新型4-氨基喹唑啉衍生物的合成及其抗肿瘤活性*
2015-04-23李文举徐海丽欧阳贵平
李文举,徐海丽,欧阳贵平
(1.贵州省环境监测中心站,贵州贵阳 550081;2.贵州师范学院化学与生命科学学院,贵州贵阳 550018;3.贵州大学精细化工研究开发中心,贵州贵阳 550025)
近些年来,4-氨基喹唑啉类化合物因具有优良的生物活性,备受人们的广泛关注,成为生物学界和化学界学者们研究的热点。4-氨基喹唑啉衍生物还具有广泛的生物活性,对 EGF受体或PDGF受体酪氨酸激酶产生较好的抑制作用,表现出具有抗肺癌、胃癌、胆囊癌和前列腺癌等的功效,抗菌,抗HIV,抗炎,治疗糖尿病等作用,如吉非替尼、厄洛替尼、二甲苯磺酸拉帕替尼和卡纽替尼二盐酸盐等上市药物。
为了寻找新型的4-氨基喹唑啉衍生物,并研究其抗肿瘤活性,本文以香草醛(1)为原料,与1-溴-3-氯丙烷反应后,再与盐酸羟胺反应制得4-(3-氯丙氧基)-3-甲氧基苯腈(3);3经硝酸硝化,铁粉还原,与N,N-二甲基甲酰胺二甲缩醛(DMFDMA)反应后,再与3-氯-4-氟苯胺合环,最后与芳香醚(8a~8g)经取代反应合成了7个新型的4-氨基喹唑啉衍生物(9a~9g,Scheme 1),其结构经1H NMR,13C NMR,IR和元素分析表征。并测定了9a~9g抑制人乳腺癌细胞Bcap-37的体外增殖性。
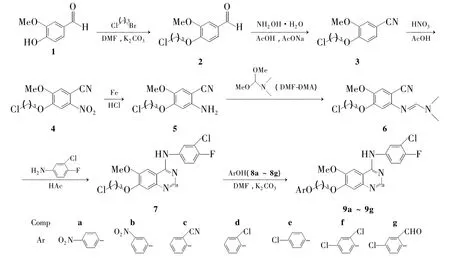
Scheme 1
1 实验部分
1.1 仪器与试剂
X-4型显微熔点仪(温度未校正);ZF-I型三用紫外分析仪;ECX-500型核磁共振仪(DMSO-d6为溶剂,TMS为内标);Shimadzu IR Prestige-21型红外光谱仪(KBr压片);Vario III型元素分析仪。
1.2 合成
(1)4-(3-氯丙氧基)-3-甲氧基苯甲醛(2)的合成
在三口瓶中依次加入1 1.5 g(9.8 mmol),1-溴-3-氯丙烷2 mL(20 mmol),DMF 10 mL和碳酸钾2 g,搅拌下于85℃反应4 h(TLC跟踪)。搅拌下将反应液倒入冰水中静置析晶,抽滤,滤饼用冷水洗涤2次~3次后用乙酸乙酯重结晶得白色固体2,产率 89.8%,m.p.88 ℃ ~91 ℃;1H NMR(CDCl3)δ:2.31 ~2.36(m,2H,CH2CH2CH2),3.77 ~3.79(t,2H,CH2Cl),3.92(s,3H,OCH3),4.25 ~4.27(t,2H,CH2O),7.00 ~7.02(d,J=7.56 Hz,1H,ArH),7.26(s,1H,ArH),7.41(d,J=7.82 Hz,1H,ArH),9.85(s,1H,CHO)。
(2)3的合成
在三口瓶中依次加入2 1.4 g(6 mmol),盐酸羟胺0.7 g,醋酸钠0.8 g 及醋酸 15 mL,搅拌下于106℃反应8 h(TLC跟踪)。冷却至室温,倒入饱和氯化钠溶液中,有白色固体析出,抽滤,滤饼用乙酸乙酯溶解,无水硫酸镁干燥,蒸出溶剂得白色固体3,产率 94.9%,m.p.89 ℃ ~91 ℃;1H NMR(CDCl3)δ:2.30 ~2.32(m,2H,CH2CH2CH2),3.77 ~3.78(t,J=6.3 Hz,2H,CH2Cl),3.88(s,3H,OCH3),4.20 ~ 4.21(t,J=5.73 Hz,2H,CH2O),6.92 ~6.93(d,J=8.05 Hz,1H,ArH),7.08(s,1H,ArH),7.26 ~7.28(d,J=7.96 Hz,1H,ArH)。
(3)4-(3-氯丙氧基)-5-甲氧基-2-硝基苯腈(4)的合成
在三口瓶中依次加入3 0.4 g(1.7 mmol),AcOH 5 mL及AC2O 5 mL,搅拌下于<5℃滴加HNO34 mL,滴毕,于室温反应7 h(TLC跟踪)。搅拌下倒入冰水中,用乙酸乙酯(3×10 mL)萃取,合并有机层,依次用饱和碳酸氢钠溶液(3×10 mL)和食盐水(3×10 mL)洗涤,无水硫酸镁干燥,旋蒸除溶得黄色固体4,产率90.6%,m.p.130 ℃ ~133 ℃;1H NMR(CDCl3)δ:2.33~2.35(m,2H,CH2CH2CH2),3.75 ~3.78(t,J=6.3 Hz,2H,CH2Cl),3.98(s,3H,OCH3),4.29 ~4.31(t,J=6.0 Hz,2H,OCH2),7.19 ~7.25(s,1H,ArH),7.81(s,1H,ArH)。
(4)2-氨基-4-(氯丙氧基)-5-甲氧基苯腈(5)的合成
在三口瓶中依次加入乙酸6 mL,甲醇4 mL及铁粉1 g,搅拌下于70℃ ~75℃反应10 min。于20 min内分批加入4 1.2 g,加毕,反应2 h(TLC跟踪)。趁热过滤,滤饼用乙醇(3×10 mL)洗涤,合并滤液与洗液,旋蒸除去部分溶剂后加入水中,用乙酸乙酯(3×10 mL)萃取,合并有机层,依次用饱和碳酸氢钠溶液(3×10 mL)和食盐水(3×10 mL)洗涤,无水硫酸镁干燥,旋蒸除溶得棕色固体5,产率63.4%,m.p.120 ℃ ~122 ℃;1H NMR(CDCl3) δ:2.28 ~ 2.31(m,2H,CH2CH2CH2),3.74 ~ 3.76(t,J=6.3 Hz,2H,CH2Cl),3.77(s,3H,OCH3),4.13 ~4.15(t,J=5.7 Hz,2H,OCH2),4.16(b,2H,NH2),6.27(s,1H,ArH),6.80(s,1H,ArH)。
(5)N-[5-(3-氯丙氧基)]-2-腈基-4-甲氧苯基-N,N-二甲基甲酰胺(6)的合成
在三口瓶中依次加入5 1 g,DMF-DMA 1 mL,甲苯20 mL及乙酸3滴,装上分水器,搅拌下回流(106℃)反应2 h(TLC跟踪)。降至室温,旋蒸除甲苯,残余物静置析晶,过滤,滤饼干燥得白色固体6,产率 76.8%,m.p.108 ℃ ~109 ℃;1H NMR(CDCl3)δ:2.28 ~2.31(m,2H,CH2CH2CH2),3.06(s,6H,CH3),3.74 ~ 3.77(t,J=6.3 Hz,2H,CH2Cl),3.80(s,3H,OCH3),4.17 ~4.19(t,J=5.7 Hz,2H,OCH2),6.49(s,1H,ArH),6.93(s,1H,ArH),7.56(s,1H,NCH)。
(6)N-(3-氯-4-氟苯胺基)-7-(3-氯丙氧基)-6-甲氧基喹唑啉-4-胺(7)的合成
在三口瓶中依次加入6 0.5 g(1.7 mmol),3-氯-4-氟苯胺0.6 g(4.1 mmol)及乙酸15 mL,搅拌下回流(120℃)反应2.5 h(TLC跟踪)。降至室温,倒入冰水中,溶液变混浊,用氨水调至pH 8~9。加乙酸乙酯10 mL,搅拌2 h,有固体析出,过滤,滤饼倒入混合溶剂[V(甲醇)∶V(盐酸)=10∶1]中搅拌,过滤,滤饼用蒸馏20 mL溶解,用氨水调至pH 8~9。过滤,滤饼干燥得灰白色固体 7,产率 61.3%,m.p.221 ℃ ~226 ℃;1H NMR δ:2.28 ~2.32(m,2H,CH2CH2CH2),3.82 ~3.85(t,J=6.25 Hz,2H,CH2Cl),4.01(s,3H,OCH3),4.28 ~4.31(t,J=6.6 Hz,2H,OCH2),7.33(s,1H,ArH),7.50 ~7.54(t,J=8.55 Hz,1H,ArH),7.76 ~ 7.69(m,1H,ArH),8.06 ~8.08(m,1H,ArH),8.82(s,1H,a-H),10.99(s,1H,NH)。
(7)9a~9g的合成(以9a为例)
在三口瓶中依次加入7 0.5 g(1.26 mmol),对硝基苯酚(8a)0.5 g(3.5 mmol),DMF 15 mL及K2CO32 g,搅拌下于85℃反应4 h~7 h(TLC跟踪)。倒入水中静置析晶,过滤,滤饼经硅胶柱层析(洗脱剂:乙酸乙酯)纯化得黄色固体9a。
用类似的方法合成黄色固体9b和白色固体9c~9g。
N-(3-氯-4-氟苯基)-6-甲氧基-7-[3-(4-硝基苯氧基)丙氧基]喹唑啉-4-胺(9a):产率80%,m.p.182 ℃ ~ 186 ℃;1H NMR δ:2.30 ~ 2.32(m,2H,CH2CH2CH2),3.96(s,3H,OCH3),4.32 ~4.34(t,J=6.3 Hz,4H,OCH2),7.19 ~7.25(d,2H,ArH),7.25(s,1H,ArH),7.43 ~7.47(t,J=9.15 Hz,1H,ArH),7.78 ~ 7.81(m,2H,ArH),8.11 ~8.13(m,1H,ArH),8.20 ~8.22(d,2H,ArH),8.50(s,1H,a-H),9.57(s,1H,NH);13C NMR δ:28.61,56.77,65.57,65.88,102.29,108.34,109.31,115.59,117.00,117.17,119.25,122.93,124.09,126.44,141.32,147.28,149.59,153.17,154.03,156.60,164.32;IR ν:3 427,2 933,1 624,1 591,1 506,1 419,1 263,1 112,1 018,864,690,547 cm-1;Anal.calcd for C24H20N4O5FCl(498.9):C 57.78,H 4.04,N 11.23;found C 57.24,H 4.31,N 10.64。
N-(3-氯-4-氟苯基)-6-甲氧基-7-[3-(3-硝基苯氧基)丙氧基]-喹唑啉-4-胺(9b):产率 83.2%,m.p.185 ℃ ~186 ℃;1H NMR δ:2.29 ~2.33(m,2H,CH2CH2CH2),3.96(s,3H,OCH3),4.29 ~4.33(t,4H,OCH2),7.24(s,1H,ArH),7.43 ~7.47(t,3H,ArH),7.56 ~ 7.60(t,1H,ArH),7.74(s,1H,ArH),7.78 ~7.81(m,3H,ArH),7.89 ~7.90(d,2H,ArH),8.12 ~8.13(m,1H,ArH),8.50(s,1H,a-H),9.55(s,1H,NH);13C NMR δ:28.69,56.77,65.61,65.70,102.31,108.48,109.26,116.08,116.98,117.16,119.22,119.37,122.47,122.77,123.95,131.24,137.31,147.46,149.26,149.56,153.17,154.01,156.51,159.52;IR ν:3 442,2 931,1 683,1 577,1 498,1 247,1 215,1 026,856,738,542 cm-1;Anal.calcd for C24H20N4O5FCl(498.9):C 57.78,H 4.04,N 11.23;found C 57.51,H 4.39,N 11.15。
2-{3-[4-(3-氯-4-氟苯胺基)-6-甲氧基喹唑啉-7-基氧基]丙氧基}苯腈(9c):产率59.3%,m.p.226 ℃ ~229 ℃;1H NMR δ:2.29 ~2.34(m,2H,CH2CH2CH2),3.84(s,3H,OCH3),4.33 ~4.34(t,J=6.3 Hz,2H,OCH2),4.35 ~ 4.36(t,J=6.3 Hz,2H,OCH2),7.08 ~ 7.11(t,J=7.45 Hz,1H,ArH),7.24(s,1H,ArH),7.31 ~7.33(d,J=8.0 Hz,1H,ArH),7.43 ~7.47(t,J=9.15 Hz,1H,ArH),7.65 ~7.68(m,1H,ArH),7.72 ~7.73(m,1H,ArH),7.78 ~7.82(m,2H,ArH),8.12 ~8.14(m,1H,ArH),9.50(s,1H,a-H),9.58(s,1H,NH);13C NMR δ:28.67,56.79,65.48,65.94,101.19,102.4,108.46,113.62,121.72,122.81,122.87,123.98,134.22,135.69,147.49,149.64,153.2,153.12,154.02,154.64,156.12,156.57,160.52,169.69,218.95;IR ν:3 385,2 933,1 627,1 577,1 498,1 450,1 421,1 261,1 217,1 111,752,540 cm-1;Anal.calcd C25H20N4O3FCl(478.9):C 62.70,H 4.21,N 11.70;found C 62.69,H 4.19,N 11.66。
N-(3-氯-4-氟苯基)-7-[3-(2-氯苯氧基)丙氧基]-6-甲氧基喹唑啉-4-胺(9d):产率56%,m.p.103 ℃ ~105 ℃;1H NMR δ:2.29 ~2.32(m,2H,CH2CH2CH2),3.69(s,3H,OCH3),4.25 ~4.27(t,J=6.40 Hz,2H,OCH2),4.33 ~4.36(t,J=6.20 Hz,2H,OCH2),6.94 ~6.98(m,1H,ArH),7.20 ~7.24(m,2H,ArH),7.29 ~7.32(m,1H,ArH),7.41 ~7.47(m,2H,ArH),7.78 ~7.82(m,2H,ArH),8.12~8.13(m,1H,ArH),8.51(s,1H,a-H),9.58(s,1H,NH);13C NMR δ:28.80,56.78,65.51,65.64,102.34,108.36,109.33,114.47,116.99,117.16,119.22,119.37,121.94,122.13,122.84,123.96,128.91,130.45,137.30,147.49,149.57,153.19,154.02,154.24,156.52;IR ν:3 442,2 962,1 663,1 581,1 555,1 423,1 286,1 220,1 080 cm-1;Anal.calcd for C24H20N3O3FCl(476.9):C 59.03,H 4.13,N 8.60;found C 59.12,H 4.03,N 8.69。
N-(3-氯-4-氟 苯 基)-7-[3-(4-氯 苯 基)丙 氧基]-6-甲氧基喹唑啉-4-胺(9e):产率60%,m.p.89 ℃ ~92 ℃;1H NMR δ:2.24 ~2.29(m,2H,CH2CH2CH2),3.97(s,3H,OCH3),4.15 ~4.17(t,J=5.7 Hz,2H,OCH2),4.28 ~4.31(t,J=6.3 Hz,2H,OCH2),7.99 ~7.01(d,J=8.6 Hz,2H,ArH),7.22(s,1H,ArH),7.32 ~ 7.33(d,J=9.15 Hz,2H,ArH),7.43 ~7.46(t,J=8.6 Hz,1H,ArH),7.80 ~ 7.81(m,2H,ArH),8.13 ~8.15(m,1H,ArH),8.51(s,1H,a-H),9.55(s,1H,NH);13C NMR δ:28.82,56.74,65.03,65.59,102.25,108.37,109.29,116.71,116.90,117.08,119.21,119.36,122.67,123.87,124.79,129.74,137.31,147.43,149.52,153.12,153.97,156.45,157.80;IR ν:3 423,2 939,1 627,1 581,1 473,1 411,1 288,1 244,1 055,864,540 cm-1;Anal.calcd for C24H20N3O3FCl(476.9):C 59.03,H 4.13,N 8.60;found C 59.06,H 4.10,N 8.65。
N-(3-氯-4-氟苯基)-7-[3-(2,4-二氯苯氧基)丙氧基]-6-甲氧基喹唑啉-4-胺(9f):产率49.3%,m.p.99 ℃ ~103 ℃;1H NMR δ:2.28 ~2.31(m,2H,CH2CH2CH2),3.96(s,3H,OCH3),4.25 ~4.28(t,J=6.3 Hz,2H,OCH2),4.32 ~ 4.35(t,J=6.3 Hz,2H,OCH2),7.22 ~ 7.24(t,2H,ArH),7.26 ~7.38(m,1H,ArH),7.43 ~7.47(t,J=9.2 Hz,1H,ArH),7.56 ~7.57(d,J=2.85 Hz,1H,ArH),7.79 ~ 7.82(m,2H,ArH),8.12 ~8.14(m,1H,ArH),8.50(s,1H,a-H),9.56(s,1H,NH);13C NMR δ:28.72,56.77,66.47,66.17,102.33,108.38,109.34,115.61,116.96,117.13,119.22,119.37,112.74,122.97,123.93,128.67,129.78,137.33,147.46,149.55,153.01,153.17,153.39,153.98,156.50;IR ν:3 340,2 943,1 683,1 653,1 562,1 456,1 420,1 219,1 055,578 cm-1。
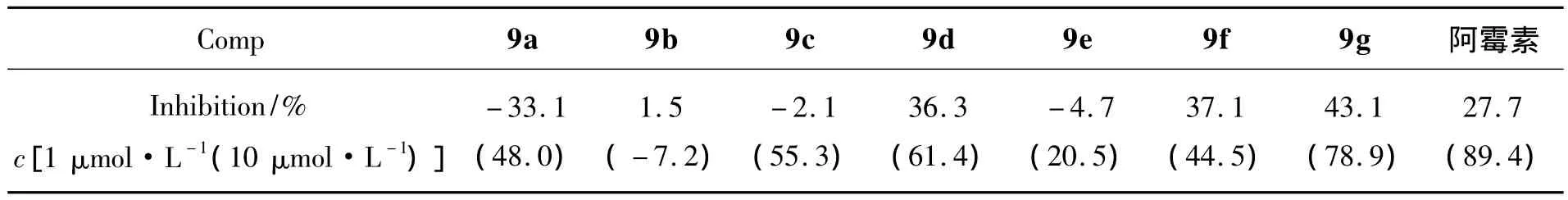
表1 9a~9g对Bcap-37的抑制活性Table 1 Inhibition activities of 9a~9g on Bcap-37 cell in vitro
5-氯-2-【3-【{4-[(3-氯-4-氟苯基)胺]-6-甲氧基喹唑啉}-7-氧】丙氧基】苯甲醛(9g):产率60.4%,m.p.110 ℃ ~112 ℃;1H NMR δ:2.31 ~2.36(m,2H,CH2CH2CH2),2.95(s,3H,CH3),4.33 ~ 4.36(t,J=5.7 Hz,2H,OCH2),4.37 ~4.39(t,J=5.7 Hz,2H,OCH2),7.26(s,1H,ArH),7.33 ~ 7.35(d,J=6.3 Hz,2H,ArH),7.43 ~7.47(t,J=7.5 Hz,1H,ArH),7.61 ~7.62(t,J=7.5 Hz,1H,ArH),7.68 ~7.71(m,1H,ArH),7.81(s,1H,ArH),8.11 ~8.13(m,1H,ArH),8.57(s,1H,ArH),9.57(s,1H,NH),10.36(s,1H,CHO);13C NMR δ:28.86,56.48,56.79,65.54,102.36,108.54,109.33,111.47,112.08,117.00,117.17,119.23,119.38,122.87,123.98,126.62,130.13,147.47,148.79,149.60,153.18,154.07,154.92,156.54,191.95;IR ν:3 460,2 932,1 683,1 653,1 558,1 458,1 280,1 217,1 136,1 020,659 cm-1。
1.3 体外抗癌活性测试
由贵州大学精细化工开发中心重点实验室细胞生物学实验室完成9a~9g的体外抗癌活性初步测试。
以DMSO作为参照物,采用MTT比色法经三次重复测定了9a~9g和对照药阿霉素对人乳癌细胞Bcap-37的抑制率。
MTT试验方法:将Bcap-37细胞接种于96孔细胞培养板中,每孔2 200个细胞,在37℃,5%CO2,饱和湿度的条件下培养24 h。加入含处理因子的1 640培养基(200 μL/孔),设空白对照和溶媒对照,每组4个平行,继续培养72 h,加入5 mg·mL-1MTT 20 μL 培养 4 h,在 570 nm 处,于酶标仪上测定其吸光度(OD),按下式计算细胞增殖抑制率。

2 结果与讨论
2.1 合成
在2~7的合成中,其产物不需纯化均可直接进行下步反应;作1H NMR检测前可用大板纯化即可。
2.2 抗Bcap-37细胞活性
9a~9g对Bcap-37的抑制活性见表1。由表1可见,9d和9g对Bcap-37细胞具有相对较好的抑制活性,在用药量为 10 μmol·L-1时,对 Bcap-37细胞抑制率分别为61.4%和78.9%,略低于对照药阿霉素(89.4%),具有一定的深入研究价值。
3 结论
香草醛为原料,经烷基化反应,硝化反应,还原反应及取代反应等合成了7个新型的4-氨基喹唑啉衍生物(9a~9g)。初步生物活性测定结果表明:在用药量为 10 μmol·L-1时,N-(3-氯-4-氟苯基)-7-[3-(2-氯基苯氧基)丙氧基]-6-甲氧基喹唑 啉-4-胺 (9d)和 5-氯-2-【3-{4-[(3-氯-4-氟 苯基)胺]-6-甲氧基喹唑啉}-7-氧丙氧基】苯甲醛(9g)对人乳腺癌细胞Bcap-37的抑制率分别为61.4%和78.9%,具有较好的抗肿瘤活性。
[1]陈舒忆,吕同杰,严和平,等.喹唑啉衍生物的合成及其抗肿瘤活性[J].合成化学,2013,21(1):092 -095.
[2]谢良辉,欧阳贵平.吉非替尼的合成工艺改进[J].合成化学,2010,18:523 -525.
[3]Cha M Y,Lee K O,Kim J W,et al.Discovery of a novel Her-1/Her-2 dual tyrosine kinase inhibitor for the treatment of Her-1 selective inhibitor resistant Nonsmall cell lung cancer[J].Journal of Medicinal Chemistry,2009,52:6880 -6888.
[4]Chandregowda V,Venkateshwara R G,Chandrasekhara R G.Convergent approach for commercial synthesis of gefitinib and erlotinib[J].Organic Process Research and Development,2007,11:813 -816.
[5]Chandrika P M,Yakaiah T,Rao A R,et al.Synthesis of novel 4,6-disubstituted quinazoline derivatives,their anti-inflammatory and anti-cancer activity(cytotoxic)against U937 leukemia cell lines[J].European Journal of Medicinal Chemistry,2008,43(4):846 -852.
[6]Duncton M A J,Estiarte A M,Johnson R J,et al.Preparation of heteroaryloxetanes and heteroarylazetidines by use of a minisci reaction[J].Journal of Organic Chemistry,2009,74(16):6354 -6357.
[7]Fang H,Li M Y,Xia L.Pharmacophore-guided design,synthesis and evaluation of quinazoline-arylpiperazines as new aladrenoceptor antagonists[J].Chinese Chemical Letters,2007,18:41 -44.
[8]Gilday J P,David M.Process for the preparation of 4-(3-chloro-4-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline[P].WO 2 004 024 703,2006.
[9]Jung F H,Pasquet G,Brempt C L,et al.Discovery of novel and potent thiazoloquinazolines as selective aurora A and B kinase inhibitors[J].Journal of Medicinal Chemistry,2006,49:955 -970.
[10]Mortlock A A,Foote K M,Heron N M,et al.Discovery,synthesis,and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase[J].Journal of Medicinal Chemistry,2007,50:2213-2224.
[11]Okano M,Mito J,Maruyama Y,et al.Discovery and structure-activity relation-ships of 4-aminoquinazoline derivatives,a novel class of opioid receptor like-1(ORL1)antagonists[J].Bioorganic & Medicinal Chemistry,2009,17:119 -132.
[12]Portela-Cubillo F,Scott J S,Walton J C.Microwavepromoted syntheses of quinazolines and dihydro quinazolines from 2-amino aryl alk-anone O-phenyl oximes[J].Journal of Organic Chemistry,2009,74:4934 -4940.
[13]Sirisoma N,Pervin A,Zhang H,et al.Discovery of N-(4-methoxyphenyl)-N, 2-dimethylquinazolin-4-amine,a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration[J].Journal of Medicinal Chemistry,2009,52:2341 -2351.
[14]Verhaeghe P,Azas N,Gasquet M,et al.Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquina-zolines[J].Bioorganic & Medicinal Chemistry Letters,2008,18:396-401.
