新型7-O-(芳基氨基甲酸酯)-布雷菲德菌素A的合成及其抗肿瘤活性*
2015-04-23吴植献王亚军万南微郑裕国
吴植献,王亚军,陈 玮,薛 锋,万南微,郑裕国
(浙江工业大学生物工程研究所,浙江杭州 310014)
布雷菲德菌素A(3,Chart 1)是一种具有多重生物活性,由真菌发酵产生的13元环的大环内酯类抗生素[1]。3通过与高尔基体关联的蛋白转运因子结合,阻碍蛋白质向高尔基体转运,从而表现出抗真菌、抗病毒、抗有丝分裂活性[2],而随着蛋白在内质网上的积累会造成内质网渗透压的升高,最终导致细胞的程序性死亡,使其具有了对多种癌细胞具有抑制活性[3-4]。

Scheme 1

Chart 1
美国肿瘤研究院将3作为抗肿瘤药物重点研究,视为新型抗癌候选药物[5]。但由于3自身的药代动力学性质(水溶性差、血浆半衰期短、细胞选择性差等)不理想,阻碍了其作为抗肿瘤治疗药物的临床研究[6]。研究发现,3的4-羟基是其活性的关键位点[7-9]。
本课题组曾对3的发酵和分离纯化进行过研究[10-11]。本文将芳胺(1a ~1m)或环己胺(1n)与三光气反应制得芳基异氰酸酯(2a~2n);2a~2n分别对3的7-羟基进行结构修饰,合成了14个新型布雷菲德菌素A氨基甲酸酯类衍生物——7-O-(芳基氨基甲酸酯)-布雷菲德菌素A(4a~4n),其结构经1H NMR,13C NMR和 ESIHR-MS表征。并对其抗肿瘤活性进行了研究。
1 实验部分
1.1 仪器与试剂
AvanceⅢ500 MHz型和Avance 126 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Esquire 6000型电感耦合等离子体质谱仪。
3 按文献方法[10-11]制备(含量 99.5%);其余所用试剂均为分析纯,其中甲苯经干燥重蒸后使用。
1.2 合成
(1)2a~2n的合成(以2a为例)
在反应瓶中加入三光气60 mg(0.2 mmol)和无水甲苯20 mL,搅拌使其溶解;冰浴冷却,于1 h内滴加混合液[苯胺(1a)46 mg(0.5 mmol)+催化剂三乙胺40 μL+无水甲苯50 mL],滴毕,于室温反应30 min;回流(80℃)反应3 h至反应液澄清[12]。减压蒸除溶剂得黄色固体2a 58 mg(直接进行下步反应)。
用类似的方法合成2b~2n。
(2)4a~4n的合成(以4a为例)
在反应瓶中依次加入3 110 mg(0.4 mmol),无水甲苯30 mL和吡啶200 μL,升温至回流(90℃)使其溶解;将溶液直接倒入上述2a的浓缩圆底瓶中,回流反应6 h[TLC跟踪:展开剂:A=V(乙酸乙酯)∶V(石油醚)=1∶5]。减压旋干甲苯,残余物用二氯甲烷50 mL溶解,用饱和氯化钠溶液(3×10 mL)洗涤,无水硫酸钠干燥,减压旋干溶剂后用少量甲醇溶解,经自制硅胶板(展开剂:A=1∶2)分离,取目标产物条带,将硅胶条带刮下,粉碎,经硅胶柱层析(洗脱剂:A=1∶2)纯化得淡黄色粉末4a。
用类似的方法合成淡黄色粉末4b~4n。
7-O-(苯基氨基甲酸酯)-布雷菲德菌素 A(4a):产率 31.7%;1H NMR δ:7.41 ~7.19(m,6H),5.92(d,J=15.6 Hz,1H),5.82 ~ 5.57(m,1H),5.12(dd,J=8.7 Hz,4.5 Hz,1H),4.85(dd,J=10.4 Hz,5.7 Hz,1H),4.11(d,J=8.7 Hz,1H),2.49 ~2.05(m,4H),2.03(d,J=27.9 Hz,1H),1.93 ~1.78(m,3H),1.77 ~1.69(m,1H),1.67 ~ 1.57(m,1H),1.69 ~1.58(m,1H),1.57 ~ 1.45(m,1H),1.26(t,J=6.2 Hz,3H),0.92(dd,J=19.6 Hz,12.7 Hz,1H);13C NMR δ:166.28,154.88,151.61,136.04,131.50,130.90,129.46,129.05,121.68,121.62,117.69,75.81,75.69,71.82,52.22,43.98,43.94,40.23,38.74,34.98,31.83,26.67,20.84;ESI-HR-MS m/z:Calcd for C23H29NO5Na{[M+Na]+}422.2,found 422.1。
7-O-(苄基氨基甲酸酯)-布雷菲德菌素 A(4b):产率 26.4%;1H NMR δ:7.41 ~7.19(m,6H),5.92(d,J=15.6 Hz,1H),5.82 ~ 5.57(m,1H),5.12(dd,J=8.7 Hz,4.5 Hz,1H),4.85(dd,J=10.4 Hz,5.7 Hz,1H),4.48 ~4.19(m,2H),4.11(d,J=8.7 Hz,1H),2.49 ~2.05(m,4H),2.03(d,J=27.9 Hz,1H),1.93 ~1.78(m,3H),1.77 ~1.69(m,1H),1.67 ~1.57(m,1H),1.69 ~1.58(m,1H),1.57 ~1.45(m,1H),1.26(t,J=6.2 Hz,3H),0.92(dd,J=19.6 Hz,12.7 Hz,1H);13C NMR δ:166.28,156.23,151.68,138.50,136.14,130.70,130.56,128.66,128.63,127.53,127.47,117.60,75.80,75.73,71.72,52.14,45.01,43.91,40.26,38.71,34.15,31.80,26.65,20.83;ESI-HR-MS m/z:Calcd for C24H31NO5Na{[M+Na]+}436.2,found 436.1。
7-O-(邻甲基苯氨基甲酸酯)-布雷菲德菌素A(4c):产率32.0%;1H NMR δ:7.54 ~7.01(m,5H),5.91(s,1H),5.23(d,J=64.1 Hz,2H),4.85(s,1H),4.41(s,1H),4.09(s,1H),2.31(t,J=33.2 Hz,6H),1.86(s,3H),1.75 ~1.62(m,3H),1.45(d,J=80.0 Hz,2H),1.28 ~1.08(m,3H),0.93(s,1H);13C NMR δ:166.33,156.67,151.84,136.00,135.34,131.54,131.01,130.74,130.37,127.05,126.75,117.53,76.18,75.59,71.72,52.01,43.86,40.17,38.60,34.04,31.77,26.62,20.80,17.67;ESI-HR-MS m/z:Calcd for C24H31NO5Na{[M+Na]+}436.2,found 436.1。
7-O-(对甲苯基氨基甲酸酯)-布雷菲德菌素A(4d):产率 31.8%;1H NMR δ:8.02 ~6.50(m,5H),5.80(m,1H),5.62 ~4.98(m,2H),4.87(s,1H),4.87(s,1H),4.40(s,1H),4.40(s,1H),3.04 ~2.11(m,6H),2.01(s,1H),1.78(dd,J=78.0 Hz,16.3 Hz,4H),1.50(d,J=39.1 Hz,2H),1.25(t,J=20.2 Hz,3H),0.93(s,1H);13C NMR δ:166.30,155.05,151.72,136.08,135.11,130.80,129.99,129.56,129.52,123.38,122.00,117.62,75.76,75.64,71.79,52.20,43.93,40.21,38.74,34.09,31.82,26.68,20.85,20.74;ESI-HR-MS m/z:Calcd for C24H31NO5Na{[M+Na]+}436.2,found 436.1。
7-O-(对氯苯基氨基甲酸酯)-布雷菲德菌素A(4e):产率 30.5%;1H NMR δ:7.51 ~ 7.44(m,3H),7.34(t,J=14.0 Hz,3H),5.72(s,2H),5.33 ~5.21(m,2H),5.03(s,1H),4.72(s,1H),4.03(s,1H),2.27 ~2.07(m,4H),1.77 ~1.61(m,4H),1.50(d,J=8.3 Hz,3H),1.20(t,J=10.2 Hz,3H),0.76(s,1H);13C NMR(DMSO)δ:196.96,165.63,154.11,153.12,138.26,136.43,130.59,130.03,129.11,128.59,123.44,119.60,116.94,75.49,73.96,70.88,51.90,42.69,40.02,38.29,33.45,31.42,26.53,20.70;ESI-HR-MS m/z:Calcd for C23H28NO5ClNa{[M+Na]+}456.2,found 456.1。
7-O-(邻氯苯基氨基甲酸酯)-布雷菲德菌素A(4f):产率 28.2%;1H NMR δ:7.33(t,J=26.3 Hz,4H),7.11(s,1H),7.00(s,1H),5.94(s,J=15.7 Hz,1.8 Hz,1H),5.74(s,J=15.0 Hz,10.2 Hz,4.6 Hz,1H),5.33 ~ 5.22(m,1H),5.22 ~5.12(m,1H),4.92 ~4.79(m,1H),4.14(d,J=9.0 Hz,1H),2.37 ~2.27(m,3H),1.86(d,J=6.9 Hz,3H),1.72(d,J=6.6 Hz,2H),1.52(d,J=16.4 Hz,2H),1.27(t,3H),0.96(s,1H);13C NMR δ:166.17,152.79,151.45,135.89,134.67,131.01,129.06,127.72,125.80,125.37,123.73,117.75,76.54,75.76,71.73,52.04,43.93,41.97,40.18,38.62,34.11,31.79,27.00,26.63,20.83;ESI-HR-MS m/z:Calcd for C23H28NO5ClNa{[M+Na]+}456.2,found 456.1。
7-O-(2,4-二氯苯胺氨基甲酸酯)-布雷菲德菌素 A(4g):产率 28.9%;1H NMR δ:7.33(t,J=26.3 Hz,4H),7.11(s,1H),7.00(s,1H),5.94(d,J=15.7 Hz,1.8 Hz,1H),5.74(d,J=15.0 Hz,10.2 Hz,4.6 Hz,1H),5.33 ~5.22(m,1H),5.22 ~5.12(m,1H),4.92 ~4.79(m,1H),4.14(d,J=9.0 Hz,1H),2.37 ~2.27(m,3H),1.86(d,J=6.9 Hz,3H),1.72(d,J=6.6 Hz,2H),1.52(d,J=16.4 Hz,2H),1.27(t,J=5.3 Hz,3H),0.96(s,1H);13C NMR δ:166.17,165.67,152.66,151.38,147.11,136.22,135.82,133.52,131.13,128.76,127.90,117.83,75.77,71.77,52.04,43.95,40.19,38.65,34.15,31.80,26.62,20.83;ESI-HR-MS m/z:Calcd for C23H27NO5Cl2Na{[M+Na]+}490.1,found 490.4。
7-O-(对氟苯基氨基甲酸酯)-布雷菲德菌素A(4h):产率 30.5%;1H NMR(DMSO-d6)δ:7.53(s,1H),7.47(s,2H),7.36(d,J=15.6 Hz,1H),7.27(s,1H),7.12(d,J=8.8 Hz,2H),5.74(d,J=15.4 Hz,2H),5.25(d,J=11.0 Hz,3H),5.02(s,1H),4.72(s,2H),4.09(d,J=43.8 Hz,2H),2.49(d,J=15.7 Hz,3H),2.23(s,1H),2.07(s,1H),1.94(s,1H),1.80(d,J=12.4 Hz,4H),1.64(s,1H),1.50(d,J=11.2 Hz,2H),1.21(t,J=10.7 Hz,3H),0.75(s,1H);13C NMR δ:166.10,157.06,154.58,153.75,136.92,131.00,130.48,125.38,125.31,116.93,115.80,115.62,75.81,74.45,71.36,52.38,43.16,41.47,38.77,33.92,31.89,26.87,21.16;ESI-HR-MS m/z:Calcd for C23H28NO5FNa{[M+Na]+}440.2,found 440.1。
7-O-(2,4-二氟苯基氨基甲酸酯)-布雷菲德菌素 A(4i):产率 30.3%;1H NMR δ:7.32(d,J=15.6 Hz,3H),7.27(s,1H),7.12(d,J=8.8 Hz,2H),5.74(d,J=15.4 Hz,2H),5.25(d,J=11.0 Hz,3H),5.02(s,1H),4.72(s,2H),4.09(d,J=43.8 Hz,2H),2.49(d,J=15.7 Hz,3H),2.23(s,1H),2.07(s,1H),1.94(s,1H),1.80(d,J=12.4 Hz,4H),1.64(s,1H),1.50(d,J=11.2 Hz,2H),1.21(t,J=10.7 Hz,3H),0.75(s,1H);13C NMR δ:166.26,158.47,154.29,151.54,136.06,130.90,130.88,120.59,117.72,114.59,114.29,100.53,75.83,71.80,55.53,52.24,43.95,40.23,38.75,34.14,31.84,26.65,20.85;ESI-HR-MS m/z:Calcd for C23H28NO5F2Na{[M+Na]+}458.2,found 458.1。
7-O-(对二氟氧甲基苯基氨基甲酸酯)-布雷菲德菌素 A(4j):产率 26.3%;1H NMR δ:7.41(m,2H),7.28(t,3H),7.18(t,1H),7.02(d,J=4.6 Hz,2H),5.92 ~ 5.98(d,J=15.7 Hz,1H),5.72(s,2H),5.33 ~5.21(m,2H),5.03(s,1H),4.72(s,1H),4.03(s,1H),2.27 ~2.07(m,4H),1.77 ~1.61(m,4H),1.50(d,J=8.3 Hz,3H),1.20(t,J=10.2 Hz,3H),0.76(s,1H);13C NMR δ:169.43,166.55,153.30,151.97,136.04,130.87,124.45,123.11,120.85,120.57,117.56,116.05,113.96,75.82,72.05,52.28,43.94,40.20,38.83,34.06,31.86,26.69,20.82;ESI-HR-MS m/z:Calcd for C23H29NO6F2Na{[M+Na]+}488.2,found 488.1。
7-O-(邻三氟甲基苯氨基甲酸酯)-布雷菲德菌素 A(4k):产率 28.7%;1H NMR δ:7.55(s,2H),7.38(s,1H),7.28(s,1H),7.19(s,1H),6.88(s,1H),5.92 ~ 5.98(d,J=15.7 Hz,1H),5.72(s,2H),5.33 ~5.21(m,5H),5.03(s,1H),4.72(s,1H),4.03(s,1H),2.27 ~2.07(m,4H),1.77 ~ 1.61(m,4H),1.50(d,J=8.3 Hz,3H),1.20(t,J=10.2 Hz,3H),0.76(s,1H);13C NMR δ:166.26,166.19,153.06,151.43,135.81,135.61,132.93,131.07,129.17,126.10,126.05,123.61,117.80,75.79,71.77,52.02,43.95,40.19,38.54,34.14,31.81,26.61,20.83;ESI-HR-MS m/z:Calcd for C23H28NO5F3Na{[M+Na]+}490.2,found 490.5。
7-O-(对氧甲基苯氨基甲酸酯)-布雷菲德菌素 A(4l):产率29.7%;1H NMR δ:7.37(d,J=15.7 Hz,1H),7.28(s,1H),6.87(d,J=5.2 Hz,1H),5.92 ~ 5.98(d,J=15.7 Hz,1H),5.72(s,2H),5.33 ~ 5.21(m,2H),5.03(s,1H),4.72(s,1H),4.03(s,1H),2.27 ~2.07(m,4H),1.77 ~1.61(m,4H),1.50(d,J=8.3 Hz,3H),1.20(t,J=10.2 Hz,3H),0.76(s,1H);13C NMR δ:166.19,152.99,151.41,135.89,135.18,131.44,131.10,131.07,130.76,117.80,111.33,111.16,75.75,71.79,52.16,44.16,43.94,40.18,38.70,34.15,31.83,26.64,20.84;ESI-HR-MS m/z:Calcd for C24H31NO5Na{[M+Na]+}451.2,found 451.1。
7-O-(萘基氨基甲酸酯)-布雷菲德菌素 A(4m):产率 35.6%;1H NMR δ:7.87(d,J=7.1 Hz,2H),7.68(d,J=8.2 Hz,1H),7.62 ~7.43(m,3H),7.28(s,1H),7.08(d,J=4.3 Hz,1H),5.92(d,J=15.6 Hz,1H),5.82 ~5.57(m,1H),5.12(dd,J=8.7 Hz,4.5 Hz,1H),4.85(dd,J=10.4 Hz,5.7 Hz,1H),4.11(d,J=8.7 Hz,1H),2.49 ~ 2.05(m,4H),2.03(d,J=27.9 Hz,1H),1.93 ~ 1.78(m,3H),1.77 ~ 1.69(m,1H),1.67 ~ 1.60(m,1H),1.57 ~1.45(m,1H),1.26(t,J=6.2 Hz,3H),0.92(s,1H);13C NMR δ:166.28,151.67,149.73,136.30,136.01,134.08,132.54,130.74,129.39,128.68,126.26,126.03,125.75,120.65,118.16,117.62,76.49,75.68,71.87,52.12,43.85,41.97,38.71,34.09,31.79,26.83,20.72;ESI-HR-MS m/z:Calcd for C27H31NO5FNa{[M+Na]+}472.2,found 472.1。
7-O-(环己烷氨基甲酸酯)-布雷菲德菌素A(4n):产率 18.1%;1H NMR δ:7.41 ~7.19(m,1H),5.92(d,J=15.6 Hz,1H),5.82 ~ 5.57(m,1H),5.12(d,J=8.7 Hz,4.5 Hz,1H),4.85(d,J=10.4 Hz,5.7 Hz,1H),4.11(d,J=8.7 Hz,1H),3.50(m,1H),2.49 ~ 2.05(m,4H),2.03(d,J=27.9 Hz,1H),1.93 ~1.78(m,3H),1.77 ~ 1.69(m,1H),1.67 ~1.57(m,1H),1.69 ~ 1.58(m,3H),1.57 ~1.45(m,4H),1.26(t,J=6.2 Hz,3H),0.92(d,J=19.6 Hz,12.7 Hz,1H);13C NMR δ:166.34,156.75,151.63,130.73,130.61,117.65,75.83,75.19,71.73,49.18,43.96,38.74,34.13,33.96,33.46,31.82,26.68,25.63,25.50,25.47,24.95,24.80,20.86;ESI-HR-MS m/z:Calcd for C23H35NO5FNa{[M+Na]+}433.2,found 433.1。
1.3 抗肿瘤活性测定
将人食管癌细胞TE-1按5.0×103细胞/孔均匀种植于细胞培养96孔板中,置于5%二氧化碳培养箱,于37℃培养24 h。以DMSO为溶剂,配制不同c(4)溶液;向各孔中添加c(4)(每个重复处理3次,取平均值),于37℃培养48 h。每孔中加入10 μL WST-1细胞增殖及细胞毒性检测试剂盒,于37℃孵育2 h,利用酶标仪测定每孔450 nm处的吸光度(A),以空白细胞培养液作为对照组,计算c(4)处理后人食管癌细胞TE-1存活率[存活率/%=(A药液-A培养液)/(A对照-A培养液)×100%]。
2 结果与讨论
2.1 合成
本文首次采用异氰酸酯对3的7-羟基进行结构修饰,合成了14个新型的布雷菲德菌素A氨基甲酸酯类衍生物。该方法具有催化剂便宜易得,反应条件较温和等优点。
2.2 抗肿瘤活性
4g,4i~4l对食管癌TE-1细胞株的肿瘤抑制活性见表1。由表1可见,在各个用药量时,他们中的大部份化合物均失去了对食管癌TE-1细胞株的抗肿瘤活性。

表1 4g,4i~4l对TE-1的抑制活性Table 1 Inhibitions activities of 4g,4i~4l against TE-1 cell
为了从靶位蛋白与小分子作用关系来解释抗肿瘤活性实验结果,我们选取4i,4j和4l与蛋白进行分子对接研究。利用DS2.5软件中的LiDOCK模块进行了分子对接,结果见图1。图1结果表明,3被带苯环的大基团修饰以后,分子空间体积变大。分子4只能结合在活性区域的外表面,而不能进入3所结合的活性区域,与特定的ASP67和TRP78两个氨基酸作用,导致4与靶位蛋白的相互作用力减弱,肿瘤抑制活性降低。这就是4丧失抑制食管癌细胞株TE-1活性的原因。
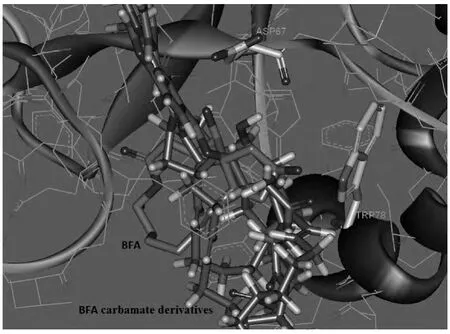
图1 4与靶点ARF1-GDP-GEF复合体的模拟对接图Figure 1 Docking for 4 in the ARF1-GDP-GEF interface
3 结论
首次采用异氰酸酯对布雷菲德菌素A的7-羟基进行结构修饰,合成了14个新型的布雷菲德菌素A氨基甲酸酯类衍生物。布雷菲德菌素A氨基甲酸酯类衍生物的合成及其生物活性评价,为布雷菲德菌素A衍生化研究提供借鉴。
[1]Singleton V L,Bohonos N,Ullstrup A J.Decumbin,a new compound from a species of Penicillium[J].Nature,1958,181:1072 -1073.
[2]Sidhu G S,Singh A K and Raghunath P N.Brefeldin A inhibits the antiviral action of interferon against encephalomyocarditis virus[J].Vir Res,1996,40:123 -133.
[3]Alexander B,Andrew I,Fishman M D,et al.Attenuation of androgenic regulation by brefeldin A in androgen-responsive prostate cancer cells[J].Urol Oncol,2013,31(1):104 -109.
[4]Verfaillie T,Garg A D,Agostinis P.Targeting ER stress induced apoptosis and inflammation in cancer[J].Cancer Letters,2013,332(2):249 -264.
[5]Nojiri H,Manya H,Isoho H.Induction of terminal differentiation and apotosis in human colonic carcinoma cells by Brefeldin A,a drug affecting fanflioside biosynthesis[J].FEBS Letters,1999,453:140 -144.
[6]Bruening A,Ishikawa T,Kneusel R E.Brefeldin A binds to glutathione S-transferase and is secreted as glutathione and cysteine conjugates by Chinese hamster ovary cells[J].J Biol Chem,1992,267:7726 -7732.
[7]Anadu N O,Davisson V,Cushman M.Synthesis and anticancer activity of brefeldin A ester derivatives[J].J Med Chem,2006,49:3897 -3905.
[8]Zhu J W,Hori H,NOjiri H.Synthesis and activity of brefeldin A analogs as induces of cancer cell differentiation and apoptosis differentiation and apoptosis[J].Bioorg Med Chem Lett,1997,7(2):139 -144.
[9]He B Y,Wang Y J,Zheng Y G,et al.Synthesis and cytotoxic evaluation of acylated brefeldin A derivatives as potential anticancer agents[J].Chem Biol Drug Des,2013,82(3):307 -316.
[10]Wang Y J,Xue F,Wu Y F.Development of macrolide lactone antibiotic brefeldin A fermentation process with Eupenicillium brefeldianum ZJB082702[J].J Biosci Bioeng,2012,114:262 -267.
[11]Wang Y J,Wu Y F,Xue F.Isolation of brefeldin A from Eupenicillium brefeldianum broth using macroporous resin adsorption chromatography[J].Chromatogr B,2012,(895):146 -153.
[12]Charalambides Y C,Moratti S C.Comparison of base-promoted and self-catalyzed conditions in the synthesis of isocyanates from amines using triphosgene[J].Synthetic Commun,2007,37:1037 -1044.
