唐氏综合征合并桥本毒症、反复脑梗死1例并文献复习
2015-04-20罗飞宏程若倩章淼滢李晓静
常 卓 罗飞宏 奚 立 程若倩 章淼滢 李晓静
·论著·
唐氏综合征合并桥本毒症、反复脑梗死1例并文献复习
常 卓 罗飞宏 奚 立 程若倩 章淼滢 李晓静
目的 通过1例唐氏综合征(DS)合并桥本毒症、反复脑梗死的报道,探讨DS合并脑梗死可能的机制。方法详细介绍1例10岁DS患儿合并桥本氏甲状腺炎、甲状腺毒症(HTT)和反复脑梗死的诊断治疗过程,系统检索文献并复习已报道的类似病例,汇总DS合并脑梗死病例的临床特征。结果10岁女童,以“消瘦,无力,多汗4月余”主诉入院,既往曾检查染色体核型47,XX,+21,诊断DS。入院后查甲状腺功能提示TPOAb明显升高、TRAb 正常,TSH降低,抗双链DNA抗体 1∶101 (+),抗组蛋白抗体 1∶101 (+),MPO 1∶101 弱(+),PR3 1∶101 弱(+),诊断为HTT。予抗甲状腺等治疗后好转出院。2月余后无明显诱因2次发生肢体无力、行走困难,MRI分别提示左侧和右侧脑梗死。予康复、抗凝、抗炎和抑制免疫治疗后,肌力恢复正常,随访1年患儿病情稳定。从Web of science、PubMed数据库、中国知网和万方数据库系统检索DS合并脑梗死的相关文献共19篇,汇总17例DS合并脑梗死病例,41.2%合并感染,52.9%合并Moyamoya病,23.5%合并甲状腺功能异常,47.1%伴有自身抗体阳性,DS合并脑梗死总体预后较好。结论DS合并桥本毒症、反复的脑梗死少见,但对生存时间较长的DS患者需要关注自身免疫性疾病发生的可能。
唐氏综合征; 桥本氏甲状腺炎; 甲状腺毒症; 脑梗死; 儿童
1 病例特征
女,10岁,因“消瘦,无力,多汗4月余”就诊于复旦大学附属儿科医院(我院)。患儿系G7P3,出生时有发绀抢救史,生后神经运动发育落后于正常儿童,6月龄抬头,13月龄独坐,2岁时独走,3岁会说话。2011年7月(8岁)曾因月经初潮来我院健康查体,查骨龄为13岁,甲状腺功能正常,染色体分析诊断为唐氏综合征(DS),核型47,XX,+21。目前10岁仍语言发育落后,体格发育同正常同年龄儿童。未予特殊治疗。
2013年3月患儿出现乏力、消瘦、多汗和夜间盗汗等症状,无咳嗽、咳痰和低热。2013年7月15日因症状无好转,外院查甲状腺功能示:FT3 28.93 ρmol·L-1(升高),FT4 78.83 ρmol·L-1(升高),TSH 0.005 μIU·mL-1(降低),2周后来我院就诊并收入院。具体病情演变及治疗过程见图1。
实验室检查示: WBC 3.0×109·L-1,甲状腺功能:TT3 514.2(正常值70~220) ng·dL-1,TT4 25.3(正常值14.5~15.4) μg·dL-1,FT3 514.2(正常值1.78~5.60) ρg·mL-1,FT4 2.45(正常值0.5~2.3) ng·dL-1,TSH 0.03(正常值0.25~7.31) μIU·mL-1,TPOAb 明显升高,TRAb正常。 ICA (64 kD) 胰岛细胞抗体 (+)。抗双链DNA抗体 1∶101 (+),抗组蛋白抗体 1∶101 (+),MPO 1∶101 弱(+),PR3 1∶101 弱(+)。ESR升高。Hb、PLT、CRP、肝肾功能、血浆抗体、补体、T细胞亚群正常。GADA 谷氨酸脱羧酶抗体 (65 kD)、IAA 胰岛素抗体、IA-2A 胰岛细胞抗体 、ICA (40 kD) 胰岛细胞抗体 、抗双链DNA 1∶10 、抗双链DNA 1∶100、抗磷脂抗体、抗核小体抗体 1∶101、ANA 1∶100、抗核糖核蛋白抗体 1∶101、CANCA 1∶10、PANCA 1∶10、GBM 1∶101、ENA-RNP/Sm 1∶101、Sm、SSA、SSB、Scl-70、Jo-1均阴性。ACTH及达菲林激发试验、骨髓细胞学检查正常。
甲状腺B超示:双侧甲状腺不均质,血供丰富。同位素甲状腺血流显像:甲状腺增大,血供增多,摄取功能增强,符合甲状腺功能亢进表现。头颅、垂体MRI平扫:右侧脑室形态失常伴室管膜下灰质异位 ,右额顶颞叶脑皮质局部增厚,垂体未见异常。颈胸腹MRA:双侧颈总动脉近端略细;左侧锁骨下动脉及颈总动脉起始部偏细,双侧锁骨下动脉局部略扩张;腹主动脉粗细欠均;左肾动脉远端显示欠清。X线胸片及超声心动图未见异常。患儿入院给予抗甲状腺和降心率等治疗2周后,甲状腺功能好转,予以出院。患儿出院后2月余无明显诱因先后发生左、右侧脑梗死,给予相应治疗后患儿肌力恢复正常,随访1年患儿病情稳定。
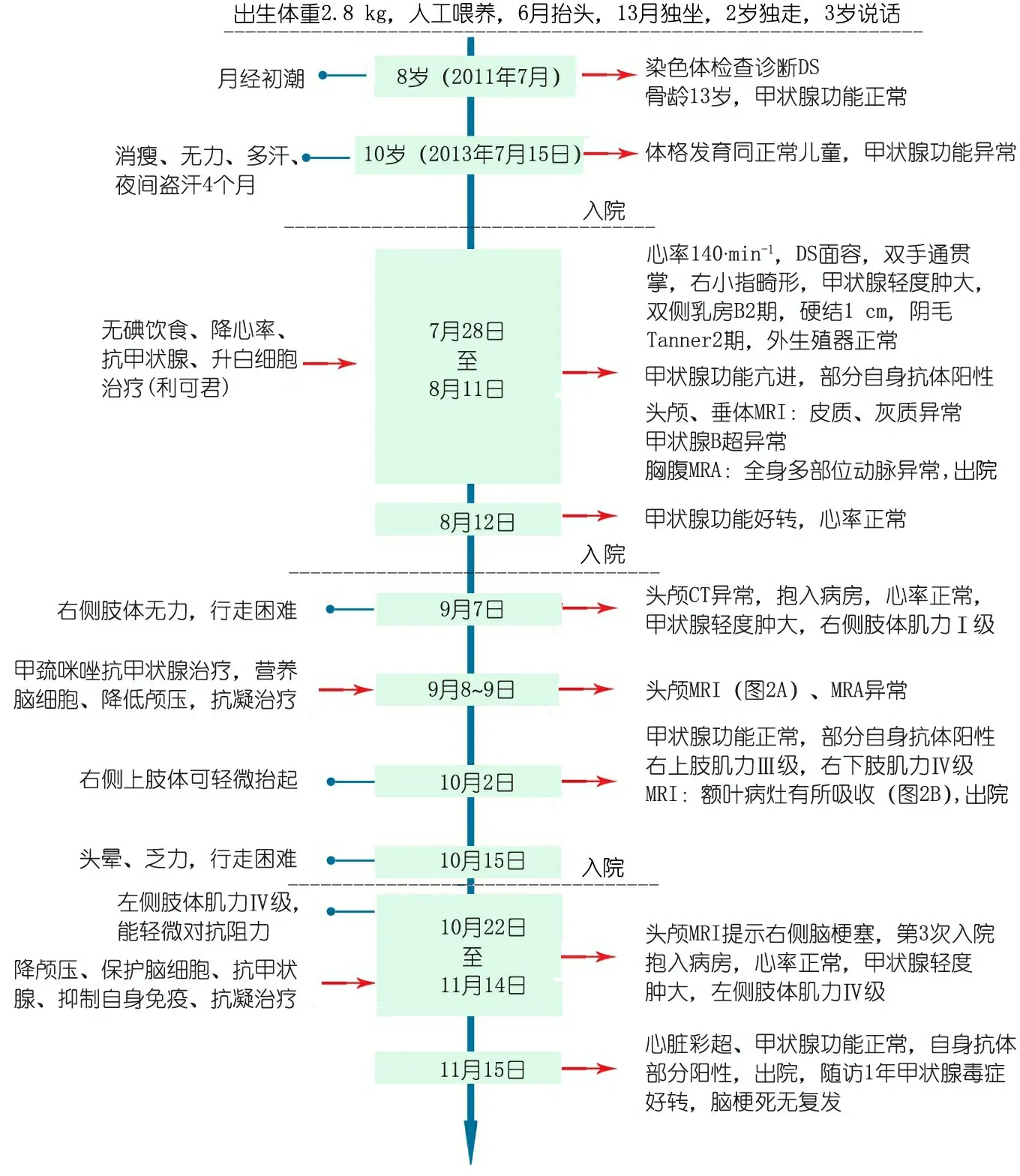
图1 本文病例重要临床信息时间轴
Fig 1 Clinical information of the case
2 检索方法及结果
以(“Down′s syndrome” OR “Mongolism ” OR “Trisomy 21”) AND “cerebral infarction”为检索式在Web of science,PubMed数据库检索,以“唐氏综合征”AND“脑梗死”为检索式在中国知网和万方数据库检索,共检索到19篇相关文献,其中可以提取临床及影像资料的病例报告12篇[1~12]共16例,本例为国内首次报告。将17例DS合并脑梗死病例的临床及影像学资料汇总见表1。
表1显示纳入的病例中<20岁12例,<15岁9例;女性11/17例。所有患者脑梗死均为急性起病,病程反复5例。4例发生大脑前动脉梗阻,12例发生大脑中动脉梗阻,3例发生大脑后动脉梗阻,6例颈内动脉梗阻,1例海绵窦血管梗阻,1例椎基底动脉梗阻,8例为双侧病变。17例中合并甲状腺功能亢进的患者2例。本文患儿为10岁女童,病初为HTT,之后先后2次(左右两侧)脑梗死。经头颅MRA提示为左侧大脑中动脉梗阻和右侧脑梗,与既往文献报道有相似性。
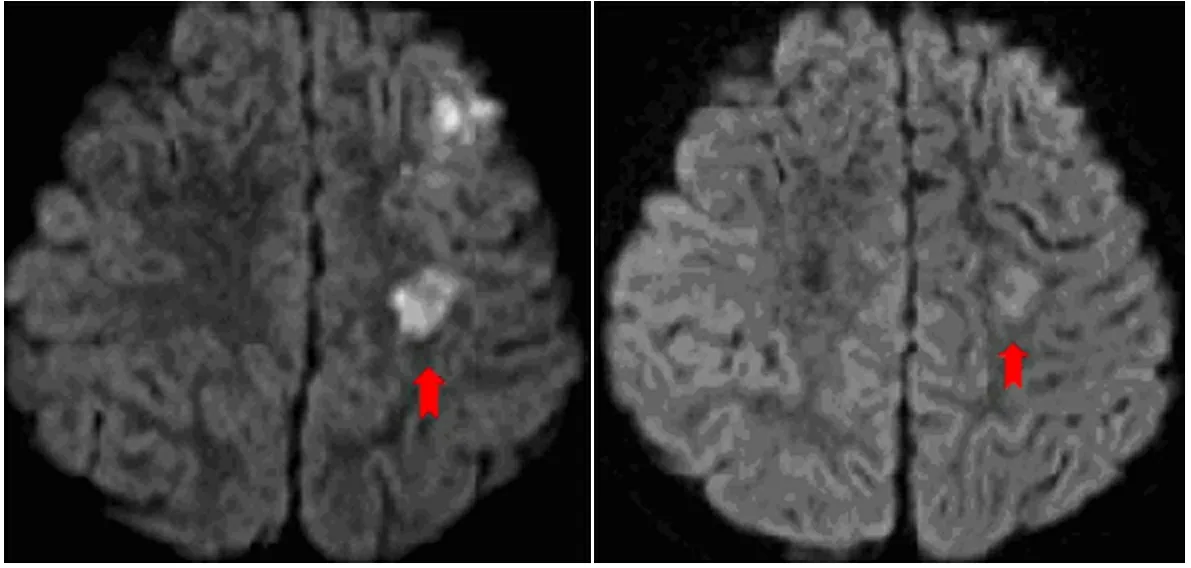
图2 本文唐氏综合征合并脑梗死患儿急性发作期和恢复期头颅MRI对比
Fig 2 MRI changes of a DS child with cerebral infarction during acute and recovery period
注 A:2013年9月9日时MRI示左侧额叶多发急性梗死;神经元移行异常,室管膜下灰质异位;B: 治疗3周后 MRI示左侧额叶病灶有所吸收
3 讨论
DS是染色体病中最常见的一种。DS患者合并甲状腺功能异常的发病率高达15%[1,13],且DS合并甲状腺疾病的危险度会随年龄增大而升高。本文DS患儿合并HTT诊断明确,但并发2次左、右侧脑梗死的病例报道少见。本文复习文献汇总了包括本文病例在内的17例DS合并脑梗死病例的临床特征,以飨读者。
3.1 感染 7/17(41.2%)合并感染(例1~5、10、11、17),其中1例虽未检查出明确感染灶,但辅助检查结果提示有疑似感染的征象。感染是脑梗死的危险因素之一,有研究显示40%的脑梗死患者合并感染[14],也有研究显示肺炎衣原体感染可以显著增加脑梗死的发生率[15],新生儿发生菌血症后脑梗死的发病率显著上升[16]。De Silva等[2]研究显示,DS合并感染后发生脑梗死患儿血清IgG4水平低于未合并感染者,提示DS合并感染后通过某种免疫介导的机制使儿童更易发生脑梗死。本文患儿第2次脑梗死后伴随感染症状,故由感染引发脑梗死可能性不大,但提示在随访中应注意防范感染,减少脑梗死复发。
表1 17例唐氏综合征合并脑梗死病例特征分析
Tab 1 Summary of clinical features and laboratory findings of Down′s syndrome in concomitant with cerebral infarction in 17 cases

病例年龄性别伴随疾病神经系统表现主要实验室检查影像学检查1[1]25月女感染左侧轻偏瘫炎症指标阳性,IgG4↓右侧大脑中动脉梗阻,右侧大脑中动脉、前动脉支配区信号异常2[2]4岁女肺部感染全身发作癫炎症指标阳性,MP抗体↑、IgG4↓左侧大脑中动脉支配脑区信号异常、左侧大脑后动脉受压3[2]6.5岁女中枢感染急性全身肌张力↓,左侧为主,双侧深反射减退炎症指标阳性,抗O滴度↑,IgG4浓度↓左侧额叶及顶叶信号异常,左侧大脑中动脉梗阻4[2]4.5岁女感染急性右侧半身瘫,表达型失语症炎症指标阳性,IgG4浓度↓左侧大脑中动脉梗阻,左侧大脑中动脉支配脑区缺血5[3]1)19岁女终末期肾功能衰竭、心包压塞、心肌梗死、肺炎、血栓左侧轻偏瘫,言语中断,左侧同向偏盲,四肢轻瘫,查体不合作,癫持续状态、感觉减退炎症指标阳性,狼疮抗凝物↑右侧大脑中动脉梗阻,左侧大脑中动脉支配区、右侧大脑后动脉支配区水肿,中线位移,左侧大脑前动脉、中动脉梗阻6[4]1)19岁女Graves病、Moy-amoya病右侧中枢性面瘫、右侧轻偏瘫、腱反射亢进甲状腺功能异常、TSH受体抗体阳性双侧大脑中动脉、海绵窦血管梗阻,左侧为主7[5]42岁男甲状腺黏膜相关淋巴瘤、亚临床甲减双下肢肌力↓,反射亢进-左侧壳核后部腔隙性梗死8[6]22岁男室间隔缺损、动脉瘤、三尖瓣关闭不全右侧轻偏瘫-大脑中动脉梗阻9[7]1)6岁女寰枢椎不稳,Moy-amoya病右侧轻偏瘫,失语炎症指标阳性右侧中央前回、顶叶、内囊处高信号,双侧颈内动脉梗阻10[8]1)11岁男感染、Moyamoya病左侧肢体肌力↓,反射亢进,眼球运动障碍C7、T1及T3脊柱裂,ANA抗体阳性,溶血,补体↓、免疫复合物↑右侧顶叶、额叶局部萎缩,双侧颈内动脉、大脑前动脉、左侧大脑中动脉梗阻,右侧大脑中动脉、椎基底动脉狭窄11[8]1)6岁女感染、过敏、Moyamoya病右侧轻偏瘫,双侧腱反射消失,巴氏征阳性,左侧轻偏瘫伴左侧面瘫-左脑室轻度增大,左大脑中动脉供血区灌注降低,双侧脑室增大,颈内动脉狭窄12[9]18月男法洛四联症、室间隔缺损、Moyamoya病左侧轻偏瘫,面瘫,语言障碍红细胞↑、血氧↓左侧大脑中动脉梗阻,双侧颈内动脉末端梗阻13[10]3岁男法洛四联症、室间隔缺损、Moyamoya病右侧轻偏瘫-左侧大脑中动脉、后动脉梗阻14[11]34月男Moyamoya病右侧轻偏瘫-大脑前、中动脉梗阻,双侧颈内动脉狭窄15[11]4岁女Moyamoya病视神经萎缩-双侧颈内动脉狭窄16[12]2岁女Moyamoya病、甲减右侧轻偏瘫、面瘫甲减征象左侧大脑动脉梗阻171)10岁女桥本氏甲状腺炎、甲状腺毒症、中耳炎右侧轻偏瘫,头晕,乏力甲状腺功能异常,部分自身抗体阳性左额叶多发梗死灶,左大脑中动脉分支较对侧少,右侧脑梗死
注 例17为本文病例;1)提示脑梗死反复发作
3.2 Moyamoya病(MMD) 9/17(52.9%)DS合并MMD(例6、9~16),9例均有反复发生脑梗死的病史。脑血管病为儿童急性偏瘫的重要病因,日本报告[17]MMD约占儿童脑血管病的50%。有研究提示DS患儿较普通人群更易患MMD[18]。MMD发病机制并不明确,21号染色体增多是MMD的危险因素[19]。21号染色体编码的一些蛋白如超氧化物歧化酶Ⅰ、γ-干扰素受体和胱硫醚β-合酶可影响动脉生理过程,引起动脉狭窄[20~22]。这些蛋白由于21号染色体的增加,而不恰当表达导致MMD血管发育不良和结构缺陷[23]。研究表明21号染色体基因编码的胶原蛋白Ⅳ异常表达和发生脑血管疾病危险相关[20,21]。也有研究表明DS易合并血管异常,如甲床毛细管形态异常、先天性心脏病、视网膜血管病变、肺动脉高压和原发性内膜纤维增生[21]。DS和MMD都可能与自身免疫有相关性:MMD的发生与抗磷脂抗体相关,而抗磷脂抗体和其他自身免疫性抗体也可在DS患者中存在,DS患者中并发MMD可能是由这些自身免疫抗体介导的,这可能是两种疾病的潜在联系[24,25]。本例患儿虽无MMD的MRI表现,但MRA检查提示部分动脉粗细不均,分布不对称等表现,提示患儿可能合并血管病变。
3.3 甲状腺功能异常与免疫功能异常 4/17(23.5%)DS患者(例6、7、16、17)甲状腺功能异常,其中2例(例6、17)为甲状腺功能亢进,2例(例7、16)为甲状腺功能减低。甲状腺激素在生长发育及物质能量代谢中发挥重要作用,其分泌异常可导致多系统多器官受损。DS患者更易患甲状腺自身免疫性疾病,以桥本氏甲状腺炎最为常见[26~29]。Goday-Arno等[30]总结了1974至2006年DS合并甲状腺功能亢进患者的文献,认为DS合并Graves占0.7%,显著高于正常人群(0.79/100 000)。Baxter等[31]指出染色体异常在某种程度上损伤了保护甲状腺的自身抗原,而母体中预先存在的甲状腺自身抗体也影响患儿染色体发育。近年来甲状腺功能亢进患者并发缺血性脑卒中的报道逐渐增多,且多数是年轻人。有研究表明甲状腺功能亢进不仅是青年人群发生脑梗死的重要危险因素[32],而且还会增加脑卒中的病死率[33]。
甲状腺功能亢进合并缺血性脑卒中的发病机制与自身免疫因素密切相关。甲状腺功能亢进患者除产生甲状腺相关抗体外,还产生一些自身抗体可导致血小板活化、聚集,激活某些淋巴因子、免疫调节蛋白(如热休克蛋白)和细胞间黏附分子等,这些免疫因子可抑制抗凝血酶原激活物的产生,抑制纤溶系统活性,损伤血管内皮细胞,导致颅内血管狭窄。在进一步的机制研究中,Tendler等[34]认为甲状腺功能亢进合并脑血管狭窄或闭塞的患者,细胞的增殖和血管调节异常与甲状腺激素的免疫刺激有关,而这种免疫刺激主要是通过T细胞实现。DS患者的T细胞亚群分布有改变,主要表现为CD4+/CD8+T细胞比值降低、gdT细胞增多。不仅如此,其功能也有所变化,主要表现为改变T细胞对特定抗原及有丝分裂原的反应、T细胞内的信号传导通路及异常地释放某些细胞因子[35],提示DS不仅使患儿更易患甲状腺功能亢进,并且可以通过改变T细胞的分布及功能的病理生理过程,使DS合并甲状腺功能亢进的患儿更易发生脑梗死。
从表1可见,合并免疫异常的有8例(例1~6、10、17),其中有4例(5、6、10、17)是自身抗体阳性。有研究显示,DS合并甲状腺功能亢进患者的突出特点不仅表现为起病年龄早、无性别差异,更重要的是易合并其他自身免疫性疾病[36~39]。而DS就较易引起自身免疫性疾病和自身免疫抗体阳性[26,27,39,40]。此外,有研究显示甲状腺自身抗体阳性是缺血性脑卒中的独立危险因素,免疫紊乱导致的动脉炎症可能是甲状腺功能亢进患者颅内血管狭窄或呈MMD的关键因素[41]。
本文患儿的脑梗死发生在甲状腺功能控制后,且有明显的免疫紊乱,在进行免疫抑制治疗前其甲状腺过氧化物酶抗体是正常值上限的200余倍,胰岛细胞相关抗体、抗双链DNA抗体 1∶101、抗组蛋白抗体 1∶101阳性,MPO 1∶101、PR3 1∶101 弱阳性,行免疫治疗后,自身抗体阳性减弱且脑梗死未复发。临床不排除患儿为自身抗体介导血管炎样病变引发的脑梗死。
3.4 先天性心脏病 4/17(23.5%)合并心功能异常(例5,8,12,13),其中有3例(8、12、13)合并先天性心脏病。先天性心脏病是脑卒中发病的重要诱因,有文献报道14.6%~25%的先天性心脏病诱发脑梗死,且多伴有心脏畸形[14,42]。先天性心脏病不仅可以引起心内血栓,而且易并发低氧血症、红细胞增多症或出现发绀,均为引起脑梗死的危险因素[43]。值得注意的是,因先天性心脏病诱发脑梗死的患儿多伴有动脉疾病,如颅外的动脉夹层或MMD[44,45],因此DS患儿存在血管和心脏的因素可能更易发生脑梗死。本例患者行心脏彩超未见心脏畸形或心功能受损。
3.5 其他相关原因 Colleran等[46]研究发现血液中游离甲状腺素水平与同型半胱氨酸、甲基丙二酸的水平呈正相关,而高同型半胱氨酸血症是脑梗死的危险因素[47],本例患儿在进行升白细胞治疗时所用的利可君为半胱氨酸衍生物,虽然本例患儿未检测同型半胱氨酸,但既往无利可君引起同型半胱氨酸血症的报道,因此该药物引起脑梗死的可能性较小。此外,据Quax等[48]研究报道,常用抗甲状腺药物丙基硫氧嘧啶可以导致甲状腺功能亢进患者形成血管炎,这种血管炎已经在绝大部分甲状腺功能亢进患者的肾、皮肤和肺等器官中观察到,也可以推测颅内血管可能也存在这种血管炎。但目前尚缺少甲基硫氧嘧啶引起血管炎的报道。本例患儿在脑梗死前2周一直服用甲基硫氧嘧啶治疗,因此药物性血管炎可能较小。
此外,尚有其他因素可能参与脑梗死的发病,如凝血异常、血流动力学改变、脑细胞的高代谢及氧消耗增加等,本例患儿无相关表现和实验室检查发现。
4 结论
DS患儿合并自身免疫性甲状腺炎等自身免疫性疾病时更易并发脑梗死,应该引起重视。在DS患者的随访过程中要注意脑梗死的发生,如一旦发现可采用温和免疫治疗方案,并适当抗凝治疗预防血栓形成。
[1]American Academy of Pediatrics: Health supervision for children with Down syndrome. Pediatrics,2001,107(2):442-449
[2]Pavone P, Falsaperla R, De Silva K, et al. Down syndrome and arterial ischemic stroke in childhood: A potential immunologic link with selective IgG4 subclass deficiency. Eur J Paediatr Neurol,2014:18(4):520-525
[3]Panichpisal K, Saigusa T, Sehli S, et al. Acute massive cerebral infarctions treated with hemodialysis. J Stroke Cerebrovasc Dis,2009,18(4):316-318
[4]Lee R, Sung K, Park YM, et al. A case of Moyamoya disease in a girl with thyrotoxicosis. Yonsei Med J,2009,50(4):594-598
[5]Yang CY, Juang SS, Chuang SS, et al. Down′s syndrome with mucosa-associated lymphoid tissue, thyroid lymphoma and cerebral infarction. Zhonghua Yi Xue Za Zhi (Taipei),2000,63(3):234-239
[6]Sass JO, Skladal D, Viertler E. Methionine loading in a Down′s syndrome patient with cerebral infarction. Ann Clin Biochem,1999,36 ( Pt 2):252-255
[7]Goldstein EM, Singer HS. Moyamoya-like disease in Down′s syndrome. Pediatr Neurosurg,1990,16(1):14-16
[8]Pearson E, Lenn NJ, Cail WS. Moyamoya and other causes of stroke in patients with Down syndrome. Pediatr Neurol,1985,1(3):174-179
[9]Takeda K, Takahashi S, Higano S, et al. Cortical laminar necrosis in a patient with moyamoya disease associated with Down syndrome: MR imaging findings. Radiat Med,1997,15(1):59-63
[10]Mito T, Becker LE. Vascular dysplasia in Down syndrome: a possible relationship to moyamoya disease. Brain Dev,1992,14(4):248-251
[11]Vicari S, Albertini G. Moyamoya disease in Down′s syndrome: a report of two cases. J Ment Defic Res,1991,35 ( Pt 4):392-397
[12]Storm W, Uhlenbrock D. Magnetic resonance imaging of moyamoya disease in a child with Down′s syndrome. J Ment Defic Res,1989,33 ( Pt 6):507-510
[13]Gibson PA, Newton RW, Selby K, et al. Longitudinal study of thyroid function in Down′s syndrome in the first two decades. Arch Dis Child,2005,90(6):574-578
[14]Steinlin M, Pfister I, Pavlovic J, et al. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics,2005,36(2):90-97
[15]Su X, Chen H. Chlamydia pneumoniae infection and cerebral infarction risk: a meta- analysis. Int J Stroke,2014,9(3):356-364
[16]Chu S, Hsu J, Lee C, et al. Neurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes. Plos One,2014,9(e10529411)
[17]Lynch JK, Han CJ. Pediatric stroke: what do we know and what do we need to know?. Semin Neurol,2005,25(4):410-423
[18]Fukushima Y, Kondo Y, Kuroki Y, et al. Are Down syndrome patients predisposed to Moyamoya disease?. Eur J Pediatr,1986,144(5):516-517
[19]Pysden K, Fallon P, Moorthy B, et al. Presumed perinatal stroke in a child with Down syndrome and moyamoya disease. Dev Med Child Neurol,2010,52(2):212-214
[20]Cramer SC, Robertson RL, Dooling EC, et al. Moyamoya and Down syndrome. Clinical and radiological features. Stroke,1996,27(11):2131-2135
[21]Dai AI, Shaikh ZA, Cohen ME. Early-onset Moyamoya syndrome in a patient with Down syndrome: case report and review of the literature. J Child Neurol,2000,15(10):696-699
[22]Korenberg JR. Toward a molecular understanding of Down syndrome. Prog Clin Biol Res,1993,384:87-115
[23]Boggs S, Hariharan SL. An uncommon presentation of stroke in a child with trisomy 21. Pediatr Emerg Care,2008,24(4):230-232
[24]Fujiwara S, Miyazono M, Tsuda H, et al. Intraventricular hemorrhage and cerebral ischemic attacks in the presence of lupus anticoagulant mimicking moyamoya disease. J Neurosurg Sci,1993,37(3):161-164
[25]Leno C, Mateo I, Cid C, et al. Autoimmunity in Down′s syndrome: another possible mechanism of Moyamoya disease. Stroke,1998,29(4):868-869
[26]Karlsson B, Gustafsson J, Hedov G, et al. Thyroid dysfunction in Down′s syndrome: relation to age and thyroid autoimmunity. Arch Dis Child,1998,79(3):242-245
[27]Idris I, O′Malley B P. Thyrotoxicosis in Down′s and Turner′s syndromes: the likelihood of Hashimoto′s thyroiditis as the underlying aetiology. Int J Clin Pract,2000,54(4):272-273
[28]Popova G, Paterson WF, Brown A, et al. Hashimoto′s thyroiditis in Down′s syndrome: clinical presentation and evolution. Horm Res,2008,70(5):278-284
[29]Ivarsson SA, Ericsson UB, Gustafsson J, et al. The impact of thyroid autoimmunity in children and adolescents with Down syndrome. Acta Paediatr,1997,86(10):1065-1067
[30]Goday-Arno A, Cerda-Esteva M, Flores-Le-Roux JA, et al. Hyperthyroidism in a population with Down syndrome (DS). Clin Endocrinol (Oxf),2009,71(1):110-114
[31]Baxter RG, Larkins RG, Martin FI, et al. Down syndrome and thyroid function in adults. Lancet,1975,2(7939):794-796
[32]Sheu JJ, Kang JH, Lin HC, et al. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke,2010,41(5):961-966
[33]Brandt F, Green A, Hegedus L, et al. A critical review and meta-analysis of the association between overt hyperthyroidism and mortality. Eur J Endocrinol,2011,165(4):491-497
[34]Tendler BE, Shoukri K, Malchoff C, et al. Concurrence of Graves′ disease and dysplastic cerebral blood vessels of the moyamoya variety. Thyroid,1997,7(4):625-629
[35]Stiehm ER, Ochs HD, Winkelstein JA. Immunological disorders in infants and children. 5th ed. USA:Elsevier Saunders,2004
[36]De Luca F, Corrias A, Salerno M, et al. Peculiarities of Graves′ disease in children and adolescents with Down′s syndrome. Eur J Endocrinol,2010,162(3):591-595
[37]Wasniewska M, Salerno M, Cassio A, et al. Prospective evaluation of the natural course of idiopathic subclinical hypothyroidism in childhood and adolescence. Eur J Endocrinol,2009,160(3):417-421
[38]Lammer C, Weimann E. Early onset of type I diabetes mellitus, Hashimoto′s thyroiditis and celiac disease in a 7-yr-old boy with Down′s syndrome. Pediatr Diabetes,2008,9(4 Pt 2):423-425
[39]Cuadrado E, Barrena MJ. Immune dysfunction in Down′s syndrome: primary immune deficiency or early senescence of the immune system?. Clin Immunol Immunopathol,1996,78(3):209-214
[40]Roizen NJ, Patterson D. Down′s syndrome. Lancet,2003,361(9365):1281-1289
[41]Cho HJ, Kim SS, Sung SM, et al. Impact of thyroid autoantibodies on functional outcome in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis,2014,23(7):1915-1920
[42]Ganesan V, Prengler M, Mcshane MA, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol,2003,53(2):167-173
[43]Mallick AA, O′Callaghan FJ. Risk factors and treatment outcomes of childhood stroke. Expert Rev Neurother,2010,10(8):1331-1346
[44]Schievink WI, Mokri B, Piepgras DG, et al. Intracranial aneurysms and cervicocephalic arterial dissections associated with congenital heart disease. Neurosurgery,1996,39(4):685-689, 689-690
[45]Lutterman J, Scott M, Nass R, et al. Moyamoya syndrome associated with congenital heart disease. Pediatrics,1998,101(1 Pt 1):57-60
[46]Colleran KM, Ratliff DM, Burge MR. Potential association of thyrotoxicosis with vitamin B and folate deficiencies, resulting in risk for hyperhomocysteinemia and subsequent thromboembolic events. Endocr Pract,2003,9(4):290-295
[47]Anan F, Takahashi N, Shimomura T, et al. Hyperhomocysteinemia is a significant risk factor for silent cerebral infarction in patients with chronic renal failure undergoing hemodialysis. Metabolism,2006,55(5):656-661
[48]Quax RA, Swaak AJ, Baggen MG. Churg-Strauss Syndrome following PTU Treatment. Int J Rheumatol,2009:504105
(本文编辑:张崇凡,李卫国)
Down′s syndrome in concomitant with Hashimoto thyrotoxicosis ,recurrent cerebral infarction: one case report and literature review
CHANGZhuo,LUOFei-hong,XILi,CHENGRuo-qian,ZHANGMiao-ying,LIXiao-jing
(DepartmentofPediatricEndocrinologyandInheritedMetabolicDisease,Children′sHospitalofFudanUniversity,Shanghai, 201102,China)
XI Li,E-mail:drxili@163.com
ObjectiveTo analyze the potential mechanisms of Down′s syndrome (DS) with cerebral infarction by reporting a case of Down′s syndrome (DS) combined with recurrent cerebral infarction, hashimoto thyrotoxicosis.MethodsThe diagnosis and treatments of a 10-years old girl with DS,Hashimoto thyrotoxicosis (HTT) and recurrent cerebral infarction were reported in detail. Clinical features of similar cases from published literatures were summarized. All literatures were retrieved systematically.ResultsA 10-years-old girl with DS(47,XX,+21) was admitted to our hospital due to weight loss, fatigue and sweating for 4 months. She was diagnosed as HTT due to obviously increased TPOAb, normal TRAb and depressed TSH. Test results were positive for anti-double-stranded DNA, anti-histone antibody, MPO and PR3. She then received antithyroid therapy and recovered. Two months after discharging from the hospital, she developed left and right sidecerebral infarction two times without obvious predisposing cause. She then accepted rehabilitation, anticoagulation and immunosuppressive therapy and myodynamia was recovered around three weeks later. Her general status kept stable more than one year before the current follow up. Nineteen related literatures concerning DS with cerebral infarction from web of science, pubmed, cnki and wanfang database were searched and seventeen of them were summarized. Infection occurred in 41.2% patients, moyamoya in 52.9%, thyroid dysfunction in 23.5% and 47.1% patients were found to have positive autoantibodies, however they had better prognosis in general.ConclusionChild with DS, cerebral infarction and HTT is extremely rare and autoimmunity disease in elder DS should be concerned.
Down′s syndrome ; Hashimoto′s thyroiditis; Thyrotoxicosis; Cerebral infarction; Children
复旦大学附属儿科医院内分泌遗传代谢科 上海,201102
奚立,E-mail:drxili@163.com
10.3969/j.issn.1673-5501.2015.03.011
2015-02-03
2015-05-20)
