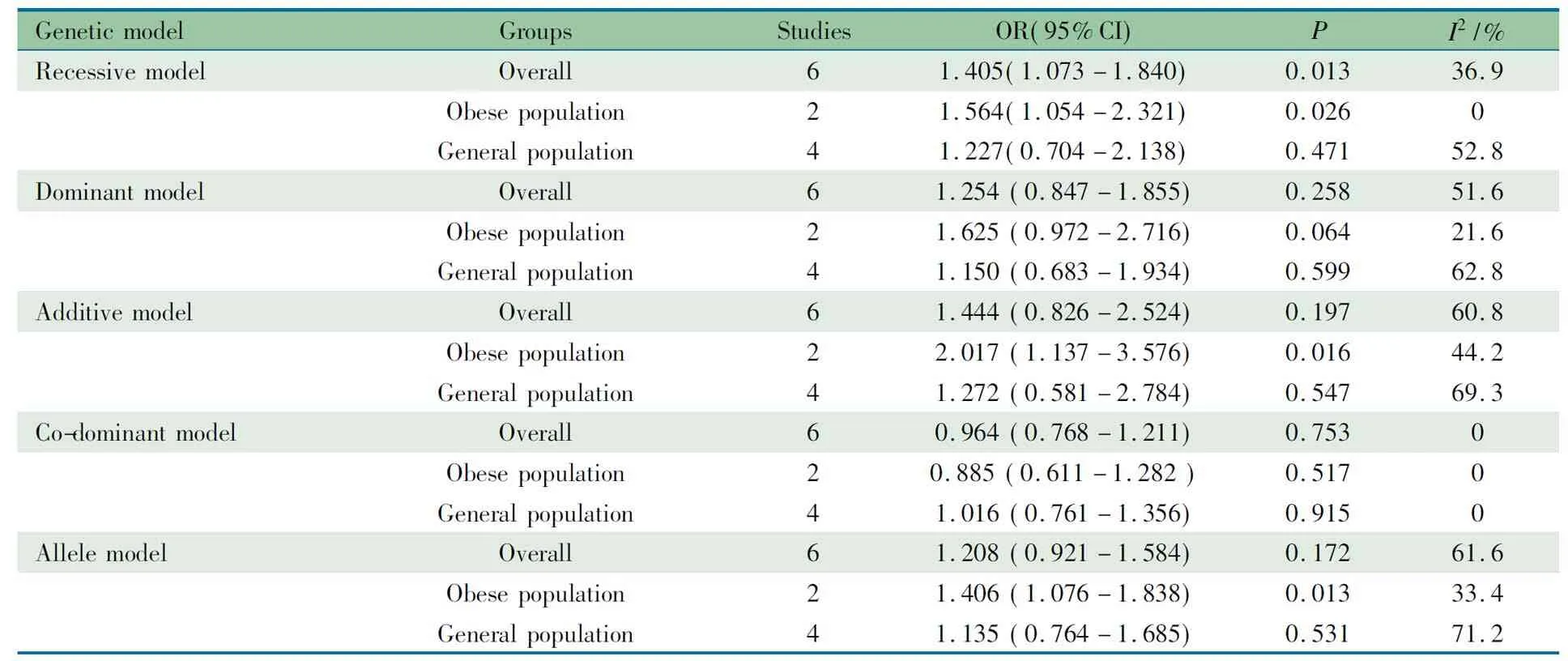
GeneticmodelGroupsStudiesOR(95%CI)PI2/%RecessivemodelOverall61.405(1.073-1.840)0.01336.9Obesepopulation21.564(1.054-2.321)0.0260Generalpopulation41.227(0.704-2.138)0.47152.8DominantmodelOverall61.254(0.847-1.855)0.25851.6Obesepopulation21.625(0.972-2.716)0.06421.6Generalpopulation41.150(0.683-1.934)0.59962.8AdditivemodelOverall61.444(0.826-2.524)0.19760.8Obesepopulation22.017(1.137-3.576)0.01644.2Generalpopulation41.272(0.581-2.784)0.54769.3Co-dominantmodelOverall60.964(0.768-1.211)0.7530Obesepopulation20.885(0.611-1.282)0.5170Generalpopulation41.016(0.761-1.356)0.9150AllelemodelOverall61.208(0.921-1.584)0.17261.6Obesepopulation21.406(1.076-1.838)0.01333.4Generalpopulation41.135(0.764-1.685)0.53171.2
Notes VV: variant homozygous; WV: heterozygous; WW: wild homozygous. Recessive model: VVvsWV+WW; dominant model: VV+WVvsWW; additive model: VVvsWW; co-dominant model: WVvsVV+WW; allele model: VvsW
表4ACED/I 多态性和儿童青少年高血压发病风险在一般人群中的亚组Meta分析结果
Tab 4 Stratification analyses of the relationship betweenACED/I polymorphism and hypertension in general population in meta-analysis
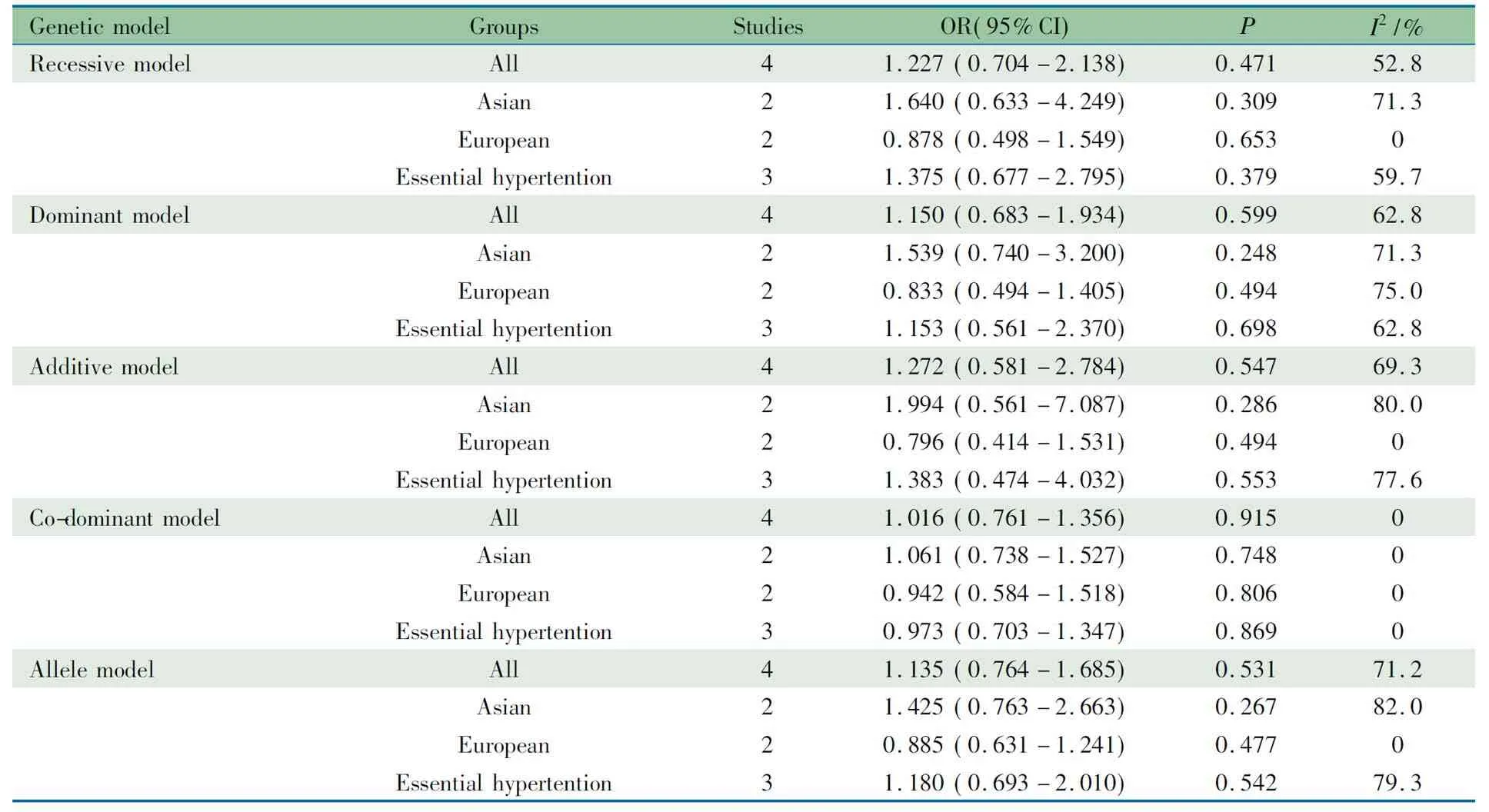
GeneticmodelGroupsStudiesOR(95%CI)PI2/%RecessivemodelAll41.227(0.704-2.138)0.47152.8Asian21.640(0.633-4.249)0.30971.3European20.878(0.498-1.549)0.6530Essentialhypertention31.375(0.677-2.795)0.37959.7DominantmodelAll41.150(0.683-1.934)0.59962.8Asian21.539(0.740-3.200)0.24871.3European20.833(0.494-1.405)0.49475.0Essentialhypertention31.153(0.561-2.370)0.69862.8AdditivemodelAll41.272(0.581-2.784)0.54769.3Asian21.994(0.561-7.087)0.28680.0European20.796(0.414-1.531)0.4940Essentialhypertention31.383(0.474-4.032)0.55377.6Co-dominantmodelAll41.016(0.761-1.356)0.9150Asian21.061(0.738-1.527)0.7480European20.942(0.584-1.518)0.8060Essentialhypertention30.973(0.703-1.347)0.8690AllelemodelAll41.135(0.764-1.685)0.53171.2Asian21.425(0.763-2.663)0.26782.0European20.885(0.631-1.241)0.4770Essentialhypertention31.180(0.693-2.010)0.54279.3
Notes VV: variant homozygous; WV: heterozygous; WW: wild homozygous. Recessive model: VVvsWV+WW; dominant model: VV+WVvsWW; additive model: VVvsWW; co-dominant model: WVvsVV+WW; allele model: VvsW. EH: essential hypertension
本文Meta分析发现,ANG基因M235T和AT1R基因A1166C多态性,在任意一种模型下与儿童青少年高血压发病风险均无统计学关联。ACE基因I/D多态性在隐性模型下与高血压的发病风险有统计学关联(OR=1.405, 95%CI: 1.073~1.840),但效应值的95%CI下限接近无效值1,结果具有显著的不精确性。有研究显示,肥胖引起的儿童青少年高血压发病风险为1.7~7.2[26~29],本文分层分析显示,ACE基因I/D多态性在隐性、相加和等位基因模型下可显著增加肥胖儿童青少年高血压的发病风险,但结果仍具显著的不精确性;在一般人群中,ACE基因I/D多态性与儿童青少年高血压的发病风险均无统计学关联;提示ACE基因I/D多态性对个体血压水平的影响可能通过肥胖介导。有研究显示ACE基因I/D多态性与肥胖相关,D等位基因为风险因素[13, 30]。
本研究的不足之处和局限性:纳入文献的数量和样本量均不大,且文献偏倚风险较高,亟待更多的研究证实RAS基因多态性与儿童青少年高血压关联。
结论:ANG基因M235T、AT1R基因A1166C与儿童青少年高血压发病风险无关联,ACE基因I/D多态性可能与肥胖儿童青少年高血压的发病风险关联。
[1] The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics, 2004,114(2 Suppl 4th Report):555-576
[2] Kavey RE, Daniels SR, Flynn JT. Management of high blood pressure in children and adolescents. Cardiol Clin, 2010,28(4):597-607
[3] Redwine KM, Acosta AA, Poffenbarger T, et al. Development of hypertension in adolescents with pre-hypertension. J Pediatr, 2012,160(1):98-103
[4] Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics, 2004,113(3 Pt 1):475-482
[5] Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation, 2008,117(25):3171-3180
[6] Munroe PB, Caulfield MJ. Genetics of hypertension. Curr Opin Genet Dev, 2000,10(3):325-329
[7] Thiel B, Weder AB. Genes for Essential Hypertension: Hype, Help, or Hope? J Clin Hypertens (Greenwich), 2000,2(3):187-193
[8] Bonnardeaux A, Davies E, Jeunemaitre X, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension, 1994,24(1):63-69
[9] Lifton RP. Molecular genetics of human blood pressure variation. Science, 1996, 272(5262):676-680
[10] 王卫平,主编. 儿科学. 第8版. 北京:人民卫生出版社, 2013.3-4
[11] Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature, 2007,447(7145):655-660
[12] Alavi-Shahri J, Behravan J, Hassany M, et al. Association between angiotensin II type 1 receptor gene polymorphism and metabolic syndrome in a young female Iranian population. Arch Med Res, 2010,41(5):343-349
[13] Lemes VA, Neves AL, Guazzelli IC, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with increased adiposity and blood pressure in obese children and adolescents. Gene, 2013,532(2):197-202
[14] Pall D, Settakis G, Katona E, et al. Angiotensin-converting enzyme gene polymorphism, carotid intima-media thickness, and left ventricular mass index in adolescent hypertension. J Clin Ultrasound, 2004,32(3):129-135
[15] Papp F, Friedman AL, Bereczki C, et al. Renin-angiotensin gene polymorphism in children with uremia and essential hypertension. Pediatr Nephrol, 2003;18(2):150-154
[16] Siklar Z, Berberoglu M, Savas Erdeve S, et al. Contribution of clinical, metabolic, and genetic factors on hypertension in obese children and adolescents. J Pediatr Endocrinol Metab,2011,24(1-2):21-24
[17] Suh I, Nam CM, Kim S, et al. Association analysis of the essential hypertension susceptibility genes in adolescents: Kangwha study. J Prev Med Public Health, 2006,39(2):177-183
[18] Wu F(吴凡), Li GL, Song XH, et al.Relationship between angiotensin converting enzyme gene polymorphism and essential hypertension in children. Chin J Contemp Pediatr(中国当代儿科杂志),2011,13(11):883-885
[19] Porto PI, Garcia SI, Dieuzeide G, et al. Renin-angiotensin-aldosterone system loci and multilocus interactions in young-onset essential hypertension. Clin Exp Hypertens, 2003,25(2):117-130
[20] Bloem LJ, Manatunga AK, Tewksbury DA, et al. The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Invest, 1995,95(3):948-953
[21] Erdos EG, Skidgel RA. The angiotensin I-converting enzyme. Lab Invest,1987,56(4):345-348
[22] Giner V, Poch E, Bragulat E, et al. Renin-angiotensin system genetic polymorphisms and salt sensitivity in essential hypertension. Hypertension, 2000,35(1 Pt 2):512-517
[23] Tiret L, Rigat B, Visvikis S, et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet, 1992, 51(1):197-205
[24] Ajala AR, Almeida SS, Rangel M, et al. Association of ACE gene insertion/deletion polymorphism with birth weight, blood pressure levels, and ACE activity in healthy children. Am J Hypertens, 2012,25(7):827-832
[25] Min J, Kim YJ, Lee H, et al. Is the association between ACE genes and blood pressure mediated by postnatal growth during the first 3 years? Early Hum Dev, 2012,88(6):425-429
[26] Dyson PA, Anthony D, Fenton B, et al. High rates of child hypertension associated with obesity: a community survey in China, India and Mexico. Paediatr Int Child Health, 2014,34(1):43-49
[27] Hosseini M, Ataei N, Aghamohammadi A, et al. The relation of body mass index and blood pressure in Iranian children and adolescents aged 7-18 years old. Iran J Public Health. 2010;39(4):126-134
[28] Lu X, Shi P, Luo CY, et al. Prevalence of hypertension in overweight and obese children from a large school-based population in Shanghai, China. BMC Public Health, 2013;13:24
[29] Monyeki KD, Kemper HC, Makgae PJ. The association of fat patterning with blood pressure in rural South African children: the Ellisras Longitudinal Growth and Health Study. Int J Epidemiol, 2006,35(1):114-120
[30] Eisenmann JC, Sarzynski MA, Glenn K, et al. ACE I/D genotype, adiposity, and blood pressure in children. Cardiovasc Diabetol, 2009,8:14
(本文编辑:丁俊杰)
The association between renin-angiotensin gene polymorphisms and risk of childhood hypertension: a systematic review and meta-analysis
YEBing-bing,SUDan-yan,LIUDong-li,QINSu-yuan,LAOJin-quan,PANGYu-sheng
(DepartmentofPediatrics,TheFirstAffiliatedHospitalofGuangxiMedicalUniversity,Nanning530021,GuangxiAutonomousRegion,China)
PANG Yu-sheng,E-mail:pangyush@163.com
ObjectiveTo explore the association between the genetic markers angiotensin converting enzyme (ACE) (I/D), angiotensinogen (ANG) (M235T) and angiotensin Ⅱ receptor-1AT1R(A1166C) and risk of hypertension in pediatric subjects.MethodsEMBASE, PubMed, Hartford User Group Exchange (HUGE), CNKI, VIP, Wanfang data and CBM database were searched for the case-control studies on the association of three gene polymorphisms with hypertension in pediatric patients from the establishment to November 27th2015. Data extraction, quality assessment and pooled analysis were conducted. Meta-analysis on the association of three gene polymorphisms with hypertension between hypertension group and control group under recessiveness, dominance, co-dominance, addition and allele gene models were performed. Statistical analysis was performed by Stata 12.0 software.ResultsA total of 824 patients and 1 731 controls from 7 case-control studies with moderate bias risk were included. An association between hypertension andACE(I/D) D variant was identified in 6 included studies in the meta-analysis under recessive model (OR=1.405, 95%CI =1.073-1.840,P=0.013). Neither ANG (M235T) polymorphism in 3 enrolled studies norAT1R(A1166C) polymorphism in 2 enrolled studies was associated with hypertension in pediatric population under recessiveness, dominance, co-dominance, addition or allele gene models. Subgroup analysis revealed thatACE(I/D) D variant was associated with an increased risk of hypertension in obese pediatric population under recessive model(OR=1.564 , 95%CI:1.054-2.321,P=0.026), additive model (OR=2.017, 95%CI:1.137-3.576,P=0.016) and allele model (OR=1.406, 95%CI: 1.076-1.838,P=0.013). There were no significant differences in the frequencies distribution ofACE(I/D) between two groups in general population by considering ethnicity and classification of hypertension. ConclusionNo significant association was found between ANG M235T,AT1RA1166C polymorphisms and hypertension in pediatric population. TheACE(I/D) polymorphism might be associated with susceptibility to hypertension in the obese pediatric population.
Gene polymorphism; Renin-angiotensin; Hypertension; Pediatrics and children; Meta-analysis; Systematic review
广西医科大学第一附属医院儿科 南宁,530021
庞玉生,E-mail:pangyush@163.com
10.3969/j.issn.1673-5501.2015.06.009
2015-07-10
2015-11-15)
